Abstract
In the current study we determined the role of IL-21 receptor signaling in Mycobacterium tuberculosis (Mtb) infection, using IL-21 receptor knockout (IL-21R KO) mice. Fifty percent of Mtb H37Rv infected IL-21R KO mice died in six months compared to no deaths in infected wild type (WT) mice. Mtb infected IL-21R KO mice had enhanced bacterial burden and reduced infiltration of antigen specific T-cells in lungs compared to Mtb infected WT mice. Antigen specific T-cells from the lungs of Mtb infected IL-21R KO mice had increased expression of T-cell inhibitory receptors, reduced expression of chemokine receptors, proliferated less and produced less IFN- γ, compared to antigen specific T cells from the lungs of Mtb infected WT mice. T cells from Mtb infected IL-21R KO mice were unable to induce optimal macrophage responses to Mtb. This may be due to a decrease in the antigen specific T cell population. We also found that IL-21R signaling is associated with reduced expression of a transcriptional factor Eomesodermin (Eomes) and enhanced functional capacity of antigen specific T-cells of Mtb infected mice. The sum of our findings suggests that IL-21 receptor signaling is essential for the optimal control of Mtb infection.
INTRODUCTION
Mycobacterium tuberculosis (Mtb) infects one-third of the world’s population and causes almost 1.2 million deaths per year (1). Approximately 90% of infected persons have protective immunity and contain infection, but 10% of infected individuals develop primary tuberculosis soon after infection or reactivation tuberculosis many years later (1). The factors responsible for development of active tuberculosis are not known.
T cells play a crucial role in protective immunity against Mtb, in part through production of IFN-γ by antigen specific T cells (2). Even though a sustained T-cell response against Mtb infection is necessary, limited information is available about the expansion and maintenance of the antigen specific T cells during Mtb infection (3, 4). The T cell receptor and costimulatory signals initiate proliferation of naïve T cells, but for the expansion of antigen specific T cells and their effector functions specific cytokine signals are essential (5–7). Members of the common γ-chain (γc) cytokine family, including IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 are critically involved in deciding transcriptional profiles of effector T cells and the development of antigen-specific T cells (8–10). These cytokines regulate T-cell functions through transcription factors like T-bet, Eomesodermin (Eomes), Bcl-6 and Blimp-1 (11). Both T-bet and Eomes are critical to regulate the expression of IFN-γ, perforin, granzyme B and effector T cells responses (8, 12).
IL-21 belongs to the common γ-chain (γc) cytokine family, is highly expressed by Th cell lineages and signals via a heterodimeric receptor complex comprised of the specific IL-21R subunit and the common receptor γ-chain (13). IL-21 is produced by natural killer T (NKT) cells and various CD4+ T cell subsets including Th17 cells and follicular helper T (Tfh) cells during viral infections (14). IL-21 stimulates the function of multiple lymphocyte subsets, including Th17 cells, follicular helper cells, B cells, NK cells, and CD8+ T cells (13). IL-21 promotes CD8+ T cell responses against tumors (13) and is required for the clearance of chronic viral infections in animal models (15–17).
In human Mtb infection, NKT cells produce IL-21 at the site of disease (18) and circulating IL-21 levels are lower in active tuberculosis (TB) patients (19). In a mouse model of Mtb infection, memory-like NK cells contribute to vaccine-induced protective immune responses against Mtb infection and IL-21 mediates the development and expansion of memory-like NK cells (20). IL-21 also enhances immunogenicity of a DNA vaccine containing Ag85A (21) but is dispensable for protective Th17 recall responses (22) and is not absolutely necessary for protective immunity against TB (23). The function of IL-21 can be compensated for by other common γ-chain (γc) cytokine family members and it is important to determine the role of IL-21 receptor signaling in Mtb infection. Recent studies found that IL-21 produced by CD4+ T cells promotes CD8+ T cell expansion and effector functions and IL-21 is essential for the optimal control of Mtb infection in mice (24).
In the current study, using IL-21 receptor knockout (IL-21R KO) mice, we further determined whether IL-21 receptor signaling has any effect on antigen specific CD4+ T-cell responses against Mtb infection. We found that IL-21 receptor signaling is associated with optimal antigen specific CD4+ T cell effector function and essential for the optimal control of Mtb infection in mice.
MATERIALS AND METHODS
Animals
All animal studies were performed on specific-pathogen-free 8-week-old female C57BL/6, C57BL/6NJ (6NJ) and IL-21R knockout (IL-21R KO) mice. The Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler approved the studies. Animal procedures involving the care and use of mice were in accordance with the guidelines of NIH / OLAW (Office of Laboratory Animal Welfare).
Aerosol infection of mice with Mtb H37Rv
Mice were infected with H37Rv using an aerosol exposure chamber, as described previously (25).
Lung cell preparation
Lungs were harvested from WT and IL-21R KO mice, and single cell suspensions were prepared at the indicated time points after Mtb challenge. The total number of viable cells in the lungs was determined by trypan blue exclusion method. For flow cytometry experiments, we gated on total lung CD45+ cells (leukocytes) and measured various cell populations like CD45+CD4+ or CD45+CD8+ cells.
Abs and other reagents
For flow cytometry, we used FITC anti-T-bet, PE anti-CD8, PE/Cy7 anti-Eomes, APC anti-CD4, APC anti-IFN-γ, APC anti-CD160, APC anti-2B4, APC anti-PD1, APC anti-CXCR5, APC anti-CXCR3, APC anti-CCR7, APC anti-IL12Rβ2, FITC anti-CD3, PE anti-CD11b, APC-anti-MHC II, FITC-anti-CD80, FITC-anti-CD86 (all from BioLegend). We used γ-irradiated Mtb H37Rv for in vitro stimulation assays (BEI Resources).
