Abstract
Myxoinflammatory fibroblastic sarcoma (MIFS) is a low grade soft tissue sarcoma with a predilection for acral sites, being associated with a high rate of local recurrence but very infrequent distant metastases. Although a t(1;10) translocation resulting in TGFBR3-MGEA5 fusion has been reported as a recurrent genetic event in MIFS, this abnormality is seen only in a subset of cases. As no studies to date have investigated the spectrum of alternative genetic alterations in TGFBR3-MGEA5 fusion negative MIFS, we undertook a genetic analysis of this particular cohort for further molecular classification. Triggered by an index case occurring in the finger of a 37 year-old female and harboring a novel TOM1L2-BRAF fusion by targeted RNA sequencing we investigated potential recurrent BRAF abnormalities by screening a large group of 19 TGFBR3-MGEA5 fusion negative MIFS by FISH. There were 6 (32%) additional MIFS with BRAF genetic abnormalities, including 5 gene rearrangements and one showing BRAF amplification. Interestingly, VGLL3 amplification, a recurrent genetic abnormality coexisting with t(1;10) in some MIFS, was also detected by FISH in 4/6 (67%) BRAF-rearranged MIFS, but not in the BRAF-amplified case. Up-regulated VGLL3 mRNA expression was also demonstrated in the index case by RNAseq. The 7 BRAF-rearranged/amplified MIFS arose in the fingers (n=3), and 1 each in wrist, forearm, foot, and knee, of adult patients (36–74 years; M:F=4:3). The histological spectrum ranged from predominantly solid growth of plump histiocytoid to epithelioid tumor cells with focal myxoid change to a predominantly myxoid background with scattered tumor cells. Varying degree of inflammatory infiltrates and large tumor cells with virocyte-like macronucleoli were observed in most cases. Immunohistochemical stains of phosphorylated ERK, a downstream effector of BRAF activation, were positive in all 4 cases tested (2 diffuse strong, 2 focal strong). Unlike t(1;10), BRAF rearrangements were only found in MIFS but not in 6 hemosiderotic fibrolipomatous tumor (HFLT) lacking TGFBR3-MGEA5 fusions (including 2 pure HFLT, 2 hybrid HFLT-MIFS, and 2 associated with pleomorphic hyalinizing angiectatic tumors).
Keywords: Myxoinflammatory fibroblastic sarcoma, BRAF, fusions, amplifications, VGLL3, MAPK
INTRODUCTION
Myxoinflammatory fibroblastic sarcoma (MIFS) is a locally recurrent and rarely metastasizing soft tissue neoplasm that often affects subcutaneous tissues of the acral regions.1–3 Microscopically, it is characterized by alternating solid and myxoid areas, a prominent inflammatory infiltrate, and the presence of Reed-Sternberg-like cells with inclusion-like macronucleoli among other less distinctive plump spindle to epithelioid tumor cells. Pseudolipoblasts with cytoplasmic vacuoles filled with mucoid substance, large tumor cells with smudgy chromatin, and emperipolesis are also common features. A t(1;10)(p22;q24) translocation, involving TGFBR3 and MGEA5 genes, has been reported as a recurrent genetic event in MIFS.4,5 The translocation juxtaposes TGFBR3 and MGEA5 in opposite directions of transcription, without forming a functional fusion transcript, but triggering instead up-regulation of FGF8 and NPM3, two neighboring genes of MGEA5.5 In addition to t(1;10), a subset of MIFS also harbor recurrent amplification of the 3p11.1–12.1 region, usually in the form of marker or ring chromosomes.5–7 This commonly amplified region includes VGLL3 (3p12.1) and CHMP2B (3p11.2), two genes showing increased expression levels associated with the amplification.5
Hemosiderotic fibrolipomatous tumor (HFLT) is a related soft tissue neoplasm that shares similar demographics and clinical features with MIFS, occurring in the ankle/foot of adult women. However, HFLTs have a benign appearance histologically, being composed of bland short spindle cells associated with hemosiderin deposition, forming cellular septa or wrapping around lobules of adipocytes creating a honeycomb pattern.8 Thus most HFLTs are morphologically distinct from MIFS, with only occasional cases showing myxoid stroma and increased nuclear atypia, resembling MIFS. Such lesions have been described as “hybrid HFLT-MIFS” in the literature and were found to have metastatic potential.6,9–12 The demonstration of identical t(1;10) genetic abnormalities with or without 3p amplifications in both pure HFLT and hybrid cases similar to MIFS has clarified their pathogenetic relationship in keeping with a morphologic spectrum.5,6,13
Despite earlier observations of recurrent t(1;10) translocations in both MIFS and HFLT,5 the incidence of this genetic abnormality is not well defined, with conflicting results from different studies, ranging from 6% to 71% for pure MIFS.6,9,10 No studies to date have attempted to investigate the genetic alterations in MIFS lacking t(1;10). Triggered by an index case with a novel BRAF-related fusion we sought to investigate the recurrent potential of this alteration in a large cohort of TGFBR3-MGEA5 fusion negative MIFS and HFLT.
MATERIALS AND METHODS
Index case and patient selection
The index case was a 37 year-old female presenting with a subcutaneous finger mass, which was excised and diagnosed as MIFS. Microscopically, the tumor showed a nodular lesion with a mildly infiltrative border into adjacent subcutaneous fat (Fig. 2A). It was composed predominantly of solid sheets of histiocytoid to epithelioid tumor cells with vesicular chromatin and prominent nucleoli, admixed with evenly distributed lymphocytes, scattered plasma cells, and occasional lymphoid aggregates. A focal myxoid component was also present, in sharp contrast from the solid areas (Fig. 2C). Characteristic Reed-Sternberg-like tumor cells with macronucleoli, large tumor cells with smudgy chromatin, and emperipolesis (Fig. 3E) were also observed. Mitotic activity was low (1/10 high power fields). Targeted RNA sequencing (RNAseq) was performed for fusion assessment. The fusion candidate identified by RNAseq fusion algorithm was further validated by FISH and PCR (see below).
