Abstract
The dehydratase domain FosDH1 from module 1 of the fostriecin polyketide synthase (PKS) catalyzed the stereospecific interconversion of (3R)-3-hydroxybutyryl-FosACP1 (5) and (E)-2-butenoyl-FosACP1 (11), as established by a combination of direct LC-MS/MS and chiral GC-MS. FosDH1 did not act on either (3S)-3-hydroxybutyryl-FosACP1 (6) or (Z)-2-butenoyl-FosACP1 (12). FosKR2, the ketoreductase from module 2 of the fostriecin PKS that normally provides the natural substrate for FosDH2, was shown to catalyze the NADPH-dependent stereospecific reduction of 3-ketobutyryl-FosACP2 (23) to (3S)-3-hydroxybutyryl-FosACP2 (8). Consistent with this finding, FosDH2 catalyzed the interconversion of the corresponding triketide substrates (3R,4E)-3-hydroxy-4-hexenoyl-FosACP2 (18) and (2Z,4E)-2,4-hexadienoyl-FosACP2 (21). FosDH2 also catalyzed the stereospecific hydration of (Z)-2-butenoyl-FosACP2 (14) to (3S)-3-hydroxybutyryl-FosACP2 (8). Although incubation of FosDH2 with (3S)-3-hydroxybutyryl-FosACP2 (8) did not result in detectable accumulation of (Z)-2-butenoyl-FosACP2 (14), FosDH2 catalyzed the slow exchange of the 3-hydroxy group of 8 with [18O]-water. FosDH2 unexpectedly could also support the stereospecific interconversion of (3R)-3-hydroxybutyryl-FosACP2 (7) and (E)-2-butenoyl-FosACP2 (13).
Graphical Abstract

INTRODUCTION
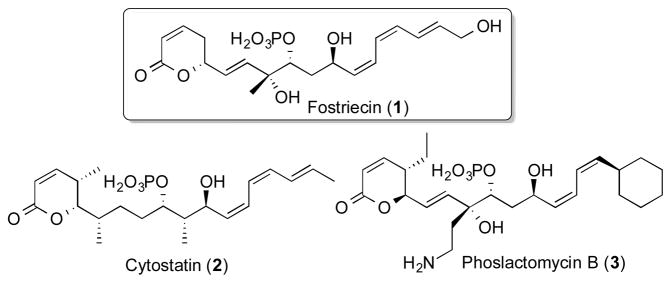
Fostriecin (1), a polyketide phosphate monoester isolated from Streptomyces pulveraceus,1 belongs to a class of selective protein phosphatase inhibitors showing anti-metastatic and antitumor activity that includes the closely related natural products cytostatin (2) and phoslactomycin B (3) (Figure 1).2 Fostriecin also exhibits antimycotic activity.3 In the biosynthesis of fostriecin (1), the parent nonaketide 4 is assembled by a polyketide synthase (PKS) consisting of 8 modules, with a loading tri-domain at the N-terminus of module 1 responsible for priming by the acetyl starter unit, while a thioesterase (TE) domain at the C-terminus of module 8 mediates polyketide chain release and lactonization (Figure 2).4a A series of tailoring reactions, including elimination of malonate to introduce the 2,3-double bond of the dihydropyrone moiety, then converts 4 to the mature product fostriecin (1).4
Figure 1.
Fostriecin (1) and related polyketides.
Figure 2.
Biosynthesis of fostriecin (1) by a modular polyketide synthase. PKS domains: ketosynthase (KS), acyl transferase (AT), acyl carrier protein (ACP), ketoreductase (KR), dehydratase (DH), enoyl reductase (ER), thioesterase (TE).
The acyclic chain of fostriecin harbors four disubstituted double bonds. The Δ16,17 and Δ6,7 E (trans) double bonds of 1 are predicted to be generated by the dehydratase domains of fostriecin modules 1 and 6, FosDH1 and FosDH6 respectively,4c while the Δ14,15 and Δ12,13 Z (cis) double bonds are attributed to the action of the corresponding FosDH2 and FosDH3 domains from fostriecin modules 2 and 3.4a Although the majority of the thousands of known polyketides harbor E double bonds,5 isomeric Z double bonds, while considerably less common, are nonetheless well represented, being found in not only 1–3, but also in the microtubule stabilizer epothilone,6 the microtubule polymerization inhibitor curacin,7 the anti-angiogenic agent borrelidin,8 and the antitubercular rifamycins.9 Surprisingly, all reported attempts to demonstrate in vitro formation of the relevant Z-double bonds by PKS-derived DH domains have been unsuccessful, resulting only in the generation of products containing the isomeric E double bonds.8b,c,9d, While PlmKR1, the ketoreductase from module 1 of the phoslactomycin PKS, has been shown to generate a (3S)-hydroxyacyl thioester that likely serves as the precursor of the Z-3-cyclohexylpropenoate produced by phoslactomycin module 1, direct biochemical evidence for the function of the paired PlmDH1 domain is still lacking.10 We now report the elucidation of the biochemical function of both FosDH1 and FosDH2, thereby confirming the predicted role of each dehydratase domain in the stereospecific formation of their respective E- and Z-disubstituted enoyl-ACP products.
RESULTS
Recombinant Fostriecin PKS Domains
FosDH1 and FosDH2 as well as FosKR2 from module 2 of the fostriecin PKS were each expressed in Escherichia coli as N-terminal His6-tagged proteins using codon-optimized synthetic genes, based on well-precedented PKS domain boundaries (Figures S1–S4). The purity and molecular mass of each recombinant protein was verified by SDS-PAGE and LC-ESI(+)-MS (Figures S6–S8). Although direct expression in E. coli of synthetic genes for FosACP1 and FosACP2 gave predominantly insoluble protein inclusion bodies, the corresponding NusA-FosACP1 and NusA-FosACP2 fusion proteins were obtained in soluble form (Figures S5, S9, and S10).9d Cleavage of the N-terminal NusA with HRV 3C protease gave apo-FosACP1 and apo-FosACP2, which were each confirmed to have the expected mass by LC-ESI(+)-MS (Figures S9 and S10).
