Abstract
Although neuraminidase (NA) is the second major viral glycoprotein of influenza virus, its immune mechanism as a vaccine target has been less considered. Here we compared the properties of antibodies and the efficacy of cross protection by N1 and N2 NA proteins, inactivated split influenza vaccines (split), and tandem repeat extracellular domain M2 on virus-like particles (M2e5x VLP). Anti-NA immune sera could confer better cross-protection against multiple heterologous influenza viruses correlating with NA inhibition activity compared to split vaccine immune sera. Whereas split vaccine was superior to NA in conferring homologous protection. NA and M2e immune sera each showed comparable survival protection. Protective efficacy by NA immune sera was lower in Fc receptor common γ-chain deficient mice but comparable in C3 complement deficient mice compared to that in wild type mice, suggesting a role of Fc receptor in NA immunity.
Keywords: Influenza virus, Neuraminidase, M2e, split vaccines
Introduction
Influenza virus is an eight-segmented negative-sense RNA virus and belongs to the family Orthomyxoviridae. Influenza A viruses are divided into different subtypes based on two types of major glycoproteins on the surface; hemagglutinin (HA) and neuraminidase (NA) [1]. Currently, 18 subtypes of HA and 11 subtypes of NA have been reported to determine the subtypes of influenza A viruses and these proteins still keep mutating to give influenza A viruses opportunities to create antigenically new strains [2].
The strategy of current influenza vaccination is to induce neutralizing antibodies targeting highly changeable HA, which does not provide effective protection against antigenically mutated viruses and pandemics [3, 4]. In the effort to overcome this limitation of current vaccines, new vaccine strategies have been investigated, targeting relatively more conserved viral antigens. The extracellular domain (M2e) of influenza virus M2 protein has been utilized in various carrier vehicles and vaccine designs [5, 6]. However, the levels of cross protection by M2e immunity are not sufficient.
Epidemiologic studies indicated that anti-NA immunity and NA inhibitors prevent severe disease or death by influenza viral infection [7]. NA targeting antibodies do not have viral neutralizing activity, providing infection-permissive protection [8]. Studies have demonstrated that NA immunity induces a broad spectrum of cross protection within the same subtype [9, 10]. Nonetheless, contribution of NA antibodies to cross protection is not well understood yet in comparison with other viral surface antigens.
In this study, we investigated how NA immune responses contribute to cross protection by comparing with those induced by tandem repeat M2e virus-like particle (M2e5x VLP) and inactivated split virus (as HA) vaccines. NA antibodies were found to be more effective in conferring heterologous cross-protection than strain-specific HA antibodies. The contribution of NA immunity to protection appeared to be limited when compared to M2e immunity. Also, the roles Fc receptors and complement protein C3 in mediating protection by NA antibodies were investigated in mutant mouse models.
Materials and methods
Viruses and vaccines
A/California/04/2009 (A/Cal) H1N1, A/Puerto Rico/8/1934 (A/PR8) H1N1, A/Philippines/2/1982 (A/Phil) H3N2, A/Wisconsin/67/2005 (A/Wis) H3N2, and reassortant A/Vietnam H5N1 (rgH5N1) containing H5 HA with removed polybasic residues and NA from H5N1 A/Vietnam/1203/2004 and six internal genes from A/PR8 H1N1 were propagated in embryonated hen’s eggs as previously described [11]. N1 NA protein derived from A/Cal and N2 NA protein derived from A/Wis were provided from BEI resources. M2e5x VLP containing tandem repeat of heterologous M2e derived from human, swine, and avian influenza virus was prepared as detailed in previous study [5]. Briefly, Sf9 insect cells were co-infected with recombinant baculoviruses expressing influenza M1 matrix core protein and M2e5x. M2e5x VLP vaccine was purified from cell culture supernatants containing released VLP by sucrose gradient ultracentrifugation and characterized as reported [5]. Commercial human influenza split A/Cal vaccine was obtained from a vaccine manufacturing company (Green Flu-S; Green Cross, South Korea). Inactivated virus vaccines (A/PR8, A/Phil) were prepared by treating the virus with formalin at a final concentration of 1:4000 (v/v) as previously described [12].
