Abstract
Aim
To characterize rectal histology in an inception cohort of children newly diagnosed with ulcerative colitis (UC) and to explore its relationship with clinical indices of disease severity.
Methods
The PROTECT (Predicting Response to Standardized Pediatric Colitis Therapy) Study enrolled children ≤ 17 years newly diagnosed with UC. Baseline rectal biopsies were evaluated for acute and chronic inflammation, eosinophilic inflammation (peak eosinophil count > 32 eos/high powered field (hpf), eosinophilic cryptitis or abscesses), and architectural/non-architectural chronic changes. Correlation with clinical indices including Mayo endoscopy sub-score (MSS) and Pediatric Ulcerative Colitis Activity Index (PUCAI) was performed.
Results
Rectal biopsies from 369 patients (mean age 12.9 ± 3.1 years, 50% female) were reviewed. Cryptitis was found in 89%, crypt abscesses in 25%, and eosinophilic inflammation in 58%. Crypt distortion/atrophy was present in 98% of specimens. Higher grades of acute and chronic inflammation were associated with the presence of basal plasmacytosis (p<0.0001), basal lymphoid aggregates (p<0.0001), and surface villiform changes (p<0.0001). A severe MSS was most common among those with severe acute and chronic inflammation, though this relationship was not linear. Severe PUCAI scores were associated with the absence of or only mild eosinophilic inflammation (<32 eos/hpf) (p<0.03) and the presence of surface villiform changes (p<0.005).
Conclusion
Acute and chronic inflammation, eosinophilic inflammation and chronic changes are common in children newly diagnosed with UC. The clinical and biological implication of low to absent eosinophilic inflammation and the presence of surface villiform changes requires further study.
Keywords: Ulcerative colitis, histology, pediatric, eosinophilia
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disorder manifested by signs and symptoms of colonic dysfunction, mucosal abnormalities demonstrated at endoscopy, and mucosal biopsies that generally confirm active inflammation (1,2). However, the classic histologic signs of chronicity such as crypt architectural distortion, reduced crypt density and basal plasmacytosis may be less commonly identified in colon biopsies of children compared to adults with UC (2–6), especially in those children under the age of 10 years (5). Lack of chronic histologic changes can impact the ability to distinguish UC from acute self-limited colitis (3–5).
Histologic findings in ulcerative colitis may include increased eosinophils raising the specter of an alternative diagnosis such as eosinophilic or allergic colitis (3–5, 7, 8). Systematic evaluations of eosinophilic infiltrates have not always been reported in previous studies of children suspected to have UC (4, 5). Eosinophils may be more numerous in biopsies from pediatric patients with UC compared to biopsies from patients with allergic conditions (8). Clinical and experimental evidence suggests an important role for eosinophils in colitis: the chemokine eotaxin-1 which promotes eosinophil infiltration into tissue is increased in both gene array and PCR studies in rectosigmoid biopsies from children who have UC, and eotaxin-1 and −2 levels, and eosinophil density are increased in the colon of mice following dextran sodium sulfate-induced epithelial injury (10). Eosinophils in colonic biopsies from children (9, 11–13) are least numerous in the rectosigmoid colon compared to the more proximal colon and can be present in surface and crypt epithelium in normal biopsies (9, 12). Applying quantitative criteria to identify increased eosinophil density and eosinophilic cryptitis/abscess would aid in the ability to identify biopsies with excess eosinophils in clinical practice with more certainty, and could define more specifically the correlations between eosinophil density and endoscopic and clinical features of UC.
The PROTECT Study: Predicting Response to Standardized Pediatric Colitis Therapy was initiated in 2012 to systematically examine the response of children and adolescents newly diagnosed with UC to standardized treatment regimens. During this prospective study a large inception patient cohort was rigorously phenotyped clinically, endoscopically, and histologically. We now report the histopathologic findings from the treatment naive PROTECT patient cohort determined by centralized reading of rectal biopsies obtained at the time of the clinical diagnosis. The goals of our study were to evaluate the relationship of inflammatory and architectural changes to clinical presentation heretofore reported in smaller single center studies and to further explore the relationship of eosinophilic infiltrates to histologic, clinical, and endoscopic findings.
METHODS
Patient population
Potential patients for inclusion in the PROTECT study were recruited from 29 participating centers. Children between the ages of 4 and 17 years with a clinical history suggestive of UC (e.g. any combination of diarrhea, bleeding, and abdominal pain) were eligible. Complete demographic, clinical, and endoscopic data by ileocolonoscopy and esophagogastroduodenoscopy were obtained. Eligibility required disease extent beyond the rectum only (patients with proctitis only by endoscopic assessment were excluded), a baseline Pediatric Ulcerative Colitis Activity Index (PUCAI) (14) score of ≥10, no previous therapy for colitis, and stool testing negative for enteric bacterial pathogens (Salmonella, Shigella, Campylobacter, E. coli 0157:H7) and Clostridium difficile toxin. A clinical, endoscopic, and histologic diagnosis of ulcerative colitis was made using previously established criteria (1). Disease extent was classified as proctosigmoiditis, left-sided (to the splenic flexure), extensive (to the hepatic flexure), and pancolitis (to the cecum). Visual endoscopic evidence of inflammation was used to determine disease extent and severity of endoscopy findings was recorded as Mayo endoscopic sub-score (MSS) (15) according to a referenced 4 point pictorial scale system (0 inactive, 1 mild, 2 moderate, 3 severe) provided to all sites. The MSS was determined by the endoscopist at each participating site. Disease severity by PUCAI was scored over a range of 0 to 85. Mild PUCAI scores ranged from 10–34, moderate PUCAI ranged from 35–64 and severe PUCAI scores were ≥65 (14).