Chemoenzymatic synthesis of C4 and C6 acyl-FosACP substrates
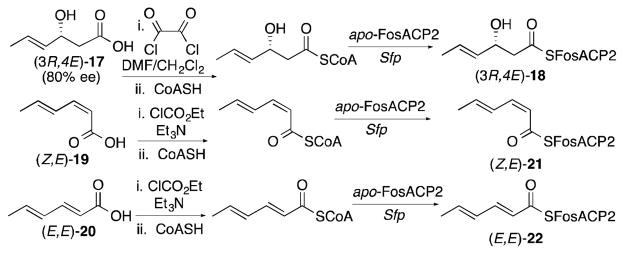
(3R)- and (3S)-3-Hydroxybutyryl-FosACP1 (5 and 6) as well as (3R)- and (3S)-3-hydroxybutyryl-FosACP2 (7 and 8) were chemo-enzymatically prepared from (3R)- and (3S)-hydroxybutyric acid (9 and 10), respectively, via the corresponding -SCoA esters, using apo-FosACP1 or apo-FosACP2 and the surfactin phosphopantetheinyl transferase Sfp (Scheme 1). Similar procedures were also used to prepare the individual unsaturated (E)- and (Z)-2-butenoyl-FosACP1 (11 and 12) as well as (E)- and (Z)-2-butenoyl-FosACP2 (13 and 14) from (E)- and (Z)-2-butenoic acid (15 and 16). For the synthesis of ACP-bound triketides, synthetic (3R,4E)-3-hydroxy-4-hexenoic acid (17, 3R/3S 90:10) was chemo-enzymatically converted via its -SCoA thioester to the corresponding (3R,4E)-3-hydroxy-4-hexenoyl-FosACP2 (18, 80% d.e.) (Scheme 2).11,12 In like manner, (2Z,4E)-2,4-hexadienoic acid (19), prepared as previously described by ring-closing metathesis–base-induced ring opening,13 and (2E,4E)-2,4-hexadienoic acid (20) were each converted via their -SCoA esters to the corresponding (2Z,4E)-2,4-hexadienoyl-FosACP2 (21) and (2E,4E)-2,4-hexadienoyl-FosACP2 (22).
Scheme 1.
Synthesis of C4 acyl-FosACP substrates.
Scheme 2.
Synthesis of C6 acyl-FosACP2 derivatives.
Fostriecin Synthase Module 1. Substrate and Product Specificity of FosDH1
Module 1 of the fostriecin PKS is predicted to produce (E)-2-butenoyl-FosACP1 (11), integrated into the parent module, as inferred from the E (trans) geometry of the derived Δ16,17 double bond of fostriecin (1) (Figure 1). The substrate for FosDH1 is expected to be (3R)-3-hydroxybutyryl-ACP1, as suggested by the presence of the characteristic highly conserved Leu-Asp-Asp motif in the paired ketoreductase domain FosKR1 (Figure S1).14 Fully consistent with these predictions, incubation of FosDH1 with (3R)-3-hydroxybutyryl-FosACP1 (5) resulted in dehydration to give exclusively (E)-2-butenoyl-FosACP1 (11) (Scheme 3). Thus treatment of the incubation mixture with PICS TE, the thioesterase from the picromycin PKS, so as to hydrolyze the ACP-bound diketide substrate and products, followed by treatment with N,Obis( trimethylsilyl)trifluoroacetamide (N,O-bis(TMS)-TFA) gave the corresponding (E)-2-butenoyl-TMS derivative whose geometry was confirmed by GC-MS analysis and direct comparison with authentic standards derived from both (E)-15 and (Z)-16. No dehydration product from 5 was detected in the absence of added FosDH1 (Figure S11).
Scheme 3.
Stereospecific dehydration and hydration by FosDH1.
In a complementary set of incubations, FosDH1 catalyzed the reverse hydration reaction by stereospecifically converting (E)-2-butenoyl-FosACP1 (11) to (3R)-3-hydroxybutyryl-FosACP1 (5) (Scheme 3). LC-ESI(+)-MS analysis of the reaction mixture showed the expected addition of water (M+H2O) for the parent ion while LC-ESI(+)-MS/MS confirmed the predicted increase of M+18 in the mass of the derived pantetheinyl ejection fragments (329 to 347 Da) (Figures S12 and S13).15 Chiral GC-MS analysis after hydrolysis of the incubation products with PICS TE and derivatization by (N,O-bis(TMS)-TFA established the exclusive formation of bis(TMS)-(3R)-3-hydroxybutyrate (bis(TMS)-(3R)-9) (rt 9.61 min, identical with an authentic reference sample) (Figure S14). Hydration of 11 was not detected in the absence of FosDH1. Neither the stereoisomeric (3S)-3-hydroxybutyryl-FosACP1 (6) nor (Z)-2-butenoyl-FosACP1 (12) underwent any detectable reaction in the presence of FosDH1.
Fostriecin Synthase Module 2. Substrate and Product Specificity of FosKR2
Module 2 of the fostriecin PKS is predicted to produce (2Z,4E)-2,4-hexadienoyl-FosACP2 (21), integrated into the parent module, as inferred from the Z (cis) geometry of the derived Δ14,15 double bond of fostriecin (1) (Figure 1). Within fostriecin synthase module 2, FosKR2 is responsible for generating the bound 3-hydroxyacyl-FosACP2 triketide that serves as the native substrate of the paired FosDH2 domain in intact fostriecin module 2. The absence of the characteristic Leu-Asp-Asp triad typical of 3R-hydroxy (D)-specific ketoreductases suggested that FosKR2 should generate the (L)-hydroxy product typical of an A-type KR domain.14 To establish the stereochemistry of this reduction, 3-ketobutyryl-FosACP2 (23), chemoenzymatically prepared by treatment of acetoacetyl-SCoA with Sfp and apo-FosACP2, was reduced with FosKR2 in the presence of NADPH (Scheme 4 and Figure S15). The exclusive product was (3S)-3-hydroxybutyryl-FosACP2 (8), as established by chiral GC-MS analysis of the corresponding bis(TMS)-derivative.