Detection of ESAT-64–17 specific T cells
PE-labeled MHC class II tetramers (I-Ab) containing the stimulatory residues 4 to 17 (QQWNFAGIEAAASA) of the early secreted antigenic target 6 kD (ESAT-6) of Mtb were obtained from the National Institutes of Health Tetramer Core Facility (Emory University Vaccine Center). For Ag-specific responses and intracellular cytokine staining, cells were incubated with ESAT-64–17aa MHC class II multimer (1:50 dilution) at 37°C 5% CO2 for 60 min in media, washed three times and cultured with ESAT-64–17aa peptide in the presence of monensin. After 5h, cells were surface stained for CD3, CD4, and CD8 at room temperature for 15 min in PBS 0.5% BSA 20% mouse serum, washed, and then fixed with 2% paraformaldehyde (Sigma-Aldrich) for 1 h. After fixation, cells were permeabilized, then washed with PBS 0.5% BSA 0.2% saponin. Cells were stained for intracellular IFN-γ (clone XMG-6.1) by incubating with anti-IFN-γ Abs in staining medium for 15 min at room temperature. Cells were washed and resuspended in 1% paraformaldehyde. Data were collected on a FACS CALIBUR and analyzed using FlowJo software 8.6.3 (Tree Star) (26).
Flow cytometry
Cells were surface stained as described previously (26) before acquisition using a FACS Calibur (BD Biosciences). In some experiments, intracellular staining for T-bet, Eomes, IFN-γ and isotype control was performed, according to the manufacturer’s instructions. For T-bet, Eomes and IFN-γ analysis, we gated on CD4+ or CD8+ lymphocytes, and determined the percentages or the number of T cells expressing specific marker.
Real-time PCR for quantification of cytokine mRNA
Total RNA was extracted from macrophages or lung cells as described previously (26). RNA was reverse transcribed, and real-time PCR was performed using the Quantitect SYBR Green PCR kit (Qiagen). All samples were normalized to the amount of β-actin/GAPDH transcript present in each sample.
Primers used in the study are mentioned in the supplemental Table I.
Carboxyfluorescein succinimidyl ester (CFSE) labeling
Mediastinal lymph node (MLN) cells were resuspended (5 × 106/ml) in 0.1% BSA in PBS. CFSE was added to a final concentration of 2 mM, and cells were incubated at 37°C for 15 min. Cells were washed once with 10% FCS in PBS, twice with 0.1% BSA in PBS, and resuspended in RPMI for subsequent stimulation.
In vitro stimulation
CFSE labeled cells were cultured for 3 d, with or without phorbol 12-myristate 13-acetate (PMA) + Ionomycin (500 ng/ml each). On day three, cell proliferation, inhibitory and chemokine receptor expression was determined by flow cytometry (BD Biosciences). Culture supernatants were collected to determine cytokine levels by ELISA (eBiosciences).
siRNA
Freshly isolated mouse MLN cells from Mtb infected IL-21R Knockout mice were transfected with siRNA for Eomes or control siRNA as described previously (26). After siRNA transfection, cells were washed and stimulated with γ-irradiated Mtb H37Rv or kept in medium alone as a control. On day three, cell proliferation of CFSE labeled cells, cytokine and transcription factor expression was determined by flow cytometry.
T-Cell isolation and purification
Effector T cells were isolated from the pooled spleen and MLN cells of WT C57BL/6NJ or IL-21R Knockout mice 3 months after infection with Mtb H37Rv. Briefly, lymphocytes were prepared from pooled cells by Ficoll density gradient. Pan T-cells were isolated from total lymphocytes by negative selection using LD columns (Miltenyi Biotech). The purity of CD3+ T-cells was > 95% as determined by flow cytometry.
Isolation of mouse peritoneal macrophages (PEMs) and infection with Mtb H37Rv
Mouse PEMs were isolated and infected with Mtb H37Rv at an MOI of 1:2.5 (one PEM and 2.5 Mtb) as described previously (26). Cells were washed to remove extracellular bacilli, and re-suspended in RPMI 1640 media for the subsequent culture with T lymphocytes.
Culturing of macrophages and T cells
Mtb infected PEMs were cultured with autologous effector T cells (1:5 ratio, one PEM and 5 T-cells) from WT or IL-21R KO mice for 5 days. Supernatants were collected to determine cytokine levels by ELISA. Bacterial load in the macrophages was enumerated by plating on 7H10 agar plates. Expression of MHC II, CD80 and CD86 was determined by flow cytometry.
Statistical analysis
Results are shown as the mean ± SE. Comparisons between groups were performed by a paired or unpaired t test, as appropriate. Mouse survival was compared using Kaplan- Meier Log-rank test.
RESULTS
IL-21R KO mice are susceptible to Mtb infection
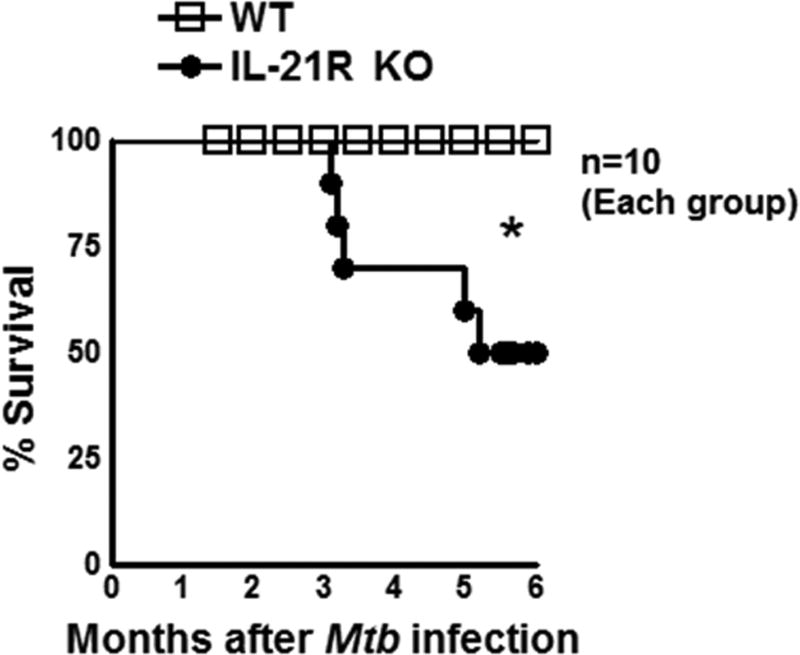
To determine the role of IL-21R in Mtb infection, we infected wild type (WT) and IL-21R KO mice with Mtb H37Rv as mentioned in the methods section. As shown in Fig. 1, fifty percent of IL-21R KO mice died between 3 and 6 months (p<0.05) post Mtb infection compared to no deaths in the Mtb infected WT mice. Due to animal welfare reasons, we have not followed these mice for a longer period of time and this was a time to death study.