Patients with clinical, endoscopic, radiologic, or histologic evidence of Crohn’s disease, use of oral corticosteroid for non-gastrointestinal indications in the past 4 weeks, other gastrointestinal or non-gastrointestinal conditions which could interfere with the study mandated therapy, (i.e. active medical or surgical disease or active liver/gastrointestinal disease) or positive testing for enteric bacterial pathogens were excluded.
Histologic Assessment
Patients enrolled in the PROTECT study could have rectal biopsy specimens collected at diagnosis and sent for central review. Each patient with rectal biopsies available for central review was included in this histologic sub-study. An experienced pediatric pathologist (M.H.C.) examined these de-identified rectal biopsies and recorded findings on a standardized form. The additional rectal biopsy obtained for central review was fixed in formalin, routinely processed in the central review pathology laboratory, and embedded in paraffin blocks from which 4–5 micron sections were cut and stained with hematoxylin-eosin. Two to three levels were examined on one slide per biopsy.
Validated histopathologic scoring systems to evaluate baseline rectal biopsies from children with UC do not exist. In order to evaluate the character of the inflammatory infiltrate including quantitative evaluation of eosinophils and chronic changes at baseline, a scoring system was devised for the PROTECT study (Table 1). Grades of acute and chronic inflammation were recorded on a 5-point scale as I= no acute inflammation and no increase in chronic inflammation; II = chronic inflammation only; III = acute inflammation including acute cryptitis but without crypt abscesses; IV = acute inflammation including crypt abscesses in ≤ 10 % of crypts; and V = acute inflammation including crypt abscesses in >10 % of crypts. These grades were combined into 3 groups for subsequent statistical analysis (grades I/II, grade III, and grades IV/V). A crypt abscess was defined as a damaged crypt that was dilated and lined by attenuated epithelium with reduced to absent mucin and acute inflammation in the pericryptal lamina propria, in the damaged crypt epithelium and in the crypt lumen (Figures 1 and 2).
Table 1.
Definitions and classifications of histological changes
| Definitions | |
|---|---|
| Acute and Chronic Inflammation Grades | |
| I | No acute inflammation and no increased chronic inflammation |
| II | Chronic inflammation only |
| III | Acute inflammation including acute cryptitis - no abscesses |
| IV | Acute crypt abscesses in ≤ 10 % of crypts |
| V | Acute crypt abscesses in > 10 % of crypts |
| Eosinophilic Inflammation Grades | |
| 1 | None or ≤ 32 eosinophils/hpf |
| 2 | Peak eosinophil count > 32/hpf but without epithelial invasion (< 2 eosinophils in surface epithelium/hpf, < 9 eosinophils in crypt epithelium/hpf) |
| 3 | Eosinophilic cryptitis (>9 intraepithelial eosinophils /hpf) |
| 4 | Few eosinophil crypt abscesses (≤ 10% of crypts) |
| 5 | Many eosinophil crypt abscesses (> 10% of crypts) |
| Chronic Architectural and Non-architectural Changes (present or absent) | |
| Ulcer/Erosion | Absence of surface epithelium with granulation tissue (ulcer) or disrupted/absent surface epithelium with fibrin deposits (erosion) |
| Crypt distortion/atrophy | Crypt abnormalities including elongated crypts, branched crypts, atrophic crypts |
| Surface villiform changes | Villiform changes at the surface |
| Basal plasmacytosis | Aggregates of plasma cells below crypts and above the muscularis mucosa |
| Basal lymphoid aggregates | Aggregates of lymphocytes below crypts and above the muscularis mucosa |
| Paneth cell metaplasia | Paneth-like cells in crypt epithelium |
| Granuloma | Sarcoid-like granulomas in uninflamed areas (pericryptal granulomas in areas of damaged/inflamed crypts were permitted) |
Figure 1.
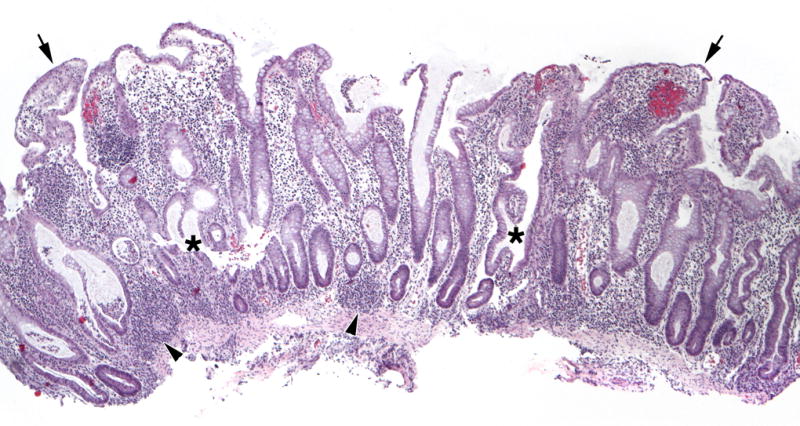
Representative Architectural and Non-architectural Features. Surface villiform changes are evident (arrows), elongated crypts are easily identified, crypts with abnormal shapes are seen (asterisk), as are subcryptal lymphoid aggregates (arrowheads). (H&E, 40×)
Figure 2.