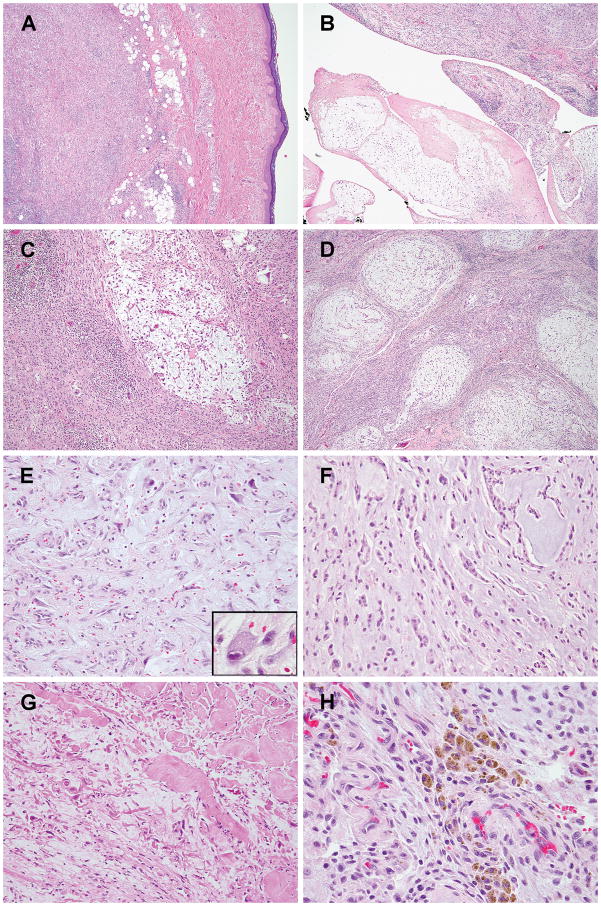
Figure 2. Morphologic spectrum of MIFS with BRAF gene rearrangements.
(A) Most of the tumors present as subcutaneous nodules with mildly infiltrative borders (case #1). (B) Effaced synovium by tumor in one of the two cases with intra-articular involvement (case #4). (C–E) Variable myxoid areas, ranging from microscopic foci with abrupt transition to a largely solid mass (C, case #1), large, multinodular with solid areas in between (D, case #4), or extensive myxoid changes with rich small vessel proliferation and scattered plump, epithelioid or stellate tumor cells (E, case #5) and occasional Reed-Sternberg-like cells with macronuceloli (inset). (F) Infrequently, the tumor cells have a cord-like or reticular pattern (case #4). (G) Prominent collagenous stroma admixed with myxoid areas in an intra-articular knee lesion (case #6). (H) Hemosiderin deposition, predominantly with a perivascular distribution, in a wrist mass involving both subcutaneous tissues and joint (case #4).
Figure 3. Histologic features of MIFS with BRAF rearrangements.
(A–C) Characteristic Reed-Sternberg-like tumor cells with macronucleoli were observed in all BRAF-rearranged cases, either intermixed among smaller histiocytoid tumor cells and neutrophilic infiltrate (A, case #2), obscured by dense inflammatory infiltrate composed of plasma cells, lymphocytes and eosinophils (B, initial excision of case #3), or scattered within the myxoid component (C, re-excision of residual lesion in case #3). (D–E) An exceptionally mitotically active (up to 40/10 HPFs) and cellular MIFS composed of sheets of epithelioid cells (D) and also areas typical for MIFS with myxoid stroma and inflammatory infiltration (E) (case #2). (F–G) Emperipolesis (F, case #1) and pseudolipoblasts (G, case #2), frequent features of MIFS, were also observed in 3 cases each.
In order to assess the incidence of this genetic abnormality, we identified 19 cases of MIFS with classic morphologic findings lacking either TGFBR3 and/or MGEA5 gene rearrangements by FISH. MIFS were defined as tumors showing alternating areas of solid and myxoid pattern, composed of histiocytoid, epithelioid or plump spindle cells, commonly associated with prominent nucleoli, inflammatory infiltration, and pseudolipoblasts. Cases without a typical morphology such as tumors with overt nuclear pleomorphism or lacking myxoid areas were excluded. Cases in the screening cohort with known locations were all located in the extremities, predominantly acral areas. In addition, 7 cases of MIFS harboring TGFBR3 and/or MGEA5 gene rearrangements were collected for morphologic comparison as well as to confirm that MGEA5 and TGFBR3 rearrangements are mutually exclusive to BRAF-gene abnormalities. Six of MIFS showed TGFBR3-MGEA5 fusions, while the remaining case had only TGFBR3 rearrangement. Representative hematoxylin and eosin slides of all cases were reviewed. FISH for BRAF rearrangements were performed on FFPE sections. Clinical and pathologic information was collected from the electronic medical records.
As a control group, we included 4 HFLT and 2 hybrid HFLT-MIFS cases negative for the t(1;10). HFLTs were defined as proliferations of bland short spindle cells exhibiting infiltrative growth among subcutaneous adipocytes and associated with hemosiderin deposition. Two of the HFLT showed areas of increased cellularity and ectatic vessels with hyalinized wall and thrombosis, in keeping with the so-called HFLT with PHAT (pleomorphic hyalinizing angiectatic tumor) component. The study was approved by the Institutional Review Board.
Targeted RNA sequencing
For the index case, RNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissues using Amsbio’s ExpressArt FFPE Clear RNA Ready kit (Amsbio LLC, Cambridge, MA). Fragment length was assessed with an RNA 6000 chip on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA-seq libraries were prepared using 20–100 ng total RNA with the Trusight RNA Fusion Panel (Illumina, San Diego, CA). Targeted RNA sequencing was performed on an Illumina MiSeq platform. Reads were independently aligned with STAR(ver 2.3) against the human reference genome (hg19) and analyzed by STAR-Fusion.