Fig. 1. IL-21R KO mice are susceptible to Mtb infection.
Wild type (WT) and IL-21R knockout (KO) mice (both C57BL/6NJ background) were infected with 50–100 CFU of Mtb H37Rv by aerosol. Survival was determined. Data are representative of two independent experiments. Ten mice per group were used in each independent experiment. In the other independent experiment, 50% of Mtb infected IL-21R KO mice died in the first six months. Survival curves were compared using log rank test (P < 0.05). Mean values, p-values and SEs are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
IL-21R is essential for optimal control of Mtb growth in mice
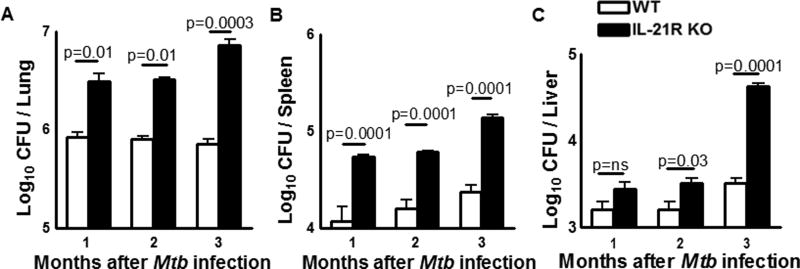
IL-21 expression was significantly upregulated in the lungs of Mtb infected WT mice compared to uninfected control mice lungs (Supplemental Fig. 1A). To determine the role of IL-21signaling in the control of Mtb growth, IL-21R KO and WT mice were infected with Mtb H37Rv by aerosol as mentioned in methods section. One, two and three months after infection, the bacterial burden in lungs, spleen, and liver was determined. There was a significant increase in the number of bacteria in the lungs of IL-21R KO mice after one month (3.4 ± 0.7 × 106 vs. 0.9 ± 0.1 × 106 CFU, p = 0.01, Fig. 2A) and two months compared to WT mice (3.2 ± 0.1 × 106 vs. 0.8 ± 0.06 × 106 CFU, p = 0.01, Fig. 2A). Ninety days after infection the bacterial burden in the lungs of IL-21R KO mice increased and it was one log higher compared to WT mice (7.3 ± 1.0 × 106 vs. 0.72 ± 0.08 × 106 CFU, p=0.0003, Fig. 2A). In the spleen (Fig. 2B) and liver (Fig. 2C) a similar increase was seen in IL-21R KO mice compared to WT mice.
Fig. 2. IL-21R is essential for optimal control of Mtb growth in mice.
A. WT and IL-21R KO mice (both C57BL/6NJ background) were infected with 50–100 CFU of H37Rv by aerosol. One, two and three months after infection, the bacterial burden in the lungs, spleen and liver was measured. Data are representative of two independent experiments. Five mice per group were used in each independent experiment. Mean values, p values and SEs are shown.
IL-21R is essential for optimal antigen specific T-cell responses during Mtb infection
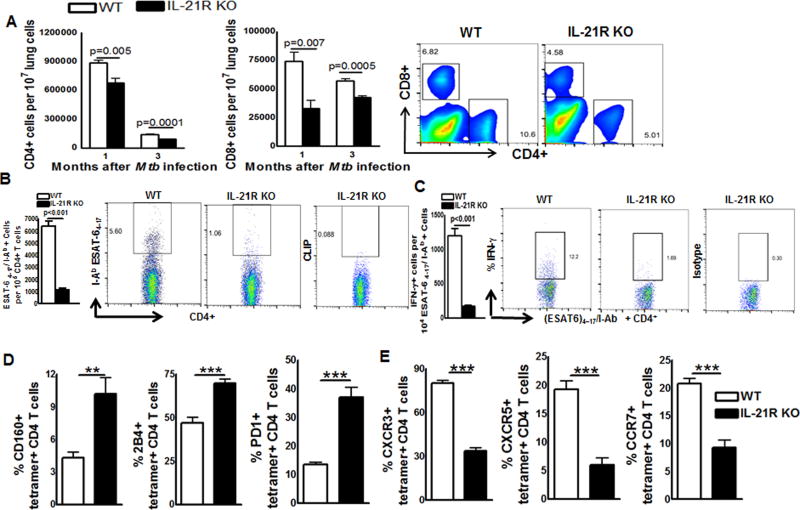
We asked whether lack of IL-21R signaling had any effect on T-cell responses during Mtb infection. WT and IL-21R KO mice were infected with Mtb and after one and three months the number of CD4+ and CD8+ cells in the lungs of control and Mtb infected mice was determined. The gating strategy is shown in Supplemental Fig. 1B. Uninfected WT and IL-21R KO mice have similar numbers of CD45+ leucocytes (Supplemental Fig. 1C). As shown in Fig. 3A, one month after infection, there was a significant decrease in total CD4+ cells (6.7 ± 0.4 × 105 vs. 8.8 ± 0.2 × 105 per 107 lung cells, p=0.005) and CD8+ cells (3.2 ± 0.6 × 104 vs. 7.4 ± 0.7 × 104 per 107 lung cells, p=0.007) in the lungs of IL-21R KO mice compared to WT mice lungs. Three months after infection the number of CD4+ cells in the IL-21R KO mice lungs was also reduced compared to WT mice (0.9 ± 0.01 × 105 vs. 1.4 ± 0.03 × 105 per 107 lung cells, p=0.0001, Fig. 3A). A Similar decrease in the number of total CD8+ cells was seen in Mtb infected IL-21R KO mice lungs compared to Mtb infected WT mice lungs (4.2 ± 0.1 × 104 vs. 5.6 ± 0.1 × 104 per 107 lung cells, p=0.0005, Fig. 3A).
Fig. 3. IL-21 is essential for optimal T-cell responses during Mtb infection.