Immunization and challenge
Adult wild type and mutant mice (6–10 weeks old) used in this study include BALB/c, C57BL/6, and C3KO (B6.129S4-C3tm1Crr/J), and were obtained from the Jackson Laboratory (Sacramento, CA). FcRγ-deficient mice (FcRγ−/− encoded by Fcer1g on the BALB/c genetic background) were purchased from Taconic Farms (Hudson, NY). Groups of each strain of mice (n= 5, males and females) were intramuscularly immunized with 5 µg of N1 or N2 NA protein with adjuvant MF59 (1:1 vol), 1 µg of split vaccines derived from A/Cal H1N1 or A/PR8 H1N1, and 10 µg of M2e5x VLP by prime-boost regimen at a 3-week interval. At 4 weeks after boost immunization, immunized mice were then challenged intranasally with a lethal dose of A/Cal H1N1 (17 X LD50) or A/Phil H3N2 (5 X LD50). Survival rates and body weight loss were daily monitored for 14 days upon infection. All animal experimental procedures in this study were approved by the Georgia State University Institutional Animal Care and Use Committee review boards.
Determination of antibody responses
Influenza virus-specific or M2e-specific antibody levels were determined by enzyme-linked immunosorbent assay (ELISA). Immune sera were serially diluted and then applied to the 96 well plate (Corning Incorporated, Tewksbury, MA) that were coated with M2e peptides or inactivated A/Cal H1N1, A/PR8 H1N1, A/Phil H3N2, A/Wis H3N2, or rgH5N1 as previously described [13]. IgG levels were determined by HRP conjugated anti-mouse IgG (SouthernBiotech, Birmingham, AL) and tetramethylbenzidine (eBioscience, San Diego, CA) as a substrate.
Neuraminidase inhibition (NI) assay
The optimal concentrations of viruses for the subsequent NI assays were determined based on the NA activity of each virus. NI activity of immune sera was measured using fetuin-based assay procedure as described [9]. Briefly, 96-well plates were coated with fetuin (25 µg/ml) and incubated overnight at 4 °C. After washing, plates were blocked with PBS containing 1% BSA for 1 hour. 2-fold serially diluted immune sera were incubated with an equal volume of virus for 1.5 hours and then added to the fetuin-coated plates and incubated for 2 hours at 37 °C. Peroxidase-labeled peanut agglutinin (1 µg/ml) was added to each well and incubated for 2 hours. The NA activity levels were determined by using tetramethylbenzidine (eBioscience, San Diego, CA) as a substrate. OD values were read at 490nm.
Hemagglutination inhibition (HI) assay
HI assay was performed as previously described [14]. Immune sera were treated with receptor destroying enzymes (Sigma Aldrich, St. Louis, MO) and then incubated at 37 °C. At 16 hours after incubation, samples were heat inactivated at 56 °C for 30 min. Serially 2-fold diluted sera were incubated with 8 HA units of A/Cal H1N1, A/PR8 H1N1, A/Phil H3N2, or rgH5N1 for 30 min, followed by adding 0.5% chicken red blood cells (Lampire Biological Laboratories, Pipersville, PA) to determine HI titers.
In vivo protection assay of immune sera
To determine the roles of immune sera in conferring protection, in vivo protection assay was performed as described previously [5]. Briefly, heat inactivated sera at 56 °C for 30 min were diluted and mixed with a lethal dose (10 X LD50) of A/Cal H1N1, rgH5N1, or A/Phil H3N2. Naïve mice were intranasally infected with a mixture (50 µl) of virus and sera (x1/4 diluted), and the survival rates and body weight changes were daily monitored for 14 days.
Statistical analysis
All results are expressed as the mean ± standard error of the mean (SEM). Significant differences among treatments were evaluated by 2-way ANOVA. P-values of less than or equal to 0.05 were considered statistically significant.