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was performed to validate the gene fusion found by targeted RNA sequencing in the index case. RNA was extracted from FFPE tissues using RNeasy FFPE (Qiagen) and reverse transcribed by SuperScript IV First-Strand Synthesis System (Invitrogen). PCR was performed by Advantage 2 PCR kit (Clontech, Mountain View, CA) with forward primer on TOM1L2 exon 10/11 1 (5′ –GAGCAGCGCAAGACGGTAACC – 3′) and reverse primer on BRAF exon 10 (5′– GCTGAGGTCCTGGAGATTTCTGTAAG –3′). The reaction was run at 68°C annealing temperature for 36 cycles. The PCR product was analyzed by gel electrophoresis and Sanger sequencing.
Fluorescence in situ hybridization (FISH)
FISH for BRAF rearrangements were performed for validation in the index case and screening in the following screening and control cohorts. Cases positive for BRAF rearrangements, were subsequently tested for TOM1L2 rearrangements. All MIFS with available material were also tested for VGLL3 amplifications. Custom probes were made by bacterial artificial chromosomes (BAC) clones flanking the target genes for BRAF and TOM1L2 and covering the target region for VGLL3, according to UCSC genome browser (http://genome.ucsc.edu) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (Oakland, CA; http://bacpac.chori.org) (Supplementary Table 1). Reference probes on 3q12.1 – 3q12.2 were used for VGLL3 (3p12.1). DNA from each BAC was isolated according to the manufacturer’s instructions. The BAC clones were labeled with fluorochromes (fluorescent-labeled dUTPs, Enzo Life Sciences, NY, USA) by nick translation and validated on normal metaphase chromosomes. The 4 μm-thick FFPE slides were deparaffinized, pretreated, and hybridized with denatured probes. After overnight incubation, the slides were washed, stained with DAPI, mounted with an antifade solution, and then examined on a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany) controlled by Isis 5 software (Metasystems).
Gene expression analysis
The mRNA expression levels of certain genes of interest, including BRAF, FGF8, and VGLL3, were evaluated by the RNAseq data of the index case, comparing it to 9 other samples run on the same RNAseq platform, including 2 CIC-rearranged round cell sarcomas, 2 synovial sarcomas, 1 alveolar rhabdomyosarcoma, 1 epithelioid hemangioma, 1 soft tissue myoepithelial carcinoma, 1 cellular neurothekeoma, 1 low-grade fibromyxoid sarcoma. In addition, whole transcriptome sequencing data of 7 BRAF-rearranged tumors including thyroid carcinomas and melanomas were downloaded from The Cancer Genome Atlas (TCGA) database to examine the expression levels of BRAF and VGLL3 in other BRAF-rearranged tumor types.14 The expression levels were compared to other sarcoma samples in our whole transcriptome sequencing database.
Immunohistochemistry for phosphorylated ERK (pERK)
To evaluate the activation of MAPK signaling pathway as a result of BRAF activation, immunohistochemical staining for pERK was performed in 4 BRAF-rearranged MIFS with available materials. Some tumors with t(1;10), including 2 MIFS, 2 hybrid HFLT-MIFS and 1 HFLT, were also tested for pERK IHC. The stains were performed on 4 μm-thick FFPE tissue sections using monoclonal rabbit antibody, phospho-p44/42MAPK (202Y284) (Cell Signaling Technology; 1:1000 dilution). The staining protocol was as reported previously.15 The staining intensity (strong, moderate, weak) and extent (focal, <50%; patchy, 50–80%; diffuse, >80%) were recorded.
RESULTS
BRAF rearrangement is a recurrent event in pure MIFS but not in other related lesions
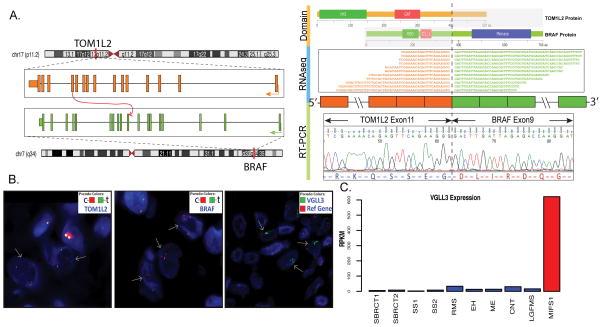
Targeted RNAseq identified a novel inter-chromosomal TOM1L2-BRAF fusion candidate in the index case, which was further confirmed by RT-PCR showing exon 11 of TOM1L2 (17p11.2) fused to exon 9 of BRAF (7q34) (Fig. 1A). The BRAF kinase domain was retained in the predicted chimeric protein. FISH validation showed unbalanced rearrangements of both BRAF and TOM1L2 genes, with increased copy number of 5′ TOM1L2 (centromeric) and 3′ BRAF (centromeric) signals (Fig. 1B). The mRNA expression levels of BRAF were not significantly increased in the index MIFS case. Unlike previously reported MIFS with TGFBR3 and MGEA5 rearrangements,5 the index MIFS case with BRAF-related fusion lacked FGF8 up-regulation.
Figure 1. Index case of MIFS with a novel TOM1L2-BRAF fusion.
(A) A t(7;17) translocation joining exon 11 of TOM1L2 with exon 9 of BRAF was identified by targeted RNA sequencing and confirmed by RT-PCR. The predicted chimeric protein from the in-frame fusion contains the VHS (Vps27-Hrs-STAM) and GAT (GAA and Tom1) domains of TOM1L2 and the protein kinase domain of BRAF. The N-terminal RBD (Raf-like RAS-binding domain) and C1_1 (Phorbol esters/diacylglycerol binding domain) domains of BRAF with auto-inhibitory functions are replaced. (B) Unbalanced rearrangements of TOM1L2 and BRAF were validated by FISH, showing separate red and green signals with increased copy number of 5′ TOM1L2 (red) and 3′ BRAF (red) (arrows). VGLL3 (3p12.1) amplification was also found by FISH in this case (arrows) (reference probe on 3q12.1–12.2). (C) VGLL3 mRNA expression is up-regulated in the index case (MIFS1) in comparison to other samples, including 2 CIC-rearranged round cell sarcomas (SBRCT1, SBRCT2), 2 synovial sarcomas (SS1, SS2), 1 alveolar rhabdomyosarcoma (RMS), 1 epithelioid hemangioma (EH), 1 soft tissue myoepithelial carcinoma (ME), 1 cellular neurothekeoma (CNT), and 1 low-grade fibromyxoid sarcoma (LGFMS), by same RNA sequencing platform expression analysis.