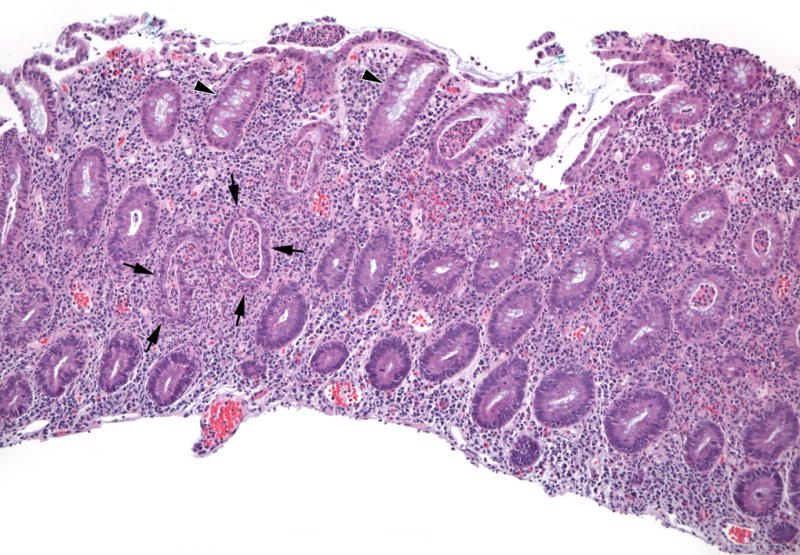
Representative Acute Inflammatory Features. In contrast to relatively well-preserved crypts (arrowheads), crypt abscesses (arrows) are dilated, lined by attenuated damaged epithelium, and contain acute inflammatory cells in the epithelium and the lumen of the crypt. (H&E, 100×)
Criteria to evaluate eosinophil density in rectal biopsies were based upon a previous study describing the presence of eosinophils in normal biopsies from children. These criteria are used in routine clinical practice by the central pathology reviewer (9). Eosinophil density was based on a peak eosinophil count. Grade 1 applied to specimens with ≤ 32 eosinophils per high power field (hpf); Grade 2 included specimens with > 32/hpf. Intraepithelial eosinophils were also scored, and grade 3 eosinophil inflammation was eosinophilic cryptitis (>9 intraepithelial eosinophils/hpf); grade 4 eosinophil inflammation was with eosinophil abscesses in ≤10% of crypts; and grade 5 eosinophil inflammation was eosinophil abscesses in > 10% of crypts.
Chronic architectural/non-architectural features were scored in a dichotomous manner as present or absent. These features were ulcer/erosion defined as the absence of surface epithelium with granulation tissue (ulcer) or disrupted/absent surface epithelium with fibrin deposits (erosion); crypt distortion/atrophy defined broadly as crypt abnormalities including elongated crypts, branched crypts, atrophic crypts; surface villiform changes defined as villiform changes at the surface; basal plasmacytosis defined as aggregates of plasma cells below crypts and above the muscularis mucosa; basal lymphoid aggregates defined as aggregates of lymphocytes below crypts and above the muscularis mucosa; Paneth cell metaplasia defined as Paneth-like cells in crypt epithelium; and granulomas defined as sarcoid-like granulomas in mucosa that was not inflamed (peri-cryptal granulomas in areas of crypt inflammation/damage were excluded from the definition of sarcoid-like granulomas). Some features required good tissue orientation for evaluation; a category of not evaluable was created for ulcer/erosion, surface villiform changes, basal plasmacytosis, and basal lymphoid aggregates for biopsies that were not well-oriented and in which those features could not be reliably evaluated. Therefore the denominator could differ between each type of architectural/non-architectural change.
Statistical approach
Demographic, clinical indices and central biopsy data were reported as summary statistics. Central biopsy data were further grouped into acute and chronic inflammation, eosinophilic inflammation and chronic architectural and non-architectural changes. Chi-square test/Fisher’s exact tests were used for categorical data and the t-test was used for continuous data. Spearman correlation was used to compare peak biopsy eosinophil count to total serum eosinophil count. A p-value of <0.05 was considered significant.
Ethical standards and Manuscript preparation
Informed consent/assent was obtained in all cases and the study was approved by the local investigational review board at all investigative sites. This study was registered with clintrials.gov (NCT01536535). The manuscript was written by the PROTECT Study Publication committee and subsequently approved by all authors.
RESULTS
A total of 431 subjects were enrolled in PROTECT with 369 (86%) having rectal biopsy material for review. The mean patient age was 12.9 ± 3.1 years, 50 % of subjects were female and 112/369 (30%) were <12 years of age. The extent of disease at diagnosis was evaluable in 346/369 (94%) of patients, while 23/369 (6%) had incomplete colonoscopies. Extensive/pancolitis was observed in 76%, 11% had left-sided colitis, and 7% proctosigmoiditis. Clinical scoring by the Mayo Endoscopy Sub-score (MSS) was evaluable in 368/369 and by the Pediatric Ulcerative Colitis Activity Index (PUCAI) in all 369 patients. Demographic and clinical characteristics of the study population are shown in Table 2.
Table 2.