A. WT and IL-21R KO mice (both C57BL/6NJ background) were infected with 50–100 CFU of H37Rv by aerosol. Three months after infection, lung cells were prepared as mentioned in methods section. Total lung CD45+ cells (leukocytes) were gated and various cell population were measured. The absolute numbers of cells was determined by calculating the percentage of gated cells multiplied by total lung cell number (excluded dead cells by Trypan blue dye exclusion). A. The number of CD4+ and CD8+ cells in the lungs was measured by flow cytometry (left) and representative plots of CD4 and CD8+ T-cells in the lung on day 90 post-infection are shown (right). B. Percentage of tetramer+ CD4 T cells in the lung (left) and representative plots of IAbESAT-64–17 MHC class II tetramer staining gated on CD4 T cells in the lung on day 90 post-infection (right). C. Percentage of IFN-γ–producing tetramer+ lung CD4 T cells (left) and representative plots of IFN-γ staining gated on tetramer+ lung CD4 T cells (right). D and E. Percentage of CD160, 2B4, PD1, CXCR3, CXCR5 and CCR7 gated on IAbESAT-64–17 tetramer+ lung CD4 T cells from WT and IL-21R KO mice. Data are representative of two independent experiments. Three mice per group were used in each independent experiment. Mean values, p values and SEs are shown. *p < 0.05, ** p < 0.01, *** p < 0.001.
To determine whether the reduced number of T cells in IL-21R KO mice also affects antigen specific responses three months after Mtb infection, we measured the frequency of (ESAT6)4–17/I-Ab MHC class II tetramer specific CD4+ cells. We found five fold less tetramer specific CD4+T-cells in the lungs of Mtb infected IL-21R KO mice compared to Mtb infected WT mice lungs (1150 ± 142.6 vs. 6400 ± 397.8, p<0.001, Fig. 3B). We also found 6.5 fold less CD4+IFN-γ+tetramer+ cells (173.0 ± 18.48 vs. 1200 ± 109.1, p<0.001, Fig. 3C,) in Mtb infected IL-21R KO mice lungs compared to Mtb infected WT mice lungs. Further we found increased frequency of CD4+tetramer+ cells that express inhibitory receptors CD160 (10.2 ± 1.4 % vs. 4.3 ± 0.5 %, p=0.006, Fig. 3D), 2B4 (69.7 ± 2.5 % vs. 47.0 ± 3.2 %, p=0.0001, Fig. 3D) and PD1 (37.0 ± 3.4 % vs. 13.4 ± 0.8 %, p=0.0006, Fig. 3D) and decreased frequency of CD4+tetramer+ cells that express chemokine receptors CXCR3 (33.4 ± 2.3 % vs. 80.2 ± 1.7 %, p=0.0001, Fig. 3E), CXCR5 (5.9 ± 1.3 % vs. 19.2 ± 1.5 %, p=0.0007, Fig. 3E) and CCR7 (9.2 ± 1.3 % vs. 20.7 ± 0.9 % p=0.0004, Fig. 3E) cells in the lungs of Mtb infected IL-21R KO mice compared to Mtb infected WT mice lungs. We found similar pattern of defective proliferation and enhanced expression of inhibitory receptors by PMA and ionomycin stimulated lung cells from IL-21R KO mice compared to uninfected WT mice (Supplemental Fig. 1D and E).
As shown in Supplemental Fig. 2A, the total number of IFN-γ+CD4+ and IFN-γ+CD8+ cells were significantly reduced in the lungs of IL-21R KO mice compared to WT mice three months after Mtb infection. The expression of IFN-γ, IL-17, IL-12, IL-1β, IL-27 and cytotoxic molecules like perforin and granzyme B were also reduced in the lungs of the above Mtb infected IL-21R KO mice compared to Mtb infected WT mice as determined by real time PCR (Supplemental Fig. 2B & 2C). In contrast, expression of TGF-β, IL-10, Type 1 interferons, TNF-α and transcription factor Eomes were significantly upregulated in the lungs of IL-21 R KO mice compared to WT mice (Supplemental Fig. 2C). We also found increased numbers (absolute) of CD4+ and CD8+ cells that express T cell inhibitory receptors like CD160, PD1, 2B4 and decreased numbers (absolute) of CXCR5, CXCR3, CCR7 and IL12Rβ2 cells in the lungs of IL-21R KO mice compared to WT mice three months after Mtb infection (Supplemental Fig. 2D & 2E).
Increased Eomes expression by T-cells of Mtb infected IL-21R KO mice
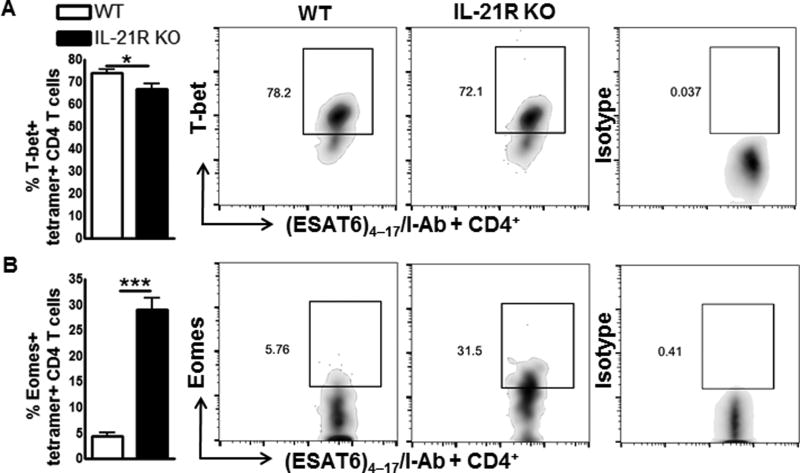
It is known that the transcription factor T-bet regulates IFN-γ production by T-cells in intracellular infections including Mtb (11, 27). Recent studies found that another transcription factor, Eomes, regulates IFN-γ production by T-cells in intracellular infections (28), but it has both positive and negative regulatory effects depending on the infection (29, 30). The role of Eomes in Mtb infection is not known. We next asked whether defective IL-21R signaling in Mtb infected mice has any effect on the expression of T-bet and Eomes. IL-21R KO and WT mice were infected with Mtb and after three months the number of CD4+ and CD8+ cells that express T-bet and Eomes in the lungs of control and Mtb infected mice was determined by flow cytometry. There is no significant difference in the number of CD4+ and CD8+ cells (Supplemental Fig.3A and 3B) that express T-bet between Mtb infected mice, but the number of Eomes+CD4+ cells and Eomes+CD8+ cells was significantly higher in Mtb infected IL-21R KO mice compared to WT Mtb infected mice (Supplemental Fig. 3A). Both Eomes and T-bet can influence memory T cell development (31). Therefore, we determined their expression in antigen specific T cells during Mtb infection. Three months after Mtb infection, we found a reduced frequency of T-bet+ESAT64–17/I-Ab MHC class II tetramer positive CD4+ T cells (66.6 ± 2.4 % vs. 73.9 ± 1.8 %, p=0.04, Fig.4A) and increased frequency of Eomes+ ESAT64–17/I-Ab MHC class II tetramer positive CD4+ T cells (29.0 ± 2.2 % vs. 4.4 ± 0.7 %, p=0.0001, Fig. 4B) in the lungs of IL-21R KO mice compared to WT mice.