Results
NA protein vaccination induces IgG antibodies cross-reactive to heterologous virus
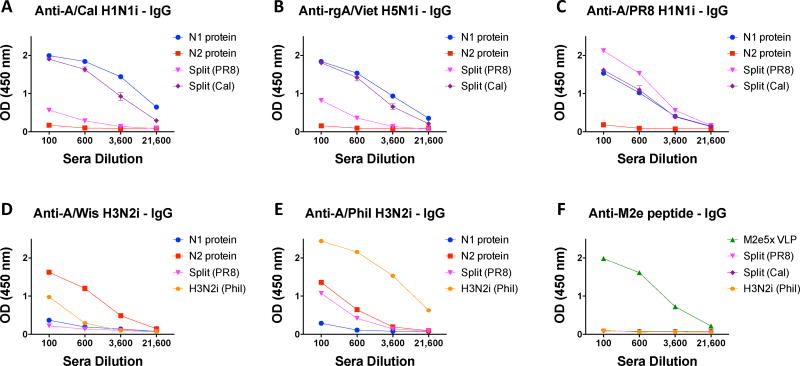
We compared the relative contributions and roles of serum antibodies specific for NA, inactivated split influenza vaccine (HA), and M2e in conferring cross protection. The groups of mice were intramuscularly immunized with N1 NA protein (5 µg, A/Cal H1N1), N2 NA protein (5 µg, A/Wis H3N2), M2e5x VLP (10 µg), inactivated split influenza vaccines (1 µg, A/PR8 H1N1 or A/Cal H1N1). Reactivity of IgG antibodies in immune sera to different strains of influenza virus was compared among the groups at 2 weeks after boost immunization (Fig. 1). N1 NA protein immune sera showed reactivity to the homologous A/Cal H1N1 and heterologous rgH5N1 and A/PR8 H1N1 viruses. A similar pattern was also observed with N2 NA protein immune sera at lower levels of IgG antibodies that bind to homologous A/Wis H3N2 virus and heterologous A/Phil H3N2 virus. However, both N1 and N2 immune sera did not show significant reactivity to different subtypes of influenza virus. M2e5x VLP immune sera exhibited high levels of IgG antibodies reactive to M2e peptide antigens (Fig. 1F). A/Cal split vaccine developed more cross-reactive IgG antibodies than A/PR8 split vaccine (Fig. 1B, C) although both showed high IgG antibodies specific for homologous virus. Overall, IgG antibodies induced by A/Cal split vaccines were cross-reactive to heterosubtypic influenza viruses but not to M2e. NA protein-induced IgG antibodies could bind to homologous and heterologous viruses within the same NA subtype.
Fig. 1. Reactivity of vaccine-specific antibodies to different strains of influenza viruses.
Immune sera were collected after boost immunization of BLAB/c mice (n= 5 per group) with N1 NA protein (A/Cal H1N1), N2 NA protein (A/Wis H3N2), M2e5x VLP, split vaccine (A/PR8 H1N1, A/Cal H1N1), or whole A/Phil H3N2 virus. Whole inactivated virus particles and M2e peptide were used as ELISA coating antigens: (A) A/Cal H1N1, (B) rgH5N1, (C) A/PR8 H1N1, (D) A/Wis H3N2, (E) A/Phil H3N2 or (F) M2e peptide. Sera were pooled and serially diluted.