Baseline Demographic and Summary Dataa
| Demographics | |
| Total subjects | 369 |
| Age (years)b | 12.9 ± 3.1 |
| Gender (% Males) | 186/369 (50.4 %) |
| Caucasian* | 304/361 (84%) |
| Disease Distribution | |
| Proctosigmoiditis | 24/369 (7 %) |
| Left sided colitis | 41/369 (11%) |
| Extensive/Pancolitis | 281/369 (76 %) |
| Unknown related to incomplete colonoscopy | 23/369 (6%) |
| Presenting symptoms | |
| Abdominal pain | 323/369 (88%) |
| Diarrhea | 340/369 (92%) |
| Rectal Bleeding | 359/369 (97%) |
| Grades of Acute and Chronic Inflammation | |
| I/II | 39/369 (11%) |
| III | 238/369 (65%) |
| IV/V | 92/369 (25%) |
| Grades of Eosinophilic inflammation | |
| 1 (None or ≤ 32 eosinophils/hpf) | 155/369 (42%) |
| 2 (Peak eosinophil count > 32/hpf) | 206/369 (56%) |
| 3 (Eosinophilic cryptitis) | 5/369 (1%) |
| 4 (Crypt abscesses (≤ 10% of crypts)) | 3/369 (1%) |
| 5 (Crypt abscesses (> 10% of crypts)) | 0 (%) |
| Chronic Architectural and Non-architectural Changes Present** | |
| Ulcer/Erosion | 16/366 (4%) |
| Crypt distortion/atrophy | 361/369 (98%) |
| Surface villiform changes | 136/366 (37%) |
| Basal plasmacytosis | 178/338 (53%) |
| Basal lymphoid aggregates | 214/338 (63%) |
| Paneth cell metaplasia | 30/369 (8%) |
| Baseline Mayo Endoscopy Sub-score (n=368)*** | |
| MSS 1 | 48/368 (13%) |
| MSS 2 | 196/368 (53%) |
| MSS 3 | 124/368 (34%) |
| Baseline PUCAI (n=369) | |
| Mild (10–34) | 83/369 (22%) |
| Moderate (35–64) | 168/369 (46%) |
| Severe (≥ 65) | 118/369 (32%) |
- counts and percentages in ()
- mean +/− standard deviation
8 patients with missing race data
Total “n” in each domain differs related to missing data or inability to evaluate that feature of the biopsy specimen
1 patient with missing MSS data
Grades of Acute and Chronic Inflammation
Grades of acute and chronic inflammation are shown in Table 2. Cryptitis was found in 238/369 (65%) of specimens while 25% (92/369) had crypt abscesses. Grades of acute and chronic inflammation did not differ by age (<12 vs. ≥12 years), gender, race, or presenting symptoms (diarrhea, abdominal pain, or rectal bleeding).
Eosinophilic inflammation
There were 206/369 (56%) biopsies that contained > 32 eosinophils /hpf (Grade 2) but without excess intraepithelial eosinophils. Only 8/369 (2%) biopsies had eosinophilic cryptitis or eosinophilic crypt abscesses (Grade 3 or 4) (Table 2). The mean peak eosinophil count was 41.6 ± 30/hpf. Eosinophil density was similar regardless of age, sex, race, disease location, or presenting symptoms. There was no relationship between the absolute eosinophil count in the serum complete blood count and the biopsy eosinophil count (p=0.16).
Chronic Architectural/Non-architectural Changes
Crypt atrophy/distortion was nearly universal occurring in 98% of specimens while ulceration/erosion was uncommon, being found in just 4% of biopsy specimens. A summary of the presence of each type of architectural/non-architectural change is shown in Table 2.
Relationship between Grades of Acute and Chronic Inflammation and Eosinophilic Inflammation
The relationship between grades of acute and chronic inflammation and eosinophilic inflammation is shown in Table 3. The distribution of eosinophilic inflammation across the degrees of acute and chronic inflammation was found to be similar (p=0.10).
Table 3.
Relationship between Grades of Acute and Chronic Inflammation and Eosinophilic Inflammation*
| Grades of Acute and Chronic Inflammation | |||||
|---|---|---|---|---|---|
| Eosinophilic Inflammation (n) |
Total 369 |
I/II 39 |
III 238 |
IV/V 92 |
p-value |
| Grade 1 | 155 | 22 (14%) | 100 (65%) | 33 (21%) | 0.10 |
| Grades 2, 3, or 4 | 214 | 17 (8%) | 138 (64%) | 59 (28%) | |
counts and percentages in ()
Relationship between Grades of Acute and Chronic Inflammation or Eosinophilic inflammation and Chronic Architectural/Non-architectural Changes
The distribution of architectural/non-architectural changes differed across the grades of acute and chronic inflammation for surface villiform changes (p<0.001), basal plasmacytosis (p<0.0001), and basal lymphoid aggregates (p<0.0001) (Table 4). Among those specimens with grade IV/V inflammation, 47/91 (52%) had surface villiform changes, 56/84 (67%) had basal plasmacytosis, and 64/84 (76%) had basal lymphoid aggregates.
Table 4.