Scheme 4.
Stereochemistry of FosKR2-catalyzed reduction.
FosDH2
Incubation of FosDH2 with the chemoenzymatically prepared form of the natural substrate stereoisomer, (3R,4E)-3-hydroxy-4-hexenoyl-FosACP2 (18) (~80% e.e.), resulted in stereospecific dehydration to give (2Z,4E)-2,4-hexadienoyl-FosACP2 (21), as determined by GC-MS analysis of the derived methyl ester (2Z,4E)-21-Me following PICS TE-catalyzed hydrolysis and treatment with TMS-CHN2, (Scheme 5, Figures S16 and S17).16 Complementary incubation of (2Z,4E)-2,4-hexadienoyl-FosACP2 (21) with FosDH2 resulted in the reverse hydration reaction to give (3R,4E)-3-hydroxy-4-hexenoyl-FosACP2 (18) as the exclusive product of DH-catalyzed hydration (Scheme 5). This result was established by chiral GC-MS analysis of the derived methyl ester, including direct comparison with reference standards of synthetic methyl (3R,4E)-17-Me and the enantiomeric methyl (3S,4E)-3-hydroxy-4-hexenoate (Figures S18 and S19). Hydration of 21 did not occur in the absence of added FosDH2, although (2Z,4E)-21 did undergo 10–15% buffer-catalyzed isomerization to (2E,4E)-22 after 2 h incubation under the same conditions.
Scheme 5.
FosDH2-catalyzed hydration and dehydration of FosACP2-bound triketide substrates.
We also carried out the analogous series of reactions with FosDH2 and the corresponding FosACP2-bound C4 substrate analogs. Thus (Z)-2-butenoyl-FosACP2 (14) underwent time-dependent, stereospecific hydration to give (3S)-3-hydroxybutyryl-FosACP2 (8) when incubated with FosDH2 (Scheme 6, Figures 3 and S20–S22). On the other hand, when FosDH2 was incubated with 8 (Figures S23 and S24) the expected dehydration product, (Z)-14, could not be directly detected, presumably due to the thermodynamically unfavorable Keq ~10−3 for dehydration to the (Z)-enoyl-ACP product.17 Evidence for the transient formation of 14 was obtained, however, by the observation that incubation of 8 with FosDH2 in [18O]-H2O for 90 min resulted in ~10% net exchange of the 3-hydroxyl oxygen of 8, as revealed by the enzyme- and time-dependent increase in the relative intensity of the [M+2] peak of the derived pantetheinate ejection fragment (349.23 Da) observed by LC-MS/MS analysis of recovered 8 (Figure S26). Isotope exchange was not detectable in the absence of FosDH2. FosDH2 was found to be unexpectedly permissive in also being able to interconvert the unnatural pair of stereoisomeric C4 analogs, (3R)-3-hydroxybutyryl-FosACP2 (7) and (E)-2-butenoyl-FosACP2 (13) (Figures S22-1 and S27–S30). Consistent with these observations, incubation of (3R)-7 with FosDH2 in [18O]-H2O resulted in essentially complete isotope exchange of the 3-hydroxyl oxygen of recovered 7 within 30 min (Figure S31).
Scheme 6.
FosDH2-catalyzed hydration and dehydration of FosACP2-bound diketide substrate analogs.
Figure 3. Hydration of (2Z,4E)-3-hydroxy-4-hexenoyl-FosACP2 (21) by FosDH2.
Chiral GC-MS of methyl (3R,4E)-3-hydroxy-4-hexenoate from incubation of 21 with FosDH2. A-1: XIC m/z 71); A-2: Co-injection with added methyl (3S,4E)-3-hydroxy-4-hexenoate, XIC m/z 71. See Figure S19 for comparisons of the TIC of reaction product, rt 36.89 min, and authentic methyl (3R,4E)-3-hydroxy-4-hexenoate.
DISCUSSION
Dehydratases of both Type I and Type II fatty acid synthases (FASs) catalyze the exclusive syn dehydration of a (3R)-3-hydroxyacyl-ACP thioester to the corresponding (E)-enoyl-ACP.18 The structure of the E. coli dehydratase (FabA) displays a characteristic hotdog fold that has been observed in all other DH structures from both FAS and PKS systems.7a,19,20 The active site of each DH harbors a universally conserved pair of His and Asp residues. Schwab, in a critical review of research on FabA and the closely related dehydratase-isomerase FabZ, has discussed a one-base, two-step mechanistic model in which the active site imidazole residue first catalyzes the stereospecific removal of 2-Hsi of the 3-hydroxyacyl-ACP substrate following which the transiently-generated imidazolium species donates its proton to the 3-hydroxyl group to promote C–O bond cleavage. 18a,21,22 Although the distinct enoyl-CoA hydratase of fatty acid oxidation differs significantly from DH enzymes in both protein fold and the presence of two essential active site Glu residues in place of the His-Asp dyad of DH domains, it catalyzes an analogous net syn hydration of (E)-2-enoyl-CoA thioesters to yield the corresponding (3S)-3-hydroxyacyl-CoA products.17a Both protons and the oxygen of the nucleophilic water are incorporated into the product, consistent with a single-base mechanism for enoyl-CoA hydratase in which one Glu residue acts sequentially, first as base and then as active site acid, while the second Glu side chain positions the active site water by an essential H-bond.23 Interestingly, while enoyl-CoA hydratase can also add water to the isomeric (Z)-2-butenoyl-CoA to give the (3R)-3-hydroxybutyryl-CoA product, the reverse dehydration could not be observed, an observation that was attributed to the calculated unfavorable Keq <10−3 for formation of the (Z)-isomer.17a
EryDH4 (from module 4 of the erythromycin PKS) and NanDH2 (from module 2 of the nanchangmycin PKS) both catalyze the syn elimination of water from a (2R,3R)-2-methyl-3-hydroxyacyl-ACP substrate to the corresponding (E)-2-enoyl-ACP product,24 while RifDH10 (from module 10 of the rifamycin PKS) catalyzes the syn dehydration of the diastereomeric (2S,3S)-2-methyl-3-hydroxyacyl-RifACP10 substrate to the (E)-2-enoyl-ACP product.9d The structures of both EryDH4 and RifDH10 display the characteristic DH double hotdog fold as well as the conserved active His and Asp residues.9d,20 While one or two of the four DH domains from the curacin PKS are thought to be responsible for formation of unsaturated intermediates possessing (Z) double bonds, all four proteins exhibit the common double hotdog fold and high levels of mutual structural homology, while the actual enzymatic formation of (Z)-enoyl-ACP products has not yet been reported.7 FosDH2 shows 71.0% mutual sequence identity (88.8% similarity) over 276 aa to the closely related PlmDH1 from module 1 of the phoslactomycin PKS, which has also been implicated in the formation of a cis double bond (Figure S32). Interestingly, FosDH1 and FosDH2 themselves show a more modest 40.8% mutual sequence identity (63.4% similarity), while retaining each of the key conserved amino acid motifs typified by the structurally characterized dehydratases EryDH4 and RifDH10.