NA protein vaccination develops NA-inhibiting cross-reactive antibodies
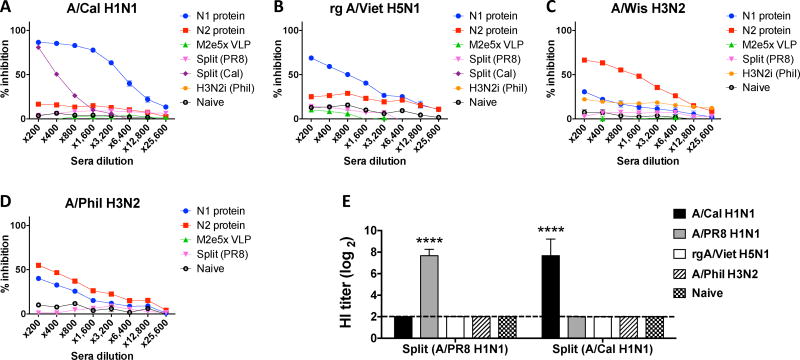
As a measure of functional antibodies, NA inhibition (NI) activity of immune sera was determined by a fetuin-based assay (Fig. 2). A/Cal N1 NA protein-induced antibodies exhibited significantly higher levels of NI activity against homologous virus than split vaccine immune sera (Fig. 2A). Also, A/Cal N1 NA immune sera showed low levels of NI activity against heterologous rgH5N1 and heterosubtypic A/Phil H3N2 virus (Fig. 2B & D). N2 NA immune sera displayed significant levels of NI activity against the homologous virus A/Wis H3N2 and heterologous A/Phil H3N2 virus (Fig. 2C & D). Split A/Cal vaccine immune sera show low NI activity against homologous but not heterologous virus. Inactivated split influenza vaccines raise high hemagglutination inhibition titers in a strain specific manner as expected (Fig. 2E). These results indicate that NA protein vaccination induces higher NI activity against both homologous and heterologous viruses than current vaccine platforms of inactivated influenza split virus. NI activity was low or hardly detected in split vaccine immunized mice.
Fig. 2. NA inhibition activity of vaccine-specific antibodies to different strains of influenza viruses.
Immune sera were collected and pooled after immunization (n=5) with N1 NA (A/Cal), N2 NA (A/Wis), M2e5x VLP, split vaccine (A/PR8, A/Cal), or inactivated A/Phil H3N2 virus. NA inhibition assays were performed against different strains of influenza viruses: (A) A/Cal H1N1, (B) rgH5N1, (C) A/Wis H3N2, or (D) A/Phil H3N2. (E) HI titers against A/Cal H1N1, A/PR8 H1N1, rgH5N1, and A/Phil H3N2 were determined from immune sera of split vaccines (A/PR8 H1N1 and A/Cal H1N1).
NA protein vaccination is less effective in homologous protection than inactivated split and in heterologous protection compared to M2e5x VLP
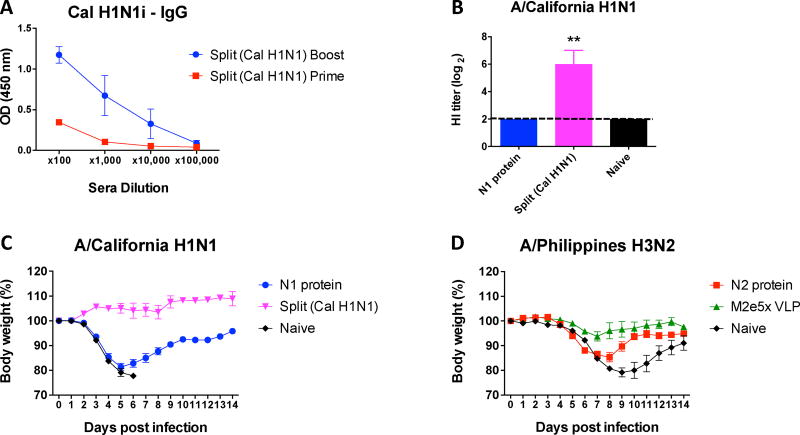
We investigated protective efficacy in actively vaccinated mice after challenge. As described for Figure 1, mice were immunized with N1 (A/Cal) or N2 (A/Wis) NA proteins, split vaccine (A/Cal), or M2e5x VLP. Immune mice with A/Cal split vaccine developed significant levels of virus-specific serum IgG antibodies (Fig. 3A) and they showed high HI activity against the homologous virus strain (Fig. 3B). Mice immunized with A/Cal split vaccine did not show body weight loss after challenge with homologous A/Cal H1N1 virus (Fig. 3C). Mice that were immunized with A/Cal N1 NA protein displayed significant weight loss (~18%), but all mice in this group survived after challenge whereas all naïve mice died of infection (Fig. 3C). Regarding A/Wis N2 NA immunization, a homologous virus challenge mouse model is not available. Thus, we determined efficacy of N2 NA vaccination after a sub-lethal dose challenge with heterologous virus A/Phil H3N2 in comparison with M2e5x VLP vaccination. The A/Wis N2 NA group exhibited lower levels of weight loss (~15%) and a quicker recovery compared to the naïve control (~21%) after A/Phil virus challenge (Fig. 3D). Interestingly the mice immunized with M2e5x VLP, a representative cross-protective vaccine, showed better cross protection (~5% weight loss) against A/Phil H3N2 virus than N2 NA protein immunized mice (Fig. 3D). Overall, these results suggest that NA protein alone is less effective as a vaccine candidate in homologous protection than HA-based split vaccine and in cross protection compared to M2e5x VLP experimental vaccine.