Relationship between Grades of Acute and Chronic Inflammation or Eosinophilic Inflammation and Chronic Architectural and Non-architectural Changes*
| Grades of Acute and Chronic Inflammation | |||||
|
| |||||
| Chronic Architectural and Non-architectural Changes | Total | I/II | III | IV/V | p-values |
|
| |||||
| Surface villiform Changes (n) | 366 | 38 | 237 | 91 | |
| Present | 136 | 3 (2%) | 86 (63%) | 47 (35%) | <0.0001 |
| Absent | 230 | 35 (15%) | 151 (66%) | 44 (19%) | |
|
| |||||
| Basal Plasmacytosis (n) | 338 | 37 | 217 | 84 | |
| Present | 178 | 7 (4%) | 115 (65%) | 56 (31%) | <0.0001 |
| Absent | 160 | 30 (19%) | 102 (64%) | 28 (18%) | |
|
| |||||
| Basal lymphoid Aggregates (n) | 338 | 37 | 217 | 84 | |
| Present | 214 | 9 (4%) | 141 (66%) | 64 (30%) | <0.0001 |
| Absent | 124 | 28 (23%) | 76 (61%) | 20 (16%) | |
|
| |||||
| Paneth cell metaplasia (n) | 369 | 39 | 238 | 92 | |
| Present | 30 | 4 (13%) | 14 (47%) | 12 (40%) | 0.09 |
| Absent | 339 | 35 (10%) | 224 (66%) | 80 (24%) | |
|
| |||||
| Eosinophilic Inflammation | |||||
|
| |||||
| Total | Grade 1 | Grades 2, 3, or 4 | p-value | ||
|
| |||||
| Surface villiform Changes (n) | 366 | 154 | 212 | ||
| Present | 136 | 47 (35%) | 89 (65%) | 0.03 | |
| Absent | 230 | 107 (47%) | 123 (53%) | ||
|
| |||||
| Basal Plasmacytosis (n) | 338 | 137 | 201 | ||
| Present | 178 | 59 (33%) | 119 (67%) | 0.004 | |
| Absent | 160 | 78 (49%) | 82 (51%) | ||
|
| |||||
| Basal lymphoid Aggregates (n) | 338 | 137 | 201 | ||
| Present | 214 | 71 (33%) | 143 (67%) | 0.0003 | |
| Absent | 124 | 66 (53%) | 58 (47%) | ||
|
| |||||
| Paneth cell metaplasia (n) | 360 | 155 | 214 | ||
| Present | 30 | 10 (33%) | 20 (67%) | 0.43 | |
| Absent | 339 | 145 (43%) | 194 (57%) | ||
counts and percentages in ()
The distribution of architectural/non-architectural changes also differed across degrees of eosinophilic inflammation for basal plasmacytosis (p=0.004), basal lymphoid aggregates (p=0.0003) and surface villiform changes (p=0.03) (Table 4). The presence of each of these architectural/non-architectural changes was more common in those with Grade 2, 3 or 4 eosinophilic inflammation compared to those with Grade 1 (Table 4).
Relationships between Degrees of Acute and Chronic Inflammation or Eosinophilic Inflammation and Mayo Endoscopy sub-scores (MSS)
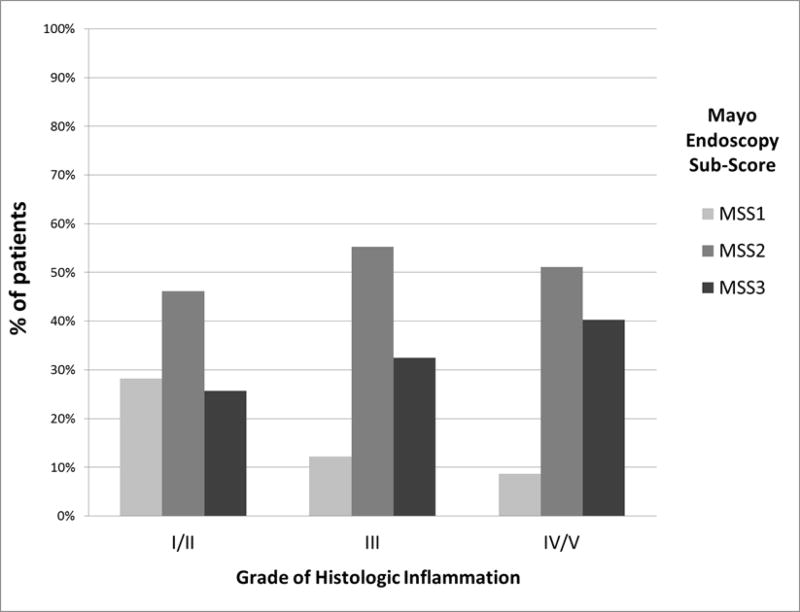
The relationship between grades of acute and chronic inflammation and MSS are shown in Table 5 and Figure 3. The distribution of MSS differed across grades of acute and chronic inflammation (p<0.03). However, this relationship was not linear as demonstrated by the fact that among those with the most severe acute and chronic inflammation (Grade IV/V), 51% had MSS 2 compared to 40% with MSS 3 (Table 5). However, the percentage of patients with a MSS of 3 increased from 26% among those with Grade I/II, to 32% in Grade III, and 40% in those with Grade IV/V.
Table 5.
Relationship between Degree of Acute and Chronic Inflammation or Eosinophilic Inflammation and Mayo Endoscopy Sub-Scores*
| Mayo Endoscopy Sub-Scores | |||||
| Grades of Acute and Chronic Inflammation (n) | Total 368 | 1 48 | 2 196 | 3 124 | p-value |
| I/II | 39 | 11 (28%) | 18 (46%) | 10 (26%) | 0.03 |
| III | 237 | 29 (12%) | 131 (55%) | 77 (32%) | |
| IV/V | 92 | 8 (9%) | 47 (51%) | 37 (40%) | |
| Mayo Endoscopy Sub-Scores | |||||
| Eosinophilic Inflammation (n) | Total 368 | 1 48 | 2 196 | 3 124 | p-value |
| Grade 1 | 155 | 26 (17%) | 68 (44%) | 61 (39%) | 0.007 |
| Grades 2, 3 or 4 | 213 | 22 (10%) | 128 (60%) | 63 (30%) | |
counts and percentages in ()
Figure 3.