In the subsequent screening cohort, FISH identified 5 additional MIFS with BRAF rearrangements among 19 TGFBR3/MGEA5-negative MIFS, indicating that BRAF rearrangement is a recurrent event in MIFS. BRAF rearrangements often showed unbalanced patterns with telomeric (5′) deletion or inversions. The 5′ fusion partners were unknown as FISH for TOM1L2 were negative in these 5 cases and no additional materials were available for RNAseq. An additional MIFS case showed BRAF amplification without rearrangement (Fig. 4C). No BRAF rearrangement or amplification was found in the 7 MIFS with TGFBR3 and/or MGEA5 rearrangements, suggesting that BRAF abnormalities are mutually exclusive from TGFBR3 and MGEA5 rearrangements.
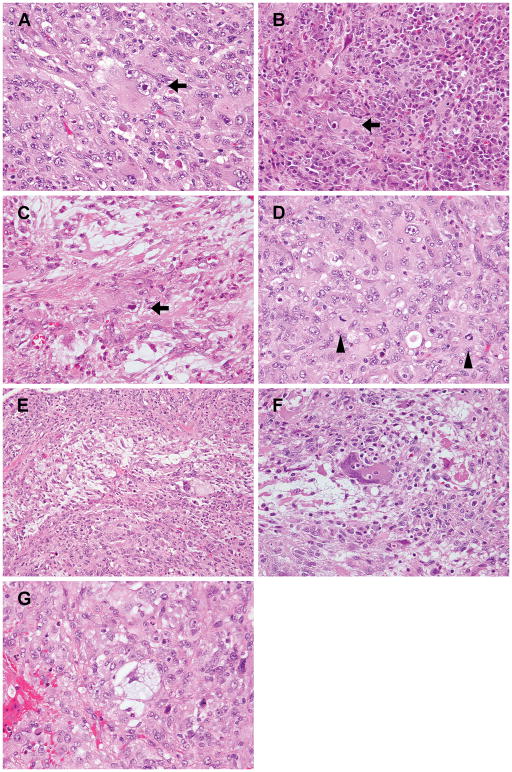
Figure 4. MIFS with BRAF amplification.
(A) Alternating myxoid and solid areas of histiocytoid tumor cells, accompanied by lymphocytic infiltrate and vascular proliferation. (B) Tumor cells with round to ovoid nuclei and occasionally prominent nucleoli (arrow). Reed-Sternberg-like tumor cells are not seen in this case. (C) BRAF amplification without rearrangement was identified by FISH.
In contrast to the BRAF rearrangements/amplifications found in some t(1;10)-negative MIFS, all the 6 cases of t(1;10)-negative HFLT, including 2 hybrid HFLT-MIFS, were negative for BRAF by FISH.
VGLL3 amplification and pERK expression is shared in both MIFS with BRAF and t(1;10) -suggesting a possible common pathogenetic mechanism
In order to assess the shared amplifications of the 3p11.1–12.1 region between MIFS with t(1;10) and MIFS with BRAF gene rearrangements/amplifications we performed VGLL3 FISH in the entire study group. VGLL3 amplifications were found in 4 of 6 BRAF-rearranged cases, including the index case (Table 1). The BRAF-amplified case was negative for VGLL3 amplification. The amplified VGLL3 signals were usually separate from the reference probe at 3q12.1 – 3q12.2 (Fig. 1B), consistent with previously reported cytogenetic findings that the amplification results in a marker or ring chromosome. The mRNA expression level examined in the index case showed an up-regulated VGLL3 expression (Fig. 1C). VGLL3 amplifications were also identified in 4 of 7 MIFS with t(1;10) and 6 of 13 MIFS lacking both BRAF and t(1;10) fusions. Overall, VGLL3 amplifications were present in 52% (14/27) of MIFS tested, with equal distribution among different MIFS subsets. All cases with VGLL3 amplifications were located in acral regions, except one MIFS from the lower leg lacking both fusions.
Table 1.
Myxoinflammatory fibroblastic sarcomas with BRAF rearrangements or amplifications
| No | Age/Sex | Site | Depth(size) | Histology | pERKIHC | FISH | Treatment; Follow-up | ||
|---|---|---|---|---|---|---|---|---|---|
| Myxoid stroma | Inflammatory cells | BRAF | VGLL3 | ||||||
| 1 | 37/F | Finger | Subcutis(1.0 cm) | 5% | Lymphocytes, plasma cells | Focal strong | Pos* | Amp | Recent case |
| 2 | 74/M | Finger | Subcutis / deep dermis (2.0 cm) | 5% | Neutrophils | Focal strong | Pos | Amp | Excision and Ray amputation; NED, DOO 6 years later (lung cancer) |
| 3 | 36/M | Finger | Subcutis(3.0 cm) | 50% | Plasma cells, eosinophils | Diffuse strong | Pos | Amp | 3 excisions; NED (5.5 years) |
| 4 | 58/M | Wrist | Subcutis, tendon, intra-articular(NA) | 90% | Lymphocytes | Diffuse strong | Pos | Amp | 1 excision; Recurred 6 months later (wide excision, radiation, re-resection); NED (3 years) |
| 5 | 52/F | Forearm | Subcutis(NA) | >90% | Not prominent | NA | Pos | Neg | 3 excisions; NED (7 months) |
| 6 | 56/M | Knee | Intra-articular(2.3 cm) | 30% | Lymphocytes | NA | Pos | Neg | NA |
| 7 | 57/F | Foot | Subcutis (NA) | 90% | Lymphocytes | NA | Amp | Neg | Chemotherapy and excision; Recurred 4 years later (excision), NED (5 years) |
pERK IHC, phosphorylated ERK immunostain; F, female; M, male; Pos, positive for rearrangement; Amp, amplified; DOO, died of other diseases; NED, no evidence of disease; NA, not available; Neg, not amplified.
fusion partner known to be TOM1L2
Immunostaining of pERK showed diffuse or focal strong staining in all 4 cases tested (Supplementary Fig. 1), compatible with the downstream effect of BRAF activation. The 2 cases with focal expression showed enhanced staining at the edges of tissues, as noted in a previous study.16 Interestingly, in addition to BRAF-rearranged MIFS, pERK immunostaining also showed positivity in 3 of 5 tumors with t(1;10) tested, including 1 of 2 MIFS (patchy strong), 1 of 2 hybrid HFLT-MIFS (diffuse strong), and 1 HFLT (focal moderate).