Fig. 4. Increased Eomes expression by tetramer+CD4+ T-cells in IL-21R KO mice.
WT and IL-21R KO mice (both C57BL/6NJ background) were infected with 50–100 CFU of H37Rv by aerosol. A and B. Percentage of T-bet (A) and Eomes (B) expressing tetramer+ lung CD4 T cells (left). Representative plots of T-bet and Eomes expression gated on IAbESAT-64–17 tetramer+ CD4 T cells from WT and IL-21R KO mice lungs on day 90 post-infection (right). Data are representative of three independent experiments. Three mice per group were used in each independent experiment. Mean values, p values and SEs are shown. *p < 0.05, ** p < 0.01, *** p < 0.001.
Eomes inhibits T-cell effector functions in Mtb infected mice
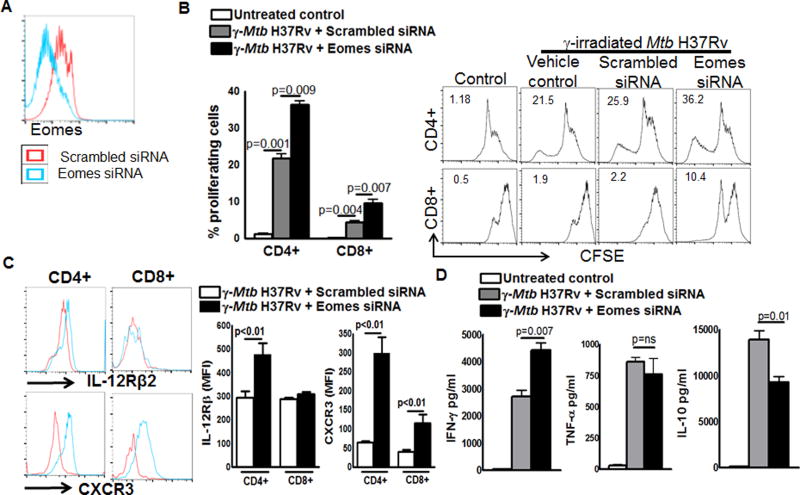
We next determined the role of Eomes on T-cell effector functions in Mtb infected mice. IL-21R knockout mice were infected with Mtb H37Rv by aerosol as mentioned in the methods section. Three months after infection, mediastinal lymph node cells were isolated and labeled with CFSE and cultured with γ-irradiated Mtb H37Rv (10 µg/ml) in the presence or absence of siRNA to Eomes or scrambled siRNA. After 72 hours, supernatants were collected and IFN-γ, TNF-α and IL-10 levels were determined by ELISA. Flow cytometry was performed on cells to determine the proliferation and expression of T-bet and Eomes. As shown in Fig. 5A, Eomes siRNA inhibited Eomes expression as determined by flow cytometry. Eomes siRNA enhanced γ-irradiated Mtb H37Rv induced CD4+ cell proliferation from 22 % to 36.5 % in CD4+ cells (p=0.009, Fig. 5B) and CD8+ cell proliferation from 4.5 % to 9.65 % (p=0.007, Fig. 5B). Eomes siRNA also significantly enhanced γ-irradiated Mtb H37Rv induced expression of CXCR3 and IL-12Rβ2 receptors on CD4+ and CD8+ T cells (Fig. 5C) and IFN-γ production (Fig. 5D). In contrast, Eomes siRNA significantly inhibited γ-irradiated Mtb H37Rv induced IL-10 production (Fig. 5D) and had no effect on γ-irradiated Mtb H37Rv induced TNF-α production (Fig. 5D). Eomes siRNA had no significant effect on the proliferation of PMA stimulated WT mice lung CD4+ T cells and IFN-γ and TNF-α production by lung cells. In contrast, Eomes siRNA significantly but marginally enhanced proliferation of CD8+ T cells and significantly inhibited IL-10 production by PMA stimulated WT mice lung cells (Supplemental Fig. 3D and 3E).
Fig. 5. Eomes inhibits T-cell effector functions in Mtb infected mice.
IL-21R KO mice were infected with 50–100 CFU of H37Rv by aerosol. Three months after infection, mediastinal lymph node cells were isolated and labelled with CFSE and cultured with γ-irradiated Mtb H37Rv (10 µg/ml) in the presence or absence siRNA to Eomes or scrambled siRNA. After 72 hours, supernatants were collected and flow cytometry was performed on cells to determine the T cell proliferation. A. Histogram plot showing the expression of Eomes by T cells. B. T-cell proliferation. C. Expression of CXCR3 and IL-12Rβ2 by CD4+ and CD8+ cells. D. IFN-γ, TNF-α and IL-10 levels in culture supernatants as determined by ELISA. Data are representative of three independent experiments. Pooled cells from two mice were used in each independent experiment. Mean values, p values and SEs are shown.