Fig. 3. Vaccination with NA proteins induces less effective protection.
Mice were prime-boost immunized with N1 (A/Cal) or N2 (A/Wis) NA proteins (5µg), M2e5x VLP (10µg), or A/Cal split vaccine (1µg). (A) Prime and boost IgG levels of A/Cal split vaccine immunized mice. (B) HI titers against A/Cal H1N1 with immune sera of N1 NA protein and split vaccine (A/Cal H1N1). At 4 weeks after boost immunization, mice were challenged with a lethal dose of influenza virus and monitored for body weights. (C) N1 NA protein and split vaccine immunized mice were challenged with a homologous A/Cal virus. (D) N2 NA protein (A/Wis H3N2) and M2e5x VLP immunized groups were challenged with a heterologous virus (A/Phil H3N2). Data represent the mean ± SEM. Statistical significances were evaluated by 2-way ANOVA. **p<0.01.
NA protein immune sera confer better cross protection against heterologous strains
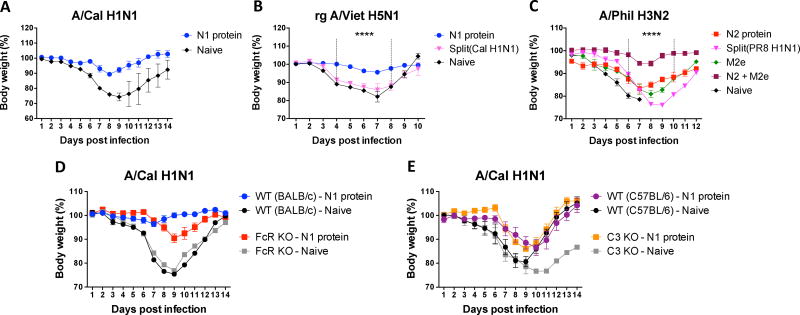
We determined the roles of different vaccine immune sera in conferring cross-protection. Naïve mice were infected with a mixture of influenza virus and immune sera collected from each vaccine group, and then daily monitored for weight changes and survival rates for 14 days (Fig. 4 A–C). Naïve sera did not confer protection against A/Cal H1N1, rgH5N1, and A/Phil H3N2 viruses as evidenced by severe weight loss (>20%) or no survival rates (Fig. 4 A–C). In contrast, N1 protein immune sera conferred protection against homologous A/Cal H1N1 virus and heterologous rgH5N1 virus although low to moderate weight loss of 5–10% was observed (Fig. 4 A, B). A/Cal split vaccine immune sera did not provide cross protection against rgH5N1 virus as shown by severe weight loss similar to that in naïve control sera (Fig. 4B). In an additional set of comparing cross protection against A/Phil H3N2 virus, the mice with N2 protein (A/Wis) immune sera showed weight loss of 16% similar to M2e5x VLP immune sera but better protection with a quicker recovery than A/PR8 split vaccine sera exhibiting severe weight loss (~25%) (Fig. 4C). Interestingly, combination of M2e5x VLP and N2 NA immune sera resulted in synergistic effects on improving protection against A/Phil virus in naïve mice (Fig. 4C). Taken together, these results suggest that NA targeting antibodies confer better cross protection compared to split vaccine-induced antibodies.