MIFS with BRAF abnormalities show a similar histologic spectrum to other MIFS subsets
The clinicopathologic features of the 7 MIFS with BRAF rearrangements or amplifications are summarized in Table 1. The tumors occurred in adult patients (36–74 years old; mean, 53; median, 56) of both genders (M:F=4:3). There is a predilection for acral regions (3 in fingers, 1 in foot) followed by non-acral extremities (one each in wrist, forearm, and knee). The tumors most commonly involved subcutaneous tissues (Fig. 2A), while tendon and intra-articular involvement was noted in 2 cases (Fig. 2B). Histologically, the tumors presented either as a single nodule with minimal infiltration of surrounding tissues (Fig. 2A) or as a multinodular mass. The degree of myxoid areas varied significantly (Table 1), including focal myxoid change in a predominantly solid tumor (Fig. 2C), multiple myxoid nodules (Fig. 2D), or diffuse myxoid stroma with or without rich vascularity (Fig. 2E). In most cases the myxoid component contained individual and scattered tumor cells (Fig. 2C, E), except for case #4 where tumor cells were arranged in a cord-like or reticular pattern (Fig. 2F). One case showed prominent collagenous stroma intermixed with the myxoid areas (Fig. 2G). Hemosiderin-laden histiocytes were only noted in one case, distributed mostly peri-vascular (Fig. 2H). Most tumor cells showed a histiocytoid or epithelioid phenotype, with plump vesicular nuclei, distinct nucleoli, and variable amount of eosinophilic cytoplasm (Fig. 3A, C–G). Reed-Sternberg-like tumor cells with macronucleoli were seen in all BRAF-rearranged cases, at least focally, present either among sheets of histiocytoid tumor cells (Fig. 3A, arrow), embedded within a dense inflammatory infiltrates (Fig. 3B, arrow), or myxoid areas (Fig. 3C, arrow). No HFLT component was identified in any of the cases. The inflammatory component varied among cases, typically composed of lymphocytes, plasma cells, eosinophils, and/or neutrophils (Fig. 3A,B; Table 1). Mitotic activity was generally below 4/10 high power fields (HPFs), except in 2 cases. Case #3 had a mitotic count of up to 6/10 HPFs, including a few atypical mitoses. Case #2 had an overall increased cellularity with a high mitotic count (up to 40/10 HPFs) (Fig. 3D, arrowheads), including atypical forms, but was otherwise morphologically typical (Fig. 3E). Emperipolesis (Fig. 3F) and pseudolipoblasts (Fig. 3G) were seen in 3 cases each. The only BRAF-amplified case was a predominantly myxoid tumor with areas of solid histiocytoid tumor cells, foci of lymphocytic infiltrate, and rich vascular proliferation (Fig. 4A). Some tumor cells showed more prominent nucleoli (Fig. 4B), but no Reed-Sternberg-like cells were identified.
MIFS with BRAF gene abnormalities show propensity for local recurrence but no distant spread
Clinical information available in 5 patients showed an indolent clinical course with an increased risk for local recurrences but no distant metastases. Two patients reported a prolonged history before the tumors were removed, with a slow-growing mass at the base of the right middle finger for 8 years before being excised as a 3.0 cm mass (case #3) and in the wrist for 2–3 years (case #4). Primary tumor size ranged from 1–3 cm in the 4 cases with known tumor size. Three patients (cases #2, #3, #5) received multiple initial surgeries to achieve clear margins of their primary lesions. No recurrent or metastatic event was noted in these 3 patients (follow-up period: 7 months – 6 years), despite the high mitotic count in case #2. One patient (case #4) developed a local recurrence 6 month after the initial excision (margin status unknown due to fragmentation), which was treated with wide excision and radiation, with no subsequent recurrence at last follow-up (3 years). The patient with BRAF-amplified MIFS (case #7) received neoadjuvant chemotherapy, followed by a surgical excision (unknown margin status) and subsequently developed a late local recurrence 4 years later, which was re-excised.
DISCUSSION
In this study, we describe a group of 6 MIFS with recurrent BRAF gene rearrangements which occurred in 22% of pure MIFS. Compared to t(1;10) positive MIFS or fusion-negative MIFS, the BRAF-rearranged cases also showed a predilection for acral locations in adult patients1–3 and typical morphologic features, with varying proportions of myxoid and solid areas, histiocytoid to plump spindle tumor cells, and characteristic Reed-Sternberg-like cells. Furthermore, patients with this novel genetic subset had a similar clinical behavior with an increased risk of local recurrence, especially if incompletely excised, but no distant metastases. Although most BRAF fusion-positive MIFS showed a low mitotic count, one outlier case showed a high proliferative activity with 40/10 HPFs (case #2) despite an otherwise typical morphology for MIFS. Despite this increased mitotic activity, the patient did not develop metastases, being NED after 6 years follow-up. MIFS with high mitotic count have been rarely reported, and their clinical significance remains unclear.16,17
A recurrent t(1;10) translocation was the initial genetic abnormality described in MIFS,4 shown later to result in the fusion of MGEA5 and TGFBR3 genes by Hallor et al in 2009.5 In their study, Hallor et al also identified a t(1;10) in a case of HFLT, suggesting for the first time their possible relationship.5 After a single case report of a hybrid MIFS-HFLT harboring t(1;10) by cytogenetics,11 our group identified MGEA5 and TGFBR3 rearrangements by FISH not only in 5 of 7 (71%) pure MIFS, but also in 12 of 14 (86%) HFLT and 3 cases with mixed MIFS/HFLT histology.6 However, further studies showed a significantly lower incidence of this fusion in pure MIFS, being present in 17% (1/6) or 6% (2/31) of the cases.9,10 As MIFS display a wide spectrum of morphologic variations, which may overlap with other entities, such discrepancy might be related to different diagnostic criteria used. In the present study, among the total cohort of 27 pure MIFS using very strict morphologic criteria, 7 (26%) cases had TGFBR3 and/or MGEA5 rearrangements (6, both genes; 1, TGFBR3 only), 7 (26%) cases had BRAF alterations (6, rearrangements; 1, amplification), and 13 (48%) cases were negative for TGFBR3, MGEA5 and BRAF gene rearrangements. These two genetic abnormalities appeared to be mutually exclusive. Our data also show that BRAF rearrangements/amplifications were restricted to pure MIFS, and were not found in TGFBR3-MGEA5 fusion negative HFLTs. Unlike TGFBR3 and MGEA5 rearrangements which do not form a fusion transcript, BRAF rearrangements resulted in an in-frame fusion transcript which was detected by RNAseq and RT-PCR in our index case.