Effect of T cells from Mtb infected IL-21R KO mice on macrophage responses to Mtb
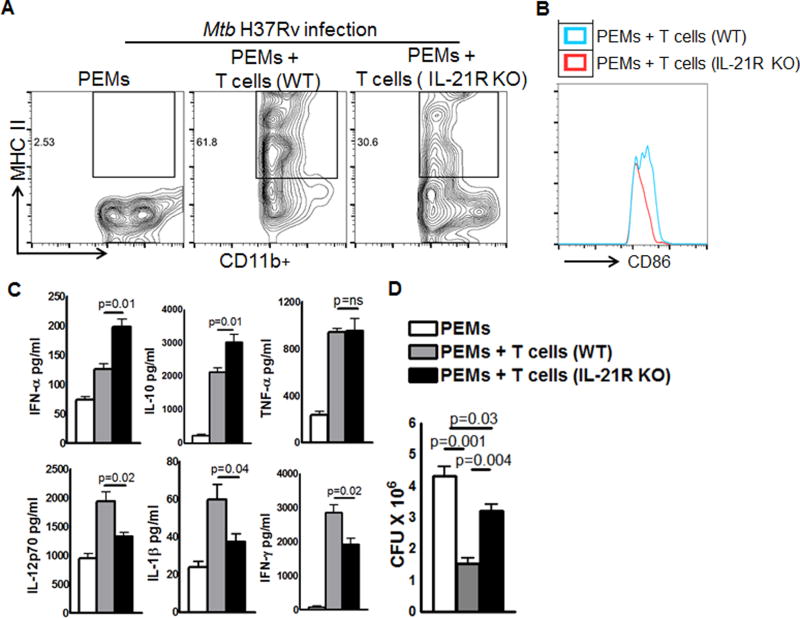
It is known that T cells enhance macrophage responses to inhibit Mtb growth. We determined the effect of T cells from Mtb infected IL-21R KO mice on macrophage responses to Mtb. Peritoneal macrophages (PEMs) were isolated from naïve mice and infected with H37Rv at an MOI of 1:2.5 (one PEM and 2.5 Mtb). After 2h, PEMs were washed to remove extracellular bacteria and then co-cultured with the pooled spleen and MLN T cells (1:5, one macrophage and five T-cells) isolated (three months after Mtb infection) from either WT or IL-21R KO Mtb infected mice. After 5 days, culture supernatants were collected to determine cytokine levels by ELISA, flow cytometry was performed to determine the expression of macrophage cell surface receptors and intracellular bacterial growth was determined as mentioned in the methods section. As shown in Fig. 6, Mtb infected 30.8 % of PEMs were MHCII+ (Fig. 6A) and the mean fluorescent intensity (MFI) of CD86 was 278.4 ± 17.3 (Fig. 6B) when the PEMs were cultured with T cells obtained from Mtb infected IL-21R KO mice, and the MHCII expression was 62.4% (Fig. 6A) and MFI of CD86 was 389.6 ± 27.3 (Fig. 6A) when Mtb infected PEMs were cultured with T cells from Mtb infected WT mice. Mtb infected PEMs produced 126.1 ± 9.9 pg/ml of IFN-α upon addition of T-cells from Mtb infected WT mice and when T-cells are from Mtb infected IL-21R KO mice this was 198.8 ± 12.8 pg/ml (p=0.01, Fig. 6C). A similar increase in IL-10 levels was observed (2130 ± 132.5 pg/ml vs. 3032 ± 236.5 pg/ml, p=0.01, Fig. 6C). However, in contrast to IFN-α and IL-10 production, Mtb infected PEMs produced less IL-1β (59.9 ± 7.8 pg/ml vs. 37.3 ± 4.2 pg/ml, p=0.04, Fig. 6C) and IL-12p70 (1944 ± 157.4 pg/ml vs. 1327 ± 76.8 pg/ml, p=0.02, Fig. 6C) upon the addition of T-cells from Mtb infected IL-21R KO mice, but not with the addition of T-cells from Mtb infected WT mice. IL-21R KO mice T cells had no effect on TNF-α production (941.0 ± 31.2 pg/ml vs. 951.7 ± 107.2, p=ns, Fig. 6C). In 3 independent experiments, 5 day post-infection, CFU in PEMs were 4.3 ± 0.2 × 106 CFU and this was reduced to 1.5 ± 0.17 × 106 CFU (p=0.001, Fig. 6D) upon the addition of T-cells from Mtb infected WT mice and to 3.2 ± 0.29 × 106 CFU (p=0.004, Fig. 6D) upon the addition of T-cells from IL-21R KO mice. The sum of these results suggest that T cells from Mtb infected IL-21R KO mice are unable to induce optimal macrophage responses to Mtb. This may be due to a decrease in the antigen specific T cell population.
Fig. 6. Effect of T cells from Mtb infected IL-21R KO mice on macrophage responses to Mtb.
WT and IL-21R KO mice were infected with 50–100 CFU of H37Rv by aerosol as mentioned in the methods section. Three months after infection, pooled spleen and MLN T-cells were isolated using magnetic selection as mentioned in the methods section. PEMs were isolated from naïve mice and infected with H37Rv at an MOI of 1:2.5 (one PEM to 2.5 Mtb). After 2 h, PEMs were washed to remove extracellular bacteria and cultured with T cells from Mtb infected WT or IL-21R KO mice (1:5, one macrophage and 5 T-cells). After 5 days, culture supernatants were collected to determine cytokine levels by ELISA, flow cytometry was performed on some cells to determine the expression of surface receptors and other cells were used to determine intracellular bacterial growth as mentioned in methods section. A. MHC class II and B. CD86 expression by Mtb infected PEM. C. IFN-α, IL-1β, IL-10, IL-12p70, IFN-γ and TNF-α levels as measured by ELISA. D. Bacterial burden of PEMs was measured by plating homogenates and counting CFU as mentioned in the methods section. Data are representative of three independent experiments. Two mice per group were used in each independent experiment. Mean values, p values and SEs are shown.
Discussion
IL-21 is a Th17 cytokine mainly produced by follicular helper CD4 T cells and NKT cells. IL-21 was shown to play a crucial role in cancer, autoimmune, inflammatory diseases and viral infections (32). Most of the studies on the effects of IL-21 and IL-21R signaling were focused on B-cell memory, differentiation and plasma cell development (33). Although IL-21 is not required for CD4+ T cell development, it is essential for the functional differentiation of CD4+ T cell subsets (34). IL-21 signaling plays an important role in maintaining the sustained functionality of CD8 T cells, allowing for the control of chronic viral infections caused by the lymphocytic choriomeningitis virus (15–17). In Mtb infected mice, IL-21 signaling has an early striking effect on CD8+ T-cell responses and is essential for optimal host resistance (24). In the current study, we found IL-21 signaling has autocrine action on CD4+ T-cell responses in Mtb infected mice. We found that in Mtb infected IL-21R knockout mice there is a defective T cell response and higher bacterial burden and mortality (50% of mice died in 6 months, Fig. 1).