In spite of the frequent occurrence of Z double bonds in complex polyketides, the experimental demonstration that FosDH2 can catalyze the interconversion of the L-3-hydroxyacyl-ACP and (Z)-2-enoylacyl-ACP thioesters is the first documented in vitro confirmation of this nominally straightforward reaction directly catalyzed by a PKS DH domain. Earlier unsuccessful attempts to observe DH-catalyzed formation of conjugated Z-enoyl thioester double bonds have been summarized above. One recent report has described the highly unusual dehydration of (3S,4S)-3,4-dihydroxypentanoyl-N-acetylcysteamine thioester to the (Z)-2-enoyl-SNAC catalyzed by a modular PKS domain formally classified as a TE on the basis of phylogenetic sequence comparisons.25 Curiously, the TE-catalyzed dehydration reaction could not be detected with the corresponding ACP thioester. The well-studied FabZ-catalyzed formation of the non-conjugated Z-3,4-decenoyl-ACP from (3R)-3-hydroxydecanoyl-ACP involves the allylic isomerization of the initially-formed (E)-2-decenoyl-ACP.18,21 Although there is molecular genetic evidence that the characteristic Z-double bond of epothilone is introduced by the DH domain from the proximal downstream module acting in trans, this transformation has not been verified at the enzyme level.26
The finding that FosKR2 stereospecifically reduces 3-ketobutyryl-FosACP2 (23) to L-(3S)-3-hydroxybutyryl-FosACP2 (8) establishes that FosDH2 never encounters the unnatural epimer, D-(3S,4E)-3-hydroxy-4-hexenoyl-FosACP2, in its native modular context. Indeed, we have now shown that FosDH2 stereospecifically interconverts L-(3R,4E)-3-hydroxy-4-hexenoyl-FosACP2 (18) and (2Z,4E)-hexadienoyl-FosACP2 (21), the predicted product of fostriecin synthase module 2. Interestingly, under the incubation conditions tested, dehydration of the C4-analog L-(3S)-8 to (Z)-14 could be detected only indirectly by FosDH2-catalyzed isotopic exchange of the 3-hydroxyl group with [18O]-water. This apparent discrepancy between the results with C6 and the C4 substrates is likely the consequence of the use of a 1.5:1 stoichiometric excess of FosDH2 to triketide alcohol 18, compared to the 5-fold lower 0.3–0.5:1 ratio that was used for the incubation of FosDH2 with the shorter chain diketide analog (3S)-8. Thus although FosDH2 cannot alter the Keq ~10−3 for free substrates and products, enzymes can differentially bind substrates and products so as to shift the ratio of bound substrates species closer to 1:1.27 For the intact fostriecin synthase module 2, the unfavorable equilibrium of the FosDH2-catalyzed dehydration reaction is overcome by metabolic coupling to the thermodynamically favorable, metabolically irreversible chain elongation reaction catalyzed by the KS domain of the fostriecin synthase module 3.
Finally, the demonstration that FosDH2 catalyzes the dehydration of L-(3R,4E)-18 to (2Z,4E)-21 does not validate the common assumption that there is a requisite correlation between an L-hydroxy configuration of the substrate and the cis geometry of the olefinic product of DH-catalyzed dehydration.14a There are already sufficient exceptions7b,8b,c,9d,23,25 to this superficially appealing generalization to establish that it does not have reliable predictive value.
EXPERIMENTAL METHODS
Materials
IPTG and kanamycin were purchased from Thermo Fisher Scientific. (E)-2-Butenoyl-CoA ((E)-Crotonyl-CoA), 3-ketobutyryl-CoA (acetoacetyl-CoA), N,O-BSTFA, TCEP, (3R)- and (3S)-3-hydroxybutyric acids, 2-butenoic acid (crotonic acid), and (2E,4E)-2,4-hexadienoic acid were purchased from Sigma-Aldrich and utilized without further purification. (Z)-2-Butenoic acid was prepared as previously described.17a,28 [18O]-H2O was purchased from Cambridge Isotope Laboratories. HRV 3C protease was obtained from Pierce. Pseudomonas fluorescens Amano lipase P was from Sigma-Aldrich. Sfp and PICS TE were each expressed and purified as previously described.9d,24,29 DNA primers were synthesized by Integrated DNA Technologies. Competent E. coli DH5α, DH10β and BL21(DE3) cloning and expression strains were purchased from New England Biolabs (NEB). Restriction enzymes, T4 DNA ligase and Phusion High-Fidelity PCR Master Mix with HF Buffer were purchased from NEB. Amicon Ultra Centrifugal Filter Units (Amicon Ultra-15 and Amicon Ultra-0.5, 3000, 10000 and 30000 MWCO) were obtained from Millipore. The 20 mL HisPrep FF 16/10 column, prepacked with precharged Ni Sepharose 6 Fast Flow, was purchased from GE Healthcare.