Distribution of Mayo endoscopy sub-scores by Grades of Acute and Chronic Inflammation
The distribution of eosinophilic inflammation also differed across the Mayo endoscopy sub-scores (p=0.007). A MSS of 3 was more common in those with absent or reduced eosinophilic inflammation (Grade 1) at 39% compared to 30% of those with Grades 2, 3 or 4 having a MSS of 3 (Table 5).
Relationship between Chronic Architectural/Non-architectural Changes and Mayo Endoscopy Sub-scores
The distribution of architectural/non-architectural changes differed across Mayo endoscopy sub-scores for surface villiform changes (p=0.007), basal plasmacytosis (p=0.0009), and basal lymphoid aggregates (p=0.01) (Table 6).
Table 6.
Relationship between Chronic Architectural and Non-architectural Changes and Mayo Endoscopy Sub-scores*
| Mayo Endoscopy Sub-scores | |||||
|---|---|---|---|---|---|
|
| |||||
| Chronic Architectural and Non-architectural Changes |
Total |
1 |
2 |
3 |
p-value |
|
| |||||
| Surface villiform Changes (n) | 365 | 48 | 194 | 123 | |
| Present | 136 | 9 (7%) | 72 (53%) | 55 (40%) | 0.007 |
| Absent | 229 | 39 (17%) | 122 (53%) | 68 (30%) | |
|
| |||||
| Basal Plasmacytosis (n) | 337 | 47 | 182 | 108 | |
| Present | 177 | 13 (7%) | 106 (60%) | 58 (33%) | 0.0009 |
| Absent | 160 | 34 (21%) | 76 (48%) | 50 (31%) | |
|
| |||||
| Basal lymphoid Aggregates (n) | 338 | 47 | 182 | 108 | |
| Present | 214 | 22 (10%) | 127 (59%) | 65 (30%) | 0.01 |
| Absent | 124 | 25 (20%) | 55 (44%) | 43 (35%) | |
|
| |||||
| Paneth cell metaplasia (n) | 368 | 48 | 196 | 124 | |
| Present | 30 | 6 (20%) | 14 (47%) | 10 (33%) | 0.48 |
| Absent | 338 | 42 (12%) | 182 (54%) | 114 (34%) | |
counts and percentages in ()
Relationships between Degrees of Acute and Chronic Inflammation or Eosinophilic Inflammation and PUCAI Scores
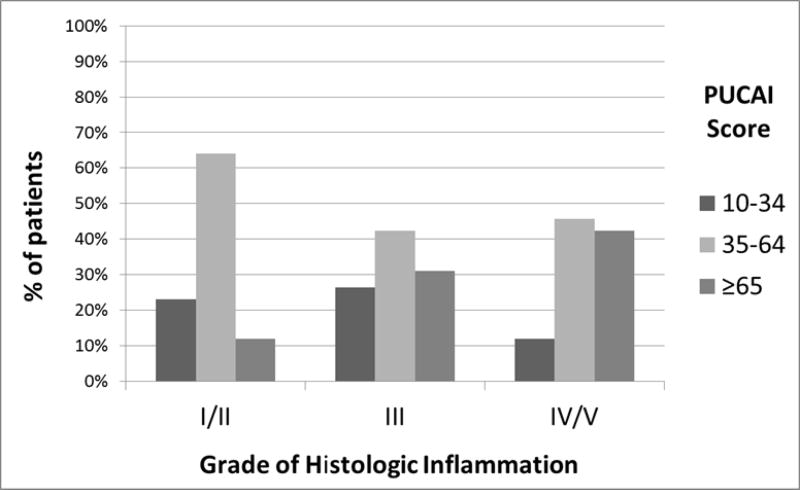
The relationship between grades of acute and chronic inflammation and clinical disease activity by PUCAI scores are noted in Table 7 and Figure 4. This distribution differed across PUCAI scores (p<0.002). The percentage of patients having severe PUCAI scores increased from 13% in those with Grade I/II, to 31% in Grade III, and 42% in Grade IV/V. However, this relationship was not linear and among those with the most severe acute and chronic inflammation Grade IV/V, 42% had severe while 46% had moderate PUCAI scores.
Table 7.
Relationship between Degrees of Acute and Chronic Inflammation or Eosinophilic Inflammation and PUCAI scores*
| PUCAI Scores | |||||
| Grades of Acute and Chronic Inflammation (n) | Total 369 | Mild (10–34) 83 | Moderate (35–64) 168 | Severe (≥65) 118 | p-value |
| I/II | 39 | 9 (23%) | 25 (64%) | 5 (13%) | 0.002 |
| III | 238 | 63 (26%) | 101 (43%) | 74 (31%) | |
| IV/V | 92 | 11 (12%) | 42 (46%) | 39 (42%) | |
| PUCAI Scores | |||||
| Eosinophilic Inflammation (n) | Total 369 | Mild (10–34) 83 | Moderate (35–64) 168 | Severe (≥65) 118 | p-value |
| Grade 1 | 155 | 36 (23%) | 57 (37%) | 62 (40%) | 0.007 |
| Grade 2, 3 or 4 | 214 | 47 (22%) | 111 (52%) | 56 (26%) | |
counts and percentages in ()
Figure 4.