Our findings in the current study demonstrate that IL-21R signaling is required for the optimal proliferation of effector T cells, production of IFN-γ, IL-17, and expression of the cytolytic molecules perforin and granzyme B in Mtb infected mice. We found that there is an association between lack of IL-21R expression and increased expression of a transcription factor Eomes in activated T cells. The sum of our findings demonstrates that IL-21R signaling is essential to maintain T-cell effector function (Fig. 3), to stimulate optimal IFN-γ production (Fig. 3), reduce bacterial burden (Fig. 2) and mortality (Fig. 1) of Mtb infected mice.
In human Mtb infection, NKT cells from plural fluids of tuberculosis patients produce IL-21 to help B cells to secrete IgG and IgA (18), pulmonary tuberculosis patients have less circulating IL-21 levels (19) and IL-21 regulates Mtb specific IFN-γ+CD4+ T cell responses (35) suggesting a role for IL-21 in human Mtb infection. In a mouse model of Mtb infection, IL-21 enhances immunogenicity of a DNA vaccine containing Ag85A (21). In Mtb infected mice, IL-21 is required for the optimal generation of lymphoid structures in lungs and is not absolutely necessary for protective immunity against TB (22, 23). These studies were performed using IL-21 knockout mice and it is possible that lack of IL-21 (soluble factor) may be compensated by other soluble mediators that can bind to IL-21 receptor to enhance signaling pathways. A recent study demonstrated that in Mtb infected mice, IL-21 signaling plays a crucial role in T cell responses during Mtb infection. In Mtb infected mice, IL-21 primes CD8+ T cells early and promotes the accumulation of T cells in the lung and enhances T cell cytokine production in Mtb infected mice (24). Our current study further demonstrates that IL-21 receptor signaling is involved in antigen specific CD4+ T-cell responses, is essential to maintain CD4+ T cell effector function and is important for the optimal control of Mtb growth and mortality of the Mtb infected mice.
We found that Mtb infected IL-21R knockout mice T cells (CD4+ and CD8+) express higher levels of inhibitory PD-1, CD160 and 2B4 receptors compared to Mtb infected WT mice. These findings are similar to previous findings that defective IL-21R signaling leads to increased expression of the inhibitory receptors TIM-3 and PD-1 (24, 36). The pattern of inhibitory-receptor co-expression and the number of receptors simultaneously expressed by the same T cell can substantially affect the severity of dysfunction (37). Our study for the first time demonstrates that IL-21R expression is an essential component for the optimal CD4+ T cell effector function during Mtb infection.
It is not known how IL-21R signaling maintains CD4+ T cell effector function. Recent studies found IL-21 inhibits the expansion of immunosuppressive Foxp3+ regulatory CD4 T cells (38), which are known to inhibit effector T cell function in viral infection (39). IL-21R KO and WT mice infected with Mtb have a similar number of Foxp3+ regulatory CD4 T cells in their lungs and spleens (Supplemental Fig. 3C) suggesting that an increased number of Foxp3+ cells may not be responsible for altered T cell function in Mtb infected IL-2R KO mice.
We found that the lack of IL-21R signaling enhances the expression of a transcriptional factor Eomes. T-bet is a known transcription factor involved in T cell mediated immune responses against Mtb (26) and infections with other intracellular pathogens (40). T-bet and Eomes are related transcription factors that show some expressional overlap, but their functional roles are not entirely reciprocal. T-bet and Eomes bind to the promoters of IFN-γ, perforin, and granzyme B and induce gene expression which is essential for optimal cytotoxic lymphocyte differentiation (41). Recently it was reported that the balance between T-bet and Eomes is vital for the maintenance of effector T cells in both HIV and CMV infection models (42). T-bet and Eomes can physically interact to form a complex in helper T cells. The properties of the interaction between T-bet and Eomes could create a scenario in which T-bet is able to control Eomes activity when the balance of the two proteins favors T-bet expression. We found that T cells from Mtb infected IL-21R knockout mice express significantly higher levels of Eomes compared to Mtb infected WT mice T cells. Eomes siRNA significantly enhanced the expression of CXCR3 and IL-12Rβ and enhanced CD4+ T cell effector functions of Mtb infected IL-21 knockout mice. Our study for the first time demonstrates that IL-21 and IL-21R signaling regulates the T-bet and Eomes ratio to favor optimal Th1 cytokine responses during Mtb infection. These studies may be applicable to other transcription factors that regulate Th1 cytokine responses to intracellular pathogen infections.
In conclusion, our studies demonstrate that the lack of IL-21 receptor signaling leads to enhanced expression of inhibitory receptors by CD4+ T cells, defective antigen specific T-cell responses and unrestricted growth of Mtb and mortality of the infected mice during the first six months of Mtb infection. Further delineation of the mechanisms through which IL-21R signaling regulates optimal Th1 responses during Mtb infection will facilitate development of therapies and also vaccines against Mtb and other intracellular pathogens.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI123310, AI120257 and A1085135 to R.V), CRDF global, The Cain Foundation for Infectious Disease Research and The Department of Pulmonary Immunology.