Methods
General methods were as previously described. 30 Growth media and conditions used for E. coli strains and standard methods for handling E. coli in vivo and in vitro were those described previously, unless otherwise noted.31 All DNA manipulations were performed following standard procedures.31 Plasmid DNA was purified using a Thermo Scientific GeneJET Plasmid mini-prep kit. DNA sequencing was carried out by Genewiz, South Plainfield, NJ. Synthetic genes, optimized for expression in E. coli, were prepared by DNA 2.0, Newark, California. All proteins were handled at 4 °C unless otherwise stated. Protein concentrations were determined according to the method of Bradford, using a Tecan Infinite M200 Microplate Reader with bovine serum albumin as standard. 32 Protein purity and size were estimated using SDS-PAGE, visualized using Coomassie Blue stain, and analyzed with a Bio-Rad ChemiDoc MP System. Gas chromatography-mass spectrometry (GC–MS) analyses were performed using an Agilent 5977A Series GC/MSD instrument (70 eV, electron impact) with a 3 min solvent delay. Protein accurate molecular weight was determined on an Agilent 6530 Accurate-Mass Q-TOF LC-MS. A Thermo LXQ equipped with Surveyor HPLC system and a Phenomenex Jupiter C4 column (150 mm × 2 mm, 5.0 μm) was utilized for analysis of acyl-ACP compounds. HPLC-ESI-MS/MS analysis was carried out in positive ion mode for analysis of pantetheinate ejection fragments, as previously described.15,33
Expression and Purification of FosDH1 and FosDH2
A synthetic gene for FosDH1, optimized for expression in E. coli and corresponding to the region from A1992 to G2294 of Fos Module 1 (Figures S1 and S2), was sub-cloned into pET28a between the NdeI and XhoI restriction sites. The FosDH2 expression plasmid corresponding to the region from A947 to A1232 of Fos Module 2 (Figure S2 and S3) was generated by subcloning the synthetic gene optimized for expression in E. coli into pET28a between the NdeI and XhoI restriction sites. Single colonies of E. coli BL21(DE3) cells that had been transformed with the individual FosDH1 or FosDH2 expression vectors were inoculated into 10 mL LB media containing 50 mg/L kanamycin and incubated overnight at 37 °C. This starter culture was then inoculated into 500 mL Super Broth (SB) containing 50 mg/L kanamycin. The culture was then grown at 37 °C at 225 rpm until OD600 = 0.5. At this point, the culture was cooled to 18 °C and then induced with 0.2 mM IPTG. Cells were harvested after 20 h by centrifugation at 4000g for 40 min. The purification was then carried out at 4 °C unless mentioned otherwise. Harvested cells were re-suspended in 50 mL start buffer (50 mM sodium phosphate, 500 mM NaCl, pH 7.5). The cells were lysed by sonication and cell debris was removed by centrifugation at 20000g for 50 min. The supernatant was loaded onto a HisPrep FF 16/10 (GE Healthcare Life Science) column pre-equilibrated with start buffer. The column was washed with 150 mL of wash buffer (50 mM sodium phosphate, 500 mM NaCl, 10 mM imidazole, pH 7.5) to elute contaminating proteins. FosDH1 or FosDH2 were then eluted with a linear gradient from 10 mM – 500 mM imidazole in the same buffer. The fractions containing FosDH1 or FosDH2 were pooled, buffer-exchanged, and concentrated to final buffer (50 mM sodium phosphate, 250 mM NaCl, 10% glycerol, pH 7.5) using an Amicon Ultra-15 (30000 MWCO) centrifugal filter. The purity and MW of FosDH1 and FosDH2 were analyzed by SDS-PAGE and LC-ESI(+)-MS (Figures S6 and S7) Aliquots of the purified proteins were stored at −80 °C.
Expression and Purification of His6-tag-NusA-FosACP1 and His6-tag-NusA-FosACP2 and Preparation of apo-FosACP1 and apo-FosACP2
Synthetic genes for FosACP1 (V3130 to T3220 of Fos Module 1 (Figures S1 and S2) and FosACP2 (region from R1696 to T1820 of Fos Module 2 (Figures S3 and S4) were each subcloned into pET28a between the NdeI and XhoI restriction sites. Since the resulting recombinant ACP proteins were produced predominantly as insoluble inclusion bodies when expressed in E. coli BL21(DE3), the corresponding NusA-FosACP1 and NusA-FosACP2 fusion proteins were then generated. In brief, the DNA regions encoding FosACP1 and FosACP2 were each amplified by PCR from the above-described pET28a constructs using the primers FosACP1/2-HRV-FP and pET-28a-RP which is complementary to the region just 3′ of the native XhoI site (Figure S5). The resultant amplified DNA harboring FosACP1 or FosACP2 was digested with NheI and XhoI before ligation into the corresponding sites of an NheI/XhoI-digested vector, immediately downstream of DNA encoding His6-NusA-HRV3C housed in a pET28 vector. The resultant expression plasmids encoded the individual NusA-FosACP1 and NusA-FosACP2 fusion proteins, each carrying an N-terminal His6-tag and an HRV 3C protease site between the N-terminal NusA and either FosACP1 or FosACP2.