Comparing the 2 genetic cohorts of MIFS, harboring either BRAF gene abnormalities or TGFBR3-MGEA5, the tumor location in both groups was limited to the extremities, with predilection for acral sites (71%). MIFS with BRAF alterations were more common in the upper than the lower extremities (5 upper, 2 lower), while MIFS with TGFBR3 and/or MGEA5 rearrangements involved lower extremities more often (2 upper, 5 lower). Morphologically, both groups showed alternating myxoid and solid areas and involved mainly subcutaneous tissues. MIFS with BRAF alterations often displayed tumor cells with vesicular chromatin, prominent nucleoli, and Reed-Sternberg-like cells in varying amount. In contrast, MIFS with TGFBR3 and/or MGEA5 rearrangements had more variable chromatin patterns ranging from vesicular, fine, to hyperchromatic and Reed-Sternberg-like cells were more difficult to find (identified only in 3/7 cases). In addition to morphologically typical areas, 2 MIFS with TGFBR3/MEGA5 alterations also showed elongated spindle cells arranged in fascicles, which were not seen in cases with BRAF alterations. Despite these minor anatomic and morphologic differences, there was a significant clinicopathologic overlap between these two molecular groups. Inflammatory cell infiltrates and pseudolipoblasts were observed in both subsets. Also of note VGLL3 gene amplifications were identified with the same frequency (4 of 7) in both MIFS cohorts, regardless of the TGFBR3-MGEA5 and BRAF alterations.
BRAF encodes a serine/threonine protein kinase that regulates the MAPK signaling pathway. BRAF gene fusions have been identified as the driver genetic events in several different tumor types, including the majority of pilocytic astrocytomas,18 a subset of pancreatic acinar cell carcinomas,15 and smaller proportions of papillary thyroid carcinomas,19,20 spitzoid melanocytic neoplasms,21–23 rare cases of Langerhans cell histiocytosis,24 prostate cancer, gastric cancer,25 lung cancer,26 etc.27 In soft tissue tumors, only a chest wall mass of a 55 year-old woman diagnosed as a malignant spindle cell neoplasm has been reported to have KIAA1549–BRAF fusion to date, although its reported immunoprofile with co-expression of both S100 and CD34 are unsual.28 In a large study cohort across different tumor types, myxofibrosarcomas and pigmented villonodular synovitis, which are common differential diagnoses of MIFS, lacked BRAF gene fusions; while no MIFS were included in that study.27 Similar to BRAF fusions in other tumors, the chimeric protein in our index case is predicted to lose the N-terminal autoinhibitory domain (encoded by exons 1–8) of wild type BRAF, and retain the C-terminal kinase domain (exons 11–18). The mRNA expression level of BRAF was not significantly up-regulated in our index case, but this finding is in keeping with reported TCGA data from BRAF-rearranged thyroid carcinomas and melanomas (data not shown). BRAF fusions involve a wide range of different 5′ fusion partners, resulting from either inter- or intra-chromosomal fusions.15,18–25,27 TOM1L2 (target of myb1 like 2 membrane trafficking protein) is a novel fusion partner which has not been previously reported. The physiologic function of TOM1L2 is associated with protein trafficking. Mouse models with reduced Tom1l2 expression developed infections and a variety of tumors, suggesting its role in immune regulation and possibly tumor suppression.29
Tumors with BRAF fusions, including pilocytic astrocytomas and melanomas, have shown evidence of clinical sensitivity to RAF or MEK inhibitors, based on in vitro experiments and few clinical reports.21,22,27,30–32 The identification of BRAF fusions in MIFS may also offer new potential therapeutic options for clinically protracted cases. In pilocytic astrocytoma with BRAF fusions, the slow growing and low-grade nature of the tumor has been attributed to oncogene-induced senescence (OIS).33 Likewise, MIFS also has a low-grade clinical behavior with rare metastasis. CEBPB, a reported OIS marker, was highly expressed in our index case, but not significantly different from other tumor types (data not shown). Therefore, the role of OIS in MIFS remains uncertain.
Unlike activating mutations and fusions, the role of BRAF amplification as an oncogenic event is less defined. BRAF gene amplifications have been described in rare spitzoid melanocytic neoplasms: one desmoplastic Spitz nevus and one atypical Spitz tumor.23 However, the downstream effects and oncogenic potential have not yet been demonstrated. In an early report of MIFS, 2 cases were found to have chromosome 7 gains by comparative genomic hybridization, but the involved different regions (7q31q32 in one case; 7p15p21 in the other) did not encompass the BRAF locus (7q34).34 Further investigations are needed to elucidate if BRAF amplification is a recurrent event in MIFS and is associated with any clinicopathologic features.