References
- 1.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. The immune response in tuberculosis. Annu. Rev. Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 2.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiley WW, Wittmer ST, Ryan LM, Eaton SM, Haynes L, Winslow GM, Woodland DL. Maintenance of peripheral T cell responses during Mycobacterium tuberculosis infection. J Immunol. Baltim. Md. 2012;1950:189. doi: 10.4049/jimmunol.1201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiley WW, Shafiani S, Wittmer ST, ’s Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. 2010;107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtsinger JM, Mescher MF. Inflammatory Cytokines as a Third Signal for T Cell Activation. Curr. Opin. Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanel A, Fonseca SG, Yassine-Diab B, Bordi R, Zeidan J, Shi Y, Benne C, Sékaly R-P. Cellular and molecular mechanisms of memory T-cell survival. Expert Rev. Vaccines. 2009;8:299–312. doi: 10.1586/14760584.8.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 8.Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naïve to memory and everything in between. Adv. Physiol. Educ. 2013;37:273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Yamane H, Paul WE. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 11.Kallies A. Distinct regulation of effector and memory T-cell differentiation. Immunol. Cell Biol. 2008;86:325–332. doi: 10.1038/icb.2008.16. [DOI] [PubMed] [Google Scholar]
- 12.Knox JJ, Cosma GL, Betts MR, McLane LM. Characterization of T-Bet and Eomes in Peripheral Human Immune Cells. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 14.Liu SM, King C. IL-21–Producing Th Cells in Immunity and Autoimmunity. J Immunol. 2013;191:3501–3506. doi: 10.4049/jimmunol.1301454. [DOI] [PubMed] [Google Scholar]
- 15.Fröhlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 16.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Li Z, Fu X, Yu S, Lao S, Yang B. Antigen-specific human NKT cells from tuberculosis patients produce IL-21 to help B cells for the production of immunoglobulins. Oncotarget. 2015;6:28633–28645. doi: 10.18632/oncotarget.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar NP, Sridhar R, Hanna LE, Banurekha VV, Nutman TB, Babu S. Decreased frequencies of circulating CD4+ T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PloS One. 2014;9:e111098. doi: 10.1371/journal.pone.0111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatasubramanian S, Cheekatla S, Paidipally P, Tripathi D, Welch E, Tvinnereim AR, Nurieva R, Vankayalapati R. IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dou J, Tang Q, Zhao F, Chu L, Chen J, Cao M, Liu C, Wang Y, Li Y, Li JL. Comparison of immune responses induced in mice by vaccination with DNA vaccine constructs expressing mycobacterial antigen 85A and interleukin-21 and Bacillus Galmette-Guérin. Immunol. Invest. 2008;37:113–127. doi: 10.1080/08820130701690741. [DOI] [PubMed] [Google Scholar]
- 22.Monin L, Griffiths KL, Slight S, Lin Y, Rangel-Moreno J, Khader SA. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 2015;8:1099–1109. doi: 10.1038/mi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, Kaushal D, Reinhart TA, Randall TD, Khader SA. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin. Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booty MG, Barreira-Silva P, Carpenter SM, Nunes-Alves C, Jacques MK, Stowell BL, Jayaraman P, Beamer G, Behar SM. IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection. Sci. Rep. 2016;6:36720. doi: 10.1038/srep36720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatasubramanian S, Dhiman R, Paidipally P, Cheekatla SS, Tripathi D, Welch E, Tvinnereim AR, Jones B, Theodorescu D, Barnes PF, Vankayalapati R. A rho GDP dissociation inhibitor produced by apoptotic T-cells inhibits growth of Mycobacterium tuberculosis. PLoS Pathog. 2015;11:e1004617. doi: 10.1371/journal.ppat.1004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan BM, Jobe O, Lazarevic V, Vasquez K, Bronson R, Glimcher LH, Kramnik I. Increased susceptibility of mice lacking T-bet to infection with Mycobacterium tuberculosis correlates with increased IL-10 and decreased IFN-gamma production. J Immunol. Baltim. Md 1950. 2005;175:4593–4602. doi: 10.4049/jimmunol.175.7.4593. [DOI] [PubMed] [Google Scholar]
- 27.van Meijgaarden KE, Haks MC, Caccamo N, Dieli F, Ottenhoff THM, Joosten SA. Human CD8+ T-cells recognizing peptides from Mycobacterium tuberculosis (Mtb) presented by HLA-E have an unorthodox Th2-like, multifunctional, Mtb inhibitory phenotype and represent a novel human T-cell subset. PLoS Pathog. 2015;11:e1004671. doi: 10.1371/journal.ppat.1004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buggert M, Tauriainen J, Yamamoto T, Frederiksen J, Ivarsson MA, Michaëlsson J, Lund O, Hejdeman B, Jansson M, Sönnerborg A, Koup RA, Betts MR, Karlsson AC. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014;10:e1004251. doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro-dos-Santos P, Turnbull EL, Monteiro M, Legrand A, Conrod K, Baalwa J, Pellegrino P, Shaw GM, Williams I, Borrow P, Rocha B. Chronic HIV infection affects the expression of the 2 transcription factors required for CD8 T-cell differentiation into cytolytic effectors. Blood. 2012;119:4928–4938. doi: 10.1182/blood-2011-12-395186. [DOI] [PubMed] [Google Scholar]
- 31.McLane LM, Banerjee PP, Cosma GL, Makedonas G, Wherry EJ, Orange JS, Betts MR. Differential localization of T-bet and Eomes in CD8 T cell memory populations. J Immunol. Baltim. Md 1950. 2013;190:3207–3215. doi: 10.4049/jimmunol.1201556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 33.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 34.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp. Med. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Jiang Y, Lao S, Yang B, Yu S, Zhang Y, Wu C. Mycobacterium tuberculosis-Specific IL-21+IFN-γ+CD4+ T Cells Are Regulated by IL-12. PloS One. 2016;11:e0147356. doi: 10.1371/journal.pone.0147356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz I, Schneider C, Fröhlich A, Frebel H, Christ D, Leonard WJ, Sparwasser T, Oxenius A, Freigang S, Kopf M. IL-21 restricts virus-driven Treg cell expansion in chronic LCMV infection. PLoS Pathog. 2013;9:e1003362. doi: 10.1371/journal.ppat.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melillo AA, Foreman O, Bosio CM, Elkins KL. T-bet regulates immunity to Francisella tularensis live vaccine strain infection, particularly in lungs. Infect. Immun. 2014;82:1477–1490. doi: 10.1128/IAI.01545-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teles RMB, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, Komisopoulou E, Kelly-Scumpia K, Chun R, Iyer SS, Sarno EN, Rea TH, Hewison M, Adams JS, Popper SJ, Relman DA, Stenger S, Bloom BR, Cheng G, Modlin RL. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339:1448–1453. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.