NusA-FosACP1 or NusA-FosACP2 were expressed in E. coli BL21(DE3) as described above for FosDH1 and FosDH2. Cells were harvested after 20 h by centrifugation at 4000g for 40 min. The recovered cells were resuspended in 50 mL start buffer and lysed by passage three times through a French Press at 10000 psi, then centrifuged at 20000g for 50 min. The pellet was discarded and the supernatant was loaded on to a HisPrep FF 16/10 (GE Healthcare Life Science) column preequilibrated with start buffer. The column was washed with 150 mL of wash buffer to elute contaminating proteins. The respective His6-NusA-FosACP1 and His6-NusA-FosACP2 proteins were then eluted with a gradient from 10 mM – 500 mM imidazole in the same buffer. The fractions containing His6-NusA-FosACP1or His6-NusA-FosACP2 were pooled. Protein was concentrated to 2–3 mL using an Amicon Ultra-15 (30000 MWCO) centrifugal filter before further purification on a Hiload 16/600, Superdex 200 pg size exclusion column pre-equilibrated with 50 mM sodium phosphate and 250 mM NaCl, pH 7.5. Fractions containing His6-NusA-FosACP1 or His6-NusA-FosACP2 were pooled and treated with 1 μL of HRV-3C protease (1U/μL) per 200 μg of purified protein. After overnight incubation at 4 °C with continuous shaking, the mixture was loaded on to a HisPrep FF 16/10 column pre-equilibrated with start buffer. Both His6-NusA up to the HRV 3C protease cleavage site, as well as the HRV-3C protease, which also carries a His6-tag, along with any undigested His6-Nus-AFosACP were retained by the column while the cleaved FosACP1 and FosACP2 eluted during the column wash step. The fractions containing cleaved FosACP1 or FosACP2 were pooled and buffer-exchanged with final buffer (50 mM sodium phosphate, 250 mM NaCl, 10% glycerol, pH 7.5) using an Amicon Ultra-15 (30000 MWCO) centrifugal filter. The purity and MW of FosACP1 and FosACP2 were analyzed by SDS-PAGE and LC-ESI(+)-MS (Figures S9 and S10). Aliquots of purified proteins were stored at −80 °C.
Expression and Purification of FosKR2
A synthetic gene for FosKR2, optimized for expression in E. coli and corresponding to the region from F1239 to L1683 of Fos Module 2 (Figures S3 and S4) was subcloned into pET28a between the NdeI and XhoI restriction sites. FosKR2 was expressed and purified by the same procedures described for FosDH1 and FosDH2 (Figure S8).
(2Z,4E)-2,4-Hexadienoic Acid (18)
(2Z,4E)-2,4-Hexadienoic acid (18) was prepared as previously described. 13 The cis-olefin geometry was verified by 1H NMR and chiral GC-MS by which the (2Z,4E)-2,4-hexadienoic acid (18) was readily separated from commercially available (2E,4E)-17 either as the carboxylic acids or derived methyl esters 17-Me and 18-Me. The carboxylic acid forms were analyzed with an Agilent ChiraSil-Dex capillary GC column, (0.32 mm ID × 25 m length × 0.25 μm film) using a temperature program (GC Method A) with a 1 min hold at 50 °C, followed by a 7.5 °C/min increment to 200 °C. Separation of the methyl esters 17-Me and 18-Me forms used a temperature program (GC Method B) with a 1 min hold at 50 °C, followed by a 1.00 °C/min increase to 90 °C, a 2 min hold at 90 °C, and then a further increment of 20.00 °C/min to 200 °C (Figure S16). The 1H NMR data for 18 matched the literature values:13 1H NMR (400 MHz, CDCl3): δ 7.35 (ddd, 1 H), 6.63 (dd, 1 H), 6.04 (dq, 1 H), 5.57 (d, 1 H), 1.90 (d, 3 H).
(3R,4E)-3-Hydroxy-4-hexenoic Acid (22) and (3S,4E)-3-Hydroxy-4-hexenoic Acid
(±)-Ethyl (3RS,4E)-3-Hydroxy-4-hexenoic acid was synthesized as previously described.34 The enantiomers were kinetically resolved using Amano lipase P (Scheme S1).12b,c In brief, (±)-ethyl (4E,3RS)-3-hydroxy-4-hexenoate (1.58 g, 10 mmol) was dissolved in 20 mL of hexane. Amano lipase P (1 g) was added with vinyl acetate (20 mmol) and the reaction mixture was stirred at room temperature for 24 h with monitoring by GC-MS. The lipase was removed by filtration and the solvent was evaporated. Ethyl (3S,4E)-3-hydroxy-4-hexenoate and ethyl (3R,4E)-3-acetoxy-4-hexenoate were separated by SiO2 flash column chromatography. The ethyl-3-hydroxy-4-hexenoate was eluted with 10% ethylacetate/90% hexane and then (3R,4E)-3-acetoxy-4-hexenoate was eluted by 20% ethylacetate/80% hexane. If the resolution was incomplete and the acetate ester contained more than 10% (3S,4E) isomer, a second round of Amano lipase P reaction was performed. Hydrolysis of the recovered ethyl (3S,4E)-3-hydroxy-4-hexenoate with LiOH (1.0 eq) at 0 °C for 1–2 h generated (3S,4E)-3-hydroxy-4-hexenoic acid. Methanolysis of (3R,4E)-3-acetoxy-4-hexenoate (700 mg, 3.5 mmol) was effected by treatment with K2CO3 (966 mg, 2.0 eq) in 12 mL MeOH with vigorous stirring at room temperature for 30 min. The solution was diluted with EtOAc (240 mL) and washed with 0.1 M aq. NaOH (240 mL). The aqueous layer was back-extracted with EtOAc (3 × 240 mL). The combined organic fractions were dried over MgSO4 and concentrated to give methyl (3R,4E)-3-hydroxy-4-hexenoate (22-Me) which was purified by flash column chromatography on silica gel (20% ethylacetate/80% hexane). Hydrolysis with LiOH (1.0 eq) at 0 °C for 1–2 h generated (3R,4E)-3-hydroxy-4-hexenoic acid (22). By chiral GC-MS analysis (Method B) of the methyl esters, the major component (~90%) of the (3R,4E)-22-Me eluted ca. 0.9 min earlier than the (3S,4E)-methyl ester (~10%), corresponding to ~80% ee (Figure S18). Chiral GC-MS analysis also established that the preparation of the enantiomeric methyl (3S,4E)-3-hydroxy-4-hexenoate was obtained in ~90% ee.