In this study, MIFS with BRAF fusions showed pERK immunoreactivity, indicating activated downstream MAPK signaling pathway, as in other tumors with BRAF fusions or activating mutations.15,18–22,24,25,35 Notably, pERK immunostain was also positive in tumors with TGFBR3-MGEA5 fusions, including MIFS, HFLT, and hybrid HFLT-MIFS. MAPK pathway activation may be a common event shared by MIFS with BRAF fusions and those with TGFBR3-MGEA5 fusions. This is of particular interest given MAPK signaling pathway is also known to be activated by FGF8,36,37 which is usually up-regulated in tumors with TGFBR3-MGEA5 rearrangements.5 In our index case, FGF8 was not up-regulated based on RNAseq data, suggesting different mechanisms for increased ERK phosphorylation.
Another common feature between MIFS with BRAF rearrangements and TGFBR3-MGEA5 rearrangements is the amplification and subsequent up-regulation of VGLL3 gene.5–7 VGLL3 functions as a cofactor of the TEAD transcription factors and may promote the proliferation and migration of tumor cells harboring VGLL3 amplification.38–40 In addition to MIFS and HFLT, VGLL3 amplifications have also been reported in dedifferentiated liposarcoma and undifferentiated pleomorphic sarcoma, presumed to be a genetic event occurring during tumor progression.38 However, MIFS with VGLL3 amplifications showed no significant prognostic difference. Of note the VGLL3 amplification detected in our index case with BRAF fusion was associated with VGLL3 mRNA overexpression. In contrast, the VGLL3 expression levels from the TCGA data of BRAF-fusion positive thyroid carcinomas and melanomas were not up-regulated.
The presence of BRAF fusions in MIFS but not in HFLT or hybrid HFLT-MIFS seems to support the hypothesis that at least some pure MIFS are unrelated to HFLT. However, the downstream pERK activation was observed in different subsets of MIFS as well as in HFLT. In addition, both tumor types share the accompanying VGLL3 amplifications in a subset of cases. Further studies are needed to clarify whether the functional role of BRAF fusions in MIFS is equivalent to that of TGFBR3-MGEA5 fusions. The on-going controversy in the literature regarding the MIFS/HFLT pathologic spectrum and its subsequent challenging subclassification is most likely a reflection of the variability in their genetic signatures.
In conclusion, we describe BRAF rearrangements as novel and recurrent gene abnormalities occurring in 22% of pure MIFS showing classic clinicopathologic features, but not in other related lesions, such as HFLT or mixed HFLT-MIFS. BRAF abnormalities coexisted with VGLL3 amplifications, which is similar to MIFS and HFLT with TGFBR3 and MGEA5 rearrangements. The activated MAPK pathway provides a potential therapeutic target for RAF or MEK inhibitor.
Supplementary Material
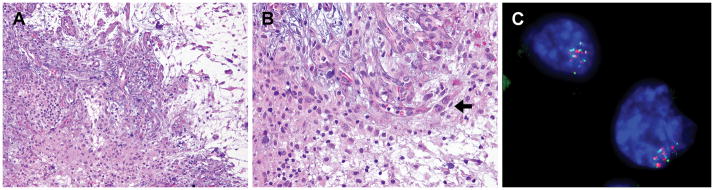
All four cases tested for pERK immunohistochemical staining showed strong staining pattern, ranging from diffuse (A, case #3) to focal (B, case #2; C, case #1).
Acknowledgments
Supported in part by: P50 CA140146-01 (CRA); P30-CA008748 (CRA); Kristen Ann Carr Foundation (CRA); Cycle for Survival (CRA)
Footnotes
Conflicts of interest: none
References
- 1.Montgomery EA, Devaney KO, Giordano TJ, et al. Inflammatory myxohyaline tumor of distal extremities with virocyte or Reed-Sternberg-like cells: a distinctive lesion with features simulating inflammatory conditions, Hodgkin’s disease, and various sarcomas. Mod Pathol. 1998;11:384–391. [PubMed] [Google Scholar]
- 2.Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22:911–924. doi: 10.1097/00000478-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Michal M. Inflammatory myxoid tumor of the soft parts with bizarre giant cells. Pathol Res Pract. 1998;194:529–533. doi: 10.1016/S0344-0338(98)80041-1. [DOI] [PubMed] [Google Scholar]
- 4.Lambert I, Debiec-Rychter M, Guelinckx P, et al. Acral myxoinflammatory fibroblastic sarcoma with unique clonal chromosomal changes. Virchows Arch. 2001;438:509–512. doi: 10.1007/s004280000376. [DOI] [PubMed] [Google Scholar]
- 5.Hallor KH, Sciot R, Staaf J, et al. Two genetic pathways, t(1;10) and amplification of 3p11–12, in myxoinflammatory fibroblastic sarcoma, haemosiderotic fibrolipomatous tumour, and morphologically similar lesions. J Pathol. 2009;217:716–727. doi: 10.1002/path.2513. [DOI] [PubMed] [Google Scholar]
- 6.Antonescu CR, Zhang L, Nielsen GP, et al. Consistent t(1;10) with rearrangements of TGFBR3 and MGEA5 in both myxoinflammatory fibroblastic sarcoma and hemosiderotic fibrolipomatous tumor. Genes Chromosomes Cancer. 2011;50:757–764. doi: 10.1002/gcc.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansoor A, Fidda N, Himoe E, et al. Myxoinflammatory fibroblastic sarcoma with complex supernumerary ring chromosomes composed of chromosome 3 segments. Cancer Genet Cytogenet. 2004;152:61–65. doi: 10.1016/j.cancergencyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Browne TJ, Fletcher CD. Haemosiderotic fibrolipomatous tumour (so-called haemosiderotic fibrohistiocytic lipomatous tumour): analysis of 13 new cases in support of a distinct entity. Histopathology. 2006;48:453–461. doi: 10.1111/j.1365-2559.2006.02360.x. [DOI] [PubMed] [Google Scholar]
- 9.Carter JM, Sukov WR, Montgomery E, et al. TGFBR3 and MGEA5 rearrangements in pleomorphic hyalinizing angiectatic tumors and the spectrum of related neoplasms. Am J Surg Pathol. 2014;38:1182–1992. doi: 10.1097/PAS.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 10.Zreik RT, Carter JM, Sukov WR, et al. TGFBR3 and MGEA5 rearrangements are much more common in “hybrid” hemosiderotic fibrolipomatous tumor-myxoinflammatory fibroblastic sarcomas than in classical myxoinflammatory fibroblastic sarcomas: a morphological and fluorescence in situ hybridization study. Hum Pathol. 2016;53:14–24. doi: 10.1016/j.humpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Elco CP, Marino-Enriquez A, Abraham JA, et al. Hybrid myxoinflammatory fibroblastic sarcoma/hemosiderotic fibrolipomatous tumor: report of a case providing further evidence for a pathogenetic link. Am J Surg Pathol. 2010;34:1723–1727. doi: 10.1097/PAS.0b013e3181f17d51. [DOI] [PubMed] [Google Scholar]