Fig. 4. Roles of immune sera in conferring protection in naïve mice.
Immune sera were collected and pooled after immunization of BALB/c mice (n=5) with N1 NA (A/Cal), N2 NA (A/Wis), split vaccine (A/Cal or A/PR8 H1N1), M2e5x VLP, or a mixture of N2 NA and M2e5x VLP. Naïve BALB/c mice were intranasally infected with a lethal dose of influenza virus mixed with immune or naïve sera, and monitored for weight changes. Multiple strains of viruses were tested: (A) A/Cal H1N1, (B) rgH5N1 and (C) A/Phil H3N2. (D) BALB/c and Fc receptor knock-out (FcR KO) or (E) C57BL/6 and C3 knock-out (C3 KO) mice (n=4 per each group) were intranasally infected with a lethal dose of influenza virus (A/ Cal) mixed with immune or naïve sera. Data represent the mean ± SEM. Statistical significances were evaluated by 2-way ANOVA. ****p<0.0001.
Fc receptor plays a role in NA antibody-mediated protection but C3 is not required
We tested whether Fc receptors are involved in NA antibody-mediated protection. Naïve wild type (BALB/c) and Fc receptor common γ-chain knock-out (FcR KO) mice were infected with a mixture of A/Cal H1N1 virus and N1 protein-immune sera (Fig. 4D). BALB/c mice showed minimal or no weight loss and were well protected against homologous A/Cal H1N1 virus. However, FcR KO mice that were inoculated with A/Cal H1N1 virus and N1 NA immune sera resulted in substantial weight loss (~12%), but they were still protected compared to the FcR KO mice with naïve serum plus virus inoculation (Fig. 4D). These results suggest that Fc receptor contributes to the protection mediated by NA antibodies.
To test whether the complement system plays a role in NA-mediated protection, naïve wild type (C57BL/6) and C3 knock-out (C3 KO) mice were infected with a mixture of A/Cal H1N1 virus and N1 protein-immune sera (Fig. 4E). No significant difference in protective efficacy was observed between wild type and C3 KO mice. Both wild type and C3 KO mice that received N1 immune sera and virus exhibited a delay and moderate levels in weight loss (12–15%), compared to naïve serum control groups displaying severe (20–25%) weight loss. It is noted that the C3 KO mice with naïve sera and virus could not fully recover weight loss. C57BL/6 mice showed a trend of lower efficacies in conferring protection than those in BALB/c mice.
Discussion
This study compared the efficacy of three different influenza vaccine antigens by using each representative vaccine: split vaccines for HA immunity, NA proteins for NA immunity, and M2e5x VLP for M2e immunity. We evaluated the protective efficacy of each antigen-specific immune sera by determining: (1) their reactivity to different subtypes of influenza A viruses, (2) enzyme inhibition activities to HA and NA, and (3) protection by active immunization or passively administrated antibodies. We found that antibodies to NA proteins confer a broader range of cross protection than HA antibodies. In addition, protection by NA-specific antibodies appears to be mediated by NA inhibition activity and Fc receptors.
Virus neutralizing activity by HA-targeting antibodies is the most effective in conferring protection against the homologous influenza virus. Immunization with split vaccines (A/Cal H1N1, A/PR8 H1N1, A/Phil H3N2) induced significant levels of antibodies binding to heterologous influenza viruses. Nonetheless, these split vaccine immune sera did not show HI activity against heterosubtypic strains and failed to induce cross protection against viral infection with different subtypes, limiting the protection to homologous virus. Based on these results, split vaccines tend to induce mainly HA immunity to homologous virus. NA protein immunization raised antibodies that are cross reactive to different influenza virus strains within the same NA type. In a similar pattern, NA protein immune sera showed high levels of NI activity to homologous and heterologous virus strains. In contrast to HA immunity by split vaccines, NA antibodies contribute to survival protection against homologous and heterologous influenza virus within the same NA subtype viruses, which is consistent with a previous study [10, 15].