(3R)- and (3S)-Hydroxybutyryl-CoA
Using the previously described method,24a 25 mg (0.24 mmol) of (3R)- or (3S)-3-hydroxybutyric acid in 1 mL THF were treated by dropwise addition of 60 mg (0.36 mmol, 1.5 eq) of 1,1′-carbonyldiimidazole (CDI) in 0.5 mL of THF. After reaction at 0 °C for 60–90 min, a solution of CoASH (20 mg in 1.5–2.0 mL H2O, 0.024 mmol, 0.1 eq) was added dropwise. The reaction was continued at room temperature and under nitrogen for 4 h. Organic solvent was removed by rotary evaporation. The aqueous phase, after extraction with ethyl acetate to remove byproducts, was purified through by HPLC (Agilent) using a Phenomenex Gemini semi-preparative C18 column (150 × 10 mm, 10 μm) equilibrated with 2% CH3CN/H2O. Elution was carried out with a linear gradient from 2% to 90% CH3CN/H2O. Collected peaks were checked for purity by LC-ESI(+)-MS using an Agilent Zorbax Extend C18 column (100 × 2.1 mm, 3.5 μm) with a linear gradient from 2% to 70% CH3CN/H2O. Peaks containing product of the desired mass, LC-ESI(+)-MS [M+H]+ 854, were collected, lyophilized and stored at −80 °C.
(E)- and (Z)-2-Butenoyl-CoA ((E)- and (Z)-Crotonyl-CoA
Using the previously described method,24a 20 mg (0.23 mmol) of (E)- or (Z)-2-butenoyl-CoA was dissolved in 2 mL anhydrous CH2Cl2 under nitrogen, then treated with 65 μL of triethylamine (47 mg, 0.47 mmol, 2 eq) followed after 10 min by 44 μL of ethylchloroformate (50 mg, 0.46 mmol, 2 eq) . The reaction mixture was stirred at 0 °C for 2 h. After removal of the organic solvent by rotary evaporation, the residue was dissolved in 2 mL THF. Insoluble salts were removed by centrifugation and the mixed anhydride was added to a round bottom flask containing 20 mg of CoASH dissolved in 2 mL of 50 mM aq. NaHCO3 (pH 8.0) and the resultant mixture was stirred for 1–3 h at room temperature under N2. After removal of excess starting materials by extraction with ethyl acetate, the aqueous phase was purified by HPLC (Agilent) using the Phenomenex Gemini semi-preparative C18 column (150 × 10 mm, 10 μm) equilibrated with 2% CH3CN/H2O. Elution was carried out with a linear gradient from 2% to 10% CH3CN/H2O. Collected peaks were checked for purity by LC-ESI(+)-MS as described above for 3-hydroxybutyryl-CoA. Peaks exhibiting the desired mass, LC-ESI(+)-MS [M+H]+ 836 were collected, lyophilized and stored at −80 °C.
L-(3R,4E)-3-Hydroxy-4-hexenoyl-CoA
L-(3R,4E)-3-Hydroxy-4-hexenoic acid (17, 26 mg, 0.2 mmol) in 1 mL anhydrous CH2Cl2 was treated with 80 μL of 2M oxalyl chloride solution (0.16 mmol, 0.8 eq) and 4 drops of anhydrous DMF in a flask fitted with a glass funnel filled with Drierite. (Note: It is important not to use an excess of oxalyl chloride in order to avoid unwanted reaction of the allylic alcohol group.) After vigorous stirring at room temperature for 2–3 h, the solvent was evaporated after dilution with ethyl acetate, resulting in co-evaporation of any unreacted oxalyl chloride. The residue was dissolved in 2 mL THF and the solution was added to 20 mg of CoASH dissolved in 1 mL of 0.4 M NaHCO3, pH 8.0. After stirring for 1–2 h at room temperature, excess starting materials were removed by extraction with ethyl acetate. The resulted aqueous crude acyl-CoA mixture was purified by HPLC using a Phenomenex Gemini semi-preparative C18 column, 150 × 10 mm, equilibrated with 10% CH3CN/H2O. The sample was eluted with a linear gradient from 10% to 100% of CH3CN/H2O. HPLC peaks were collected and lyophilized. Each fraction was analyzed by HPLC-ESI(+)-MS using an Agilent Zorbax C18 column (2.1 × 50 mm, 3.5 μm) and a linear gradient from 10% to 100% of CH3CN/H2O.
(2Z,4E)- and (2E,4E)-2,4-Hexadienoyl-CoA
(2Z,4E)-2,4-Hexadienoic acid (19) or (2E,4E)-2,4-hexadienoic acid (20) (0.2 mmol) was dissolved in 1 mL of anhydrous CH2Cl2 under N2. Triethylamine (70 μL, 47 mg, 4.0 eq) was added, followed after 10 min at 0 °C by 50 μL of ethylchloroformate (45.8 mg, 3.0 eq). The reaction mixture was stirred for 2 h at 0 °C. After evaporation of the solvent, the residue was dissolved in 2 mL THF and the insoluble salts were removed by centrifugation. The mixed anhydride was then slowly added to a separate round bottom flask containing 20 mg of CoASH in 1 mL of NaHCO3 buffer (pH 8.0). The reaction mixture was stirred 1–3 h at room temperature with monitoring by LC-MS. After removal of excess starting material by extraction with EtOAc, the aqueous crude acyl-CoA mixture was purified by HPLC using the Phenomenex Gemini semi-preparative C18 column, 150 × 10 mm, equilibrated with 10% CH3CN/H2O. The sample was eluted with a linear gradient from 10% to 100% of CH3CN/H2O. HPLC peaks were collected and lyophilized. Each fraction was analyzed by HPLC-ESI(+)-MS using an Agilent Zorbax C18 column (2.1 × 50 mm, 3.5 μm) and a linear gradient from 10% to 100% of CH3CN/H2O.