Distribution of PUCAI scores by Grades of Acute and Chronic Histologic Inflammation
The distribution of eosinophilic inflammation also differed across PUCAI scores (p<0.007). Among those with reduced or absent eosinophilic inflammation (Grade 1), 40% had severe PUCAI scores compared to just 26% of those with Grade 2, 3, or 4 eosinophilic inflammation.
Relationship between Chronic Architectural/Non-architectural Changes and PUCAI scores
The distribution of surface villiform changes differed across PUCAI scores (p<0.005) (Table 8). Among those with severe PUCAI scores, 44% (51/117) had surface villiform changes compared to 40% (67/167) of those with moderate PUCAI scores. Surface villiform changes were least common in those with mild PUCAI scores.
Table 8.
Relationship between Chronic Architectural and Non-architectural Changes and PUCAI scores*
| PUCAI Scores | |||||
|---|---|---|---|---|---|
|
| |||||
| Chronic Architectural and Non-architectural Changes |
Total |
Mild (10–34) |
Moderate (35–64) |
Severe (65) |
p-value |
|
| |||||
| Surface villiform Changes (n) | 366 | 82 | 167 | 117 | |
| Present | 136 | 18 (13%) | 67 (49%) | 51 (38%) | 0.005 |
| Absent | 230 | 64 (28%) | 100 (43%) | 66 (29%) | |
|
| |||||
| Basal Plasmacytosis (n) | 338 | 80 | 160 | 98 | |
| Present | 178 | 34 (19%) | 85 (48%) | 59 (33%) | 0.06 |
| Absent | 160 | 46 (29%) | 75 (47%) | 39 (24%) | |
|
| |||||
| Basal lymphoid Aggregates (n) | 338 | 80 | 160 | 98 | |
| Present | 214 | 47 (22%) | 104 (49%) | 63 (29%) | 0.62 |
| Absent | 124 | 33 (27%) | 56 (45%) | 35 (28%) | |
|
| |||||
| Paneth cell metaplasia (n) | 369 | 83 | 168 | 118 | |
| Present | 30 | 7 (24%) | 13 (43%) | 10 (33%) | 0.97 |
| Absent | 339 | 76 (22%) | 155 (46%) | 108 (32%) | |
counts and percentages in ()
There was a trend towards higher rates of severe PUCAI scores in those with basal plasmacytosis (p=0.06) though not statistically significant. There was no association between basal lymphoid aggregates or Paneth cell metaplasia and clinical severity.
DISCUSSION
This study provides a comprehensive review of the clinico-histopathologic manifestations of treatment naïve pediatric ulcerative colitis. Novel data that we report are relationships between grades of acute and chronic inflammation, eosinophil density, and chronic architectural and non-architectural abnormalities with clinical and endoscopic findings.
Our study greatly expands knowledge of eosinophil density and associated findings identified in baseline rectal biopsies of children newly diagnosed with UC. We found that eosinophilic inflammation was common with 56% of patients having mucosal eosinophilia. The mean peak number of eosinophils in our study was 41.6 ± 30/hpf. This expands upon prior smaller single-center studies describing the presence of mucosal eosinophilia in children with IBD (7, 8, 10). The clinical significance of mucosal eosinophilia in IBD remains uncertain. A recent study designed to identify predictors of colectomy and failure of medical therapies in adults suggested that the absence of eosinophils may be associated with need for colectomy (16). Our study found that features of chronic change including basal plasmacytosis, basal lymphoid aggregates, and surface villiform changes were found more commonly in subjects who had greater density of eosinophils in rectal biopsies compared to those who had the expected peak count.
Our data also elucidate the frequency of common architectural/non-architectural changes at the time of diagnosis. The frequency of these chronic changes described in previous studies has been inconsistent, with chronic changes ranging from 8–32% (3,4). Glickman described 18% of rectal biopsies that lacked architectural changes (4). In contrast, a study by Robert, et al found 100 % of patients had chronic architectural changes in the rectal biopsies (5). Consistent with this latter study, we found that 98% of rectal biopsies showed crypt architectural distortion. Our findings expand the understanding of the presence of Paneth cell metaplasia. A small, single-center study by Simmonds and colleagues found that Paneth cell metaplasia was found in the distal colon in 85% of children with IBD (UC and Crohn’s disease) while it was absent in age-matched healthy controls (17). In contrast, we found Paneth cell metaplasia to be uncommon, identified in only 8% of rectal biopsies. While the Simmonds’ study included multiple biopsies from rectum and more proximal colon compared to a single rectal biopsy collected for PROTECT, our study included substantially more total biopsies and may therefore be a truer estimate of the actual incidence of Paneth cell metaplasia.
We found that the presence of surface villiform changes, basal plasmacytosis, and basal lymphoid aggregates was associated with more severe grades of acute and chronic inflammation. This relationship between the presence of basal plasmacytosis at diagnosis and future clinical outcomes may be of particular importance. Prior studies in adults with IBD have shown that the presence of basal plasmacytosis in biopsies of patients in clinical and endoscopic remission was predictive of subsequent clinical relapse within the subsequent 12 months (18). Future studies from our PROTECT cohort will evaluate if the presence of basal plasmacytosis identified at diagnosis could be associated with poorer clinical outcomes.