- 12.Solomon DA, Antonescu CR, Link TM, et al. Hemosiderotic fibrolipomatous tumor, not an entirely benign entity. Am J Surg Pathol. 2013;37:1627–1630. doi: 10.1097/PAS.0b013e31829ff078. [DOI] [PubMed] [Google Scholar]
- 13.Wettach GR, Boyd LJ, Lawce HJ, et al. Cytogenetic analysis of a hemosiderotic fibrolipomatous tumor. Cancer Genet Cytogenet. 2008;182:140–143. doi: 10.1016/j.cancergencyto.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chmielecki J, Hutchinson KE, Frampton GM, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398–1405. doi: 10.1158/2159-8290.CD-14-0617. [DOI] [PubMed] [Google Scholar]
- 16.Laskin WB, Fetsch JF, Miettinen M. Myxoinflammatory fibroblastic sarcoma: a clinicopathologic analysis of 104 cases, with emphasis on predictors of outcome. Am J Surg Pathol. 2014;38:1–12. doi: 10.1097/PAS.0b013e31829f3d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vroobel K, Miah A, Fisher C, et al. Myxoinflammatory fibroblastic sarcoma of the scalp: aggressive behavior at a rare, nonextremity site. Int J Surg Pathol. 2015;23:292–297. doi: 10.1177/1066896915571452. [DOI] [PubMed] [Google Scholar]
- 18.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciampi R, Knauf JA, Kerler R, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123:4935–4944. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botton T, Yeh I, Nelson T, et al. Recurrent BRAF kinase fusions in melanocytic tumors offer an opportunity for targeted therapy. Pigment Cell Melanoma Res. 2013;26:845–851. doi: 10.1111/pcmr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson KE, Lipson D, Stephens PJ, et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin Cancer Res. 2013;19:6696–6702. doi: 10.1158/1078-0432.CCR-13-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakraborty R, Burke TM, Hampton OA, et al. Alternative genetic mechanisms of BRAF activation in Langerhans cell histiocytosis. Blood. 2016;128:2533–2537. doi: 10.1182/blood-2016-08-733790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palanisamy N, Ateeq B, Kalyana-Sundaram S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang JS, Lee A, Li J, et al. Common Oncogene Mutations and Novel SND1-BRAF Transcript Fusion in Lung Adenocarcinoma from Never Smokers. Sci Rep. 2015;5:9755. doi: 10.1038/srep09755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross JS, Wang K, Chmielecki J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer. 2016;138:881–890. doi: 10.1002/ijc.29825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subbiah V, Westin SN, Wang K, et al. Targeted therapy by combined inhibition of the RAF and mTOR kinases in malignant spindle cell neoplasm harboring the KIAA1549-BRAF fusion protein. J Hematol Oncol. 2014;7:8. doi: 10.1186/1756-8722-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girirajan S, Hauck PM, Williams S, et al. Tom1l2 hypomorphic mice exhibit increased incidence of infections and tumors and abnormal immunologic response. Mamm Genome. 2008;19:246–262. doi: 10.1007/s00335-008-9100-6. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Alberta JA, Pilarz C, et al. A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro Oncol. 2017 doi: 10.1093/neuonc/now261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selt F, Hohloch J, Hielscher T, et al. Establishment and application of a novel patient-derived KIAA1549:BRAF-driven pediatric pilocytic astrocytoma model for preclinical drug testing. Oncotarget. 2017;8:11460–11479. doi: 10.18632/oncotarget.14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzies AM, Yeh I, Botton T, et al. Clinical activity of the MEK inhibitor trametinib in metastatic melanoma containing BRAF kinase fusion. Pigment Cell Melanoma Res. 2015;28:607–610. doi: 10.1111/pcmr.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob K, Quang-Khuong DA, Jones DT, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011;17:4650–4660. doi: 10.1158/1078-0432.CCR-11-0127. [DOI] [PubMed] [Google Scholar]
- 34.Baumhoer D, Glatz K, Schulten HJ, et al. Myxoinflammatory fibroblastic sarcoma: investigations by comparative genomic hybridization of two cases and review of the literature. Virchows Arch. 2007;451:923–928. doi: 10.1007/s00428-007-0480-x. [DOI] [PubMed] [Google Scholar]
- 35.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 36.Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Sato T, Nakamura H. The Fgf8 signal causes cerebellar differentiation by activating the Ras-ERK signaling pathway. Development. 2004;131:4275–4285. doi: 10.1242/dev.01281. [DOI] [PubMed] [Google Scholar]
- 38.Helias-Rodzewicz Z, Perot G, Chibon F, et al. YAP1 and VGLL3, encoding two cofactors of TEAD transcription factors, are amplified and overexpressed in a subset of soft tissue sarcomas. Genes Chromosomes Cancer. 2010;49:1161–1171. doi: 10.1002/gcc.20825. [DOI] [PubMed] [Google Scholar]
- 39.Vassilev A, Kaneko KJ, Shu H, et al. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaudin P, Delanoue R, Davidson I, et al. TONDU (TDU), a novel human protein related to the product of vestigial (vg) gene of Drosophila melanogaster interacts with vertebrate TEF factors and substitutes for Vg function in wing formation. Development. 1999;126:4807–4816. doi: 10.1242/dev.126.21.4807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All four cases tested for pERK immunohistochemical staining showed strong staining pattern, ranging from diffuse (A, case #3) to focal (B, case #2; C, case #1).