HA targeting vaccines are superior to NA vaccines in homologous protection. This is because HI activity of anti-HA antibodies can lead to sterilizing immunity, which cannot be comparable to infection permissive cross protection. Active immunization with NA protein vaccines induced survival protection against heterologous virus challenge, but its protective efficacy was lower than that of M2e5x VLP which we previously described as for a cross protective vaccine candidate [16]. It is unclear what differences are in the protective mechanisms between NA protein and M2e5x VLP vaccines. T cells induced by M2e5x VLP vaccination were shown to play a role in conferring cross protection [17]. It is possible that in addition to M2e antibodies, T cells induced by M2e5x VLP vaccination contribute to more effective cross protection than NA protein immunization since we observed that N2 NA protein and M2e5x VLP immune sera conferred a similar level of protection against A/Phil H3N2 virus. We hypothesized that non-neutralizing antibody-mediated mechanisms would be involved in the NA antibody-mediated protection, which include antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytolysis (CDC). Fc receptor is known to be required for ADCC via phagocytic cells such as macrophages, natural killer (NK) cells, and neutrophils [18]. C3 component is an essential factor in the complement pathway leading to clearance of antigen-antibody immune complexes via CDC [6]. N1 type NA protein immune sera conferred protection against homologous viral infection in wild type (BALB/c) mice without displaying weight loss whereas a moderate level of body weight loss was observed in FcR KO mice. These results suggest that Fc receptors partially contribute to the protection by NA antibodies. In line with the roles of Fc receptors in mediating protection, HA stalk-specific antibodies were shown to induce phagocytosis of immune complexes in a FcR dependent manner [19, 20]. Similarly, Fc receptors were required for M2e immune mediated protection [13]. In contrast, a similar pattern of weight loss was observed in both wild type (C57BL/6) and C3 KO mice, which indicates that C3 is not required for protection by NA antibodies. Significantly more weight loss in C57BL/6 mice than in BALB/c mice might be due to different genetic backgrounds in these two strains of mice. C57BL/6 mice showed a defect in developing granzyme B-secreting CD8 T cells after influenza virus infection compared to BALB/c mice (unpublished data). It has been reported that C57BL/6 strain is more susceptible to influenza virus infection than BALB/c strain [21].
To overcome limitations of a single vaccine antigen in conferring cross protection, a multicomponent vaccine strategy was reported. Supplementation of inactivated influenza vaccines with M2e-based antigens resulted in inducing significantly improved cross protection in BALB/c mice [17]. It was reported that addition of NA and M2e vaccines to recombinant HA vaccines confers long-lasting cross protection against primary and secondary influenza virus infections in BALB/c mice [22]. We found that combination of N2 NA and M2e5x VLP immune sera resulted in conferring synergistically improved cross protection compared to each immune serum alone. Our unpublished data support the benefits of multicomponent vaccination that the combination of split, NA protein, and M2e5x VLP vaccines induced antibodies specific to each vaccine antigen, providing significantly improved cross protection in C57BL/6 mice than single component vaccines (data not shown). In addition, we found that the efficacy of cross protection by multicomponent vaccines was significantly lower in either CD4 or CD8 T cell deficient mice compared to that in wild type C57BL/6 mice, suggesting important roles of CD4 and CD8 T cells in cross protection (data not shown). Taken together, findings in this study highlight different roles of HA, NA, and M2e as vaccine antigens and suggest a new possible strategy for improving cross protection.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI119366 (S.M.K.), and AI093772 (S.M.K.). The neuraminidase proteins (NR-19234, NR-19237) were obtained through BEI Resources, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wohlbold TJ, Krammer F. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses. 2014;6:2465–2494. doi: 10.3390/v6062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YT, Kim KH, Ko EJ, Lee YN, Kim MC, Kwon YM, Tang Y, Cho MK, Lee YJ, Kang SM. New vaccines against influenza virus. Clinical and experimental vaccine research. 2014;3:12–28. doi: 10.7774/cevr.2014.3.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: Formulation and production strategies. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2015;94:251–263. doi: 10.1016/j.ejpb.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Subbarao K, Matsuoka Y. The prospects and challenges of universal vaccines for influenza. Trends in microbiology. 2013;21:350–358. doi: 10.1016/j.tim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MC, Song JM, Eunju O, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like Particles Containing Multiple M2 Extracellular Domains Confer Improved Cross-protection Against Various Subtypes of Influenza Virus. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YN, Kim MC, Lee YT, Kim YJ, Kang SM. Mechanisms of Cross-protection by Influenza Virus M2-based Vaccines. Immune Netw. 2015;15:213–221. doi: 10.4110/in.2015.15.5.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, Levine M, Katz JM, Ohmit SE. Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv195. [DOI] [PubMed] [Google Scholar]
- 8.Johansson BE, Cox MM. Influenza viral neuraminidase: the forgotten antigen. Expert review of vaccines. 2011;10:1683–1695. doi: 10.1586/erv.11.130. [DOI] [PubMed] [Google Scholar]
- 9.Doyle TM, Hashem AM, Li C, Van Domselaar G, Larocque L, Wang J, Smith D, Cyr T, Farnsworth A, He R, Hurt AC, Brown EG, Li X. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antiviral research. 2013;100:567–574. doi: 10.1016/j.antiviral.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio. 2015;6:e02556. doi: 10.1128/mBio.02556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song JM, Choi CW, Kwon SO, Compans RW, Kang SM, Kim SI. Proteomic characterization of influenza H5N1 virus-like particles and their protective immunogenicity. J Proteome Res. 2011;10:3450–3459. doi: 10.1021/pr200086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. Journal of virology. 2008;82:1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YN, Lee YT, Kim MC, Hwang HS, Lee JS, Kim KH, Kang SM. Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination. Immunology. 2014;143:300–309. doi: 10.1111/imm.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko EJ, Lee YT, Kim KH, Lee Y, Jung YJ, Kim MC, Lee YN, Kang T, Kang SM. Roles of Aluminum Hydroxide and Monophosphoryl Lipid A Adjuvants in Overcoming CD4+ T Cell Deficiency To Induce Isotype-Switched IgG Antibody Responses and Protection by T-Dependent Influenza Vaccine. Journal of immunology. 2017;198:279–291. doi: 10.4049/jimmunol.1600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu WC, Lin CY, Tsou YT, Jan JT, Wu SC. Cross-Reactive Neuraminidase-Inhibiting Antibodies Elicited by Immunization with Recombinant Neuraminidase Proteins of H5N1 and Pandemic H1N1 Influenza A Viruses. Journal of virology. 2015;89:7224–7234. doi: 10.1128/JVI.00585-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, Song JM, Song BM, Lee YJ, Choi JG, Kang HM, Quan FS, Compans RW, Kang SM. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22:1364–1374. doi: 10.1038/mt.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Gordan S, Lux A. FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 2015;36:325–336. doi: 10.1016/j.it.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 19.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nature medicine. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P. Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner. mBio. 2016;7 doi: 10.1128/mBio.01624-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava B, Blazejewska P, Hessmann M, Bruder D, Geffers R, Mauel S, Gruber AD, Schughart K. Host genetic background strongly influences the response to influenza a virus infections. PloS one. 2009;4:e4857. doi: 10.1371/journal.pone.0004857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schotsaert M, Ysenbaert T, Smet A, Schepens B, Vanderschaeghe D, Stegalkina S, Vogel TU, Callewaert N, Fiers W, Saelens X. Long-Lasting Cross-Protection Against Influenza A by Neuraminidase and M2e-based immunization strategies. Sci Rep. 2016;6:24402. doi: 10.1038/srep24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.