Our study also expands upon the relationship between histologic changes and clinical indices of severity. We found that these relationships are not linear but rather more severe histologic inflammation did not correlate with more severe endoscopic sub-scores (MSS). Clinical severity as captured by PUCAI scores did increase as the grades of acute and chronic inflammation increased, yet a moderate PUCAI score was found most commonly even among those with the most severe acute and chronic inflammation. In contrast, we found that a reduction or lack of eosinophilic inflammation (Grade 1) was most commonly seen in those with severe PUCAI scores, suggesting that a lack of eosinophil density in rectal biopsies may correlate with more severe clinical phenotypes of UC. Additionally, the presence of surface villiform changes differed across PUCAI scores. We found that the percentage of specimens with surface villiform changes was greatest in those with severe PUCAI scores. Future studies analyzing histologic data from the PROTECT cohort will assess the impact of these baseline findings upon future clinical outcomes.
Strengths of our study include the large number of patients included, standardized method of capturing and reporting the findings, and the inclusion only of patients with newly diagnosed and untreated UC. The primary limitation of our study is the availability of a single biopsy for central review. However, this limitation is similar to limitations in prior studies evaluating baseline histologic changes in pediatric UC. Washington’s study also analyzed only one rectal biopsy and the correlation between the findings of two pathologists was not described (3). In a subsequent study, individual pathologists reviewed biopsies from multiple areas of the colon, yet group review was performed only in cases of uncertainty (4).
In conclusion, acute and chronic inflammation, eosinophilic inflammation, and chronic architectural/non-architectural changes are common in the rectal biopsies of a large, well-characterized population of pediatric UC patients prior to therapy. Low eosinophil counts (< 32 eos/hpf) as well as surface villiform changes are associated with severe clinical disease as determined by PUCAI scores. These data lay the foundation for future studies investigating how different biological pathways contribute to the histopathologic expression of pediatric UC.
Acknowledgments
This study was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK), U01DK095745 and P30DK078392 (Integrative Morphology Core). The authors are indebted to Drs. Anthony Otley, Jennifer Strople, Keith Benkov, Maria Oliva-Hemker, Prateek Wali, Stephen Guthery, David Ziring, Dedrick Moulton, Boris Sudel, Jonathan Evans, Michael Kappelman and the site coordinators who participated in this study. We thank the PROTECT participants, their families and the participating physicians, investigators and staff for making this research possible. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Turner D, Levine A, Escher JC, et al. European Crohn’s and Colitis Organization; European Society for Paediatric Gastroenterology, Hepatology, and Nutrition. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55:340–361. doi: 10.1097/MPG.0b013e3182662233. [DOI] [PubMed] [Google Scholar]
- 2.Bousvaros A, Antonioli DA, Colletti RB, et al. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 3.Washington K, Greenson JK, Montgomery E, et al. Histopathology of ulcerative colitis in initial rectal biopsy in children. Am J Surg Pathol. 2002;26:1441–9. doi: 10.1097/00000478-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Glickman JN, Bousvaros A, Farraye FA, et al. Pediatric patients with untreated ulcerative colitis may present initially with unusual morphologic findings. Am J Surg Pathol. 2004;28:190–7. doi: 10.1097/00000478-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Robert ME, Tang L, Hao LM, et al. Patterns of inflammation in mucosal biopsies of ulcerative colitis: perceived differences in pediatric populations are limited to children younger than 10 years. Am J Surg Pathol. 2004;28:183–9. doi: 10.1097/00000478-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Markowitz J, Kahn E, Grandcher K, et al. Atypical rectosigmoid histology in children with newly diagnosed ulcerative colitis. Am J Gastroenterol. 1993;88:2034–2037. [PubMed] [Google Scholar]
- 7.Bates AW. Diagnosing eosinophilic colitis: histopathological pattern or nosological entity? Scientifica (Cairo) 2012;2012:682576. doi: 10.6064/2012/682576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pensabene L, Brundler MA, Bank JM, et al. Evaluation of mucosal eosinophils in the pediatric colon. Dig Dis Sci. 2005;50:221–9. doi: 10.1007/s10620-005-1586-0. [DOI] [PubMed] [Google Scholar]
- 9.DeBrosse CW, Case JW, Putnam PE, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210–8. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- 10.Ahrens R, Waddell A, Seidu L, et al. Intestinal Macrophage/Epithelial Cell-Derived CCL11/Eotaxin-1 Mediates Eosinophil Recruitment and Function in Pediatric Ulcerative Colitis. J Immunol. 2008;181:7390–7399. doi: 10.4049/jimmunol.181.10.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9:110–4. [PubMed] [Google Scholar]
- 12.Saad AG. Normal quantity and distribution of mast cells and eosinophils in the pediatric colon. Pediatr Develop Pathol. 2011;14:294–300. doi: 10.2350/10-07-0878-OA.1. [DOI] [PubMed] [Google Scholar]
- 13.Chernetsova E, Sullivan K, de Nanassy J, et al. Histologic analysis of eosinophils and mast cells of the gastrointestinal tract in healthy Canadian children. Hum Pathol. 2016;54:55–63. doi: 10.1016/j.humpath.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Saito H, Kusumi T, et al. Biopsy pathology predicts patients with ulcerative colitis subsequently requiring surgery. Scand J Gastroenterol. 2002;37:200–5. doi: 10.1080/003655202753416885. [DOI] [PubMed] [Google Scholar]
- 17.Simmonds N, Furman M, Karanika E, et al. Paneth cell metaplasia in newly diagnosed inflammatory bowel disease in children. BMC Gastroenterol. 2014;14:93. doi: 10.1186/1471-230X-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684–92. doi: 10.1038/ajg.2012.301. [DOI] [PubMed] [Google Scholar]