Monitoring of FosDH1 and FosDH2 Activity Using Acyl-CoA Substrates
The activity of various protein preparations of FosDH1 and FosDH2 was conveniently checked using the surrogate –SCoA substrates (E)-2-butenoyl-CoA and 3-hydroxybutyryl-CoA. Assay mixtures contained 1 mM 3-hydroxybutyryl-CoA or (E)-2-butenoylCoA in 50 mM sodium phosphate, pH 7.5 Buffer in a total volume of 100 μL. The assay mixture was divided into 50-μL portions. To one, 100 μM FosDH1 or FosDH2 was added while the blank used an equivalent volume of 50 mM sodium phosphate, pH 7.5 buffer. The assay mixtures were incubated at room temperature for 2 h, then diluted with 200 μL of H2O and passed through a Millipore 30 kDa MWCO 500 μL filter and centrifuged at 14000g to remove FosDH1 or FosDH2 by buffer exchange. The assay mixtures were analyzed by LC-ESI(+)-MS using an Agilent Zorbax Extend C18 column (100 × 2.1 mm, 3.5 μm) with a linear gradient from 2% to 70% CH3CN/H2O to monitor for the expected increase or decrease of 18 amu in the mass of the acyl-CoA components as a result of hydration or dehydration.
C4 Acyl-FosACP Substrates, 5–8 and 11–14
Small scale acylation reactions for LC-MS analysis
For preparation of acyl-FosACP substrates, each reaction contained 100–150 μM apo-FosACP1 or apo-FosACP2, and 250–300 μM (3R)- or (3S)-3-hydroxybutyryl-CoA or (E)-or (Z)-2-butenoyl-CoA, plus 2 μM Sfp, 10 mM MgCl2, and 1 mM TCEP in 50 mM sodium phosphate, pH 7.5, in a total volume of 100 μL. The reactions were incubated at room temperature for 10 min to form the corresponding acyl-FosACP1 (5–8) or acyl-FosACP2 (11–14). The reaction mixture was then concentrated using a Amicon Ultra-0.5 (3000 MWCO) centrifugal filter and centrifuged at 14000g to remove unreacted acyl-CoA by buffer exchange, with recovery of the acyl-FosACP retentate which was diluted to a total volume of 200–300 μL.
FosDH1 and FosDH2-Catalyzed Dehydration and Hydration of C4 Acyl-FosACP Substrates. LC-ESI(+)-MS and LC-ESI(+)-MS/MS Analysis
The above-described samples of acyl-FosACP were divided into 2 equal 100–150 μL portions. To one, FosDH1 or FosDH2 was added to a final concentration of 50 μM while the blank was supplemented with an equivalent volume of 50 mM sodium phosphate, pH 7.5. After parallel 60-min incubations at room temperature, each reaction mixture was diluted with formic acid/H2O and centrifuged for 5 min at 14000g. These samples were then analyzed by LC-ESI(+)-MS and LC-ESI(+)-MS/MS using an analytical Aeris widepore-C4 column (3.6 μm, 2.1 × 150 mm) from Phenomenex using a linear gradient from 30% to 70% CH3CN/H2O on a Thermo-LXQ mass spectrometer. For LC-ESI(+)-MS/MS analysis15 the M11+ ion was selected for MS/MSs such that, both the hydrated and dehydrated pPant ejection fragments could be observed together (Figures S12, S13, S23, S24, S20, S21, and S27–S30).
C4 Acyl-FosACP Substrates, 5–8 and 11–14
Larger scale acylation reaction for GC-MS analysis of FosDH-catalyzed dehydration or dehydration
Each reaction consisted of 100 μM apo-FosACP1 or apo-FosACP2 and 250–300 μM (3R)- or (3S)-3-hydroxybutyryl-CoA E)- or (Z)-2-butenoyl-CoA, plus 2 μM Sfp, 10 mM MgCl2, and 1 mM TCEP in 50 mM sodium phosphate, pH 7.5, in a total volume of 500 μL. (TCEP was omitted from the reaction with (Z)-2-butenoyl-CoA.) Each reaction was incubated at room temperature for 10 min to form the corresponding acyl-FosACP product. The reaction mixture was then concentrated using an Amicon Ultra-0.5 (3000 MWCO) centrifugal filter with centrifugation at 14000g to remove unreacted acyl-CoA by buffer exchange, with recovery of the acyl-FosACP retentate, which was diluted to a total volume of 500 μL.
FosDH1-Catalyzed Dehydration and Hydration of C4 Acyl-FosACP Substrates. Chiral GC-MS Analysis
The above-described samples of acyl-FosACP1 (5, 6, 11, and 12 were divided into 2 equal 250 μL portions. To one, FosDH1 was added to a final concentration of 40 μM while the blank was supplemented with an equivalent volume of 50 mM sodium phosphate, pH 7.5. Both mixtures, with and without FosDH1, were incubated at room temperature for 30 min before addition to each of 200 μM of PICS TE. After hydrolysis by PICS TE for 15 min, the reaction was quenched and the pH was adjusted to 3.0–3.5 by addition of ~9 μL of 1 M HCl. After centrifugation at 13000g for 5 min to remove precipitated protein, the supernatant was extracted with 4 × 800 μL of CH2Cl2. After removal of solvent by rotary evaporation, the residue was dissolved in 130 μL CH2Cl2 and then treated with 20 μL BSTFA for derivatization of the organic acids (Total 150 μl). The derivatized samples were directly analyzed by chiral GC-MS (HP GCD system) on a Varian CP ChiraSil_DEX column (25 m length, 0.25 μm diameter) using a temperature program (GC Method C) with a 1 min hold at 50 °C, followed by an increment of 8 °C/min to 150 °C, a 1 min hold, and then an increase of 20 °C/min to 210 °C, and a final hold at this temperature for 3 min (Figures S11 and S14).
FosKR2-Catalyzed Reduction of 3-Ketobutyryl-FosACP2 (23)
3-Ketobutyryl-CoA (400 μM) was reacted for 10 min at room temperature with 150 μM apo-FosACP2 in the presence of 2 μM Sfp in 50 mM sodium phosphate, pH 7.2 containing 10 mM MgCl2 and 1 mM TCEP (total vol 400 μL) to form 3-ketobutyryl-FosACP2 (23). The reaction mixture was passed through a Millipore 3 KDa MWCO 500 μL filter by centrifugation at 14000g to remove unreacted acyl-CoA by buffer exchange. The retentate containing 3-ketobutyryl-FosACP2 (23) was divided into two separate 200 μL aliquots. To each of these, 1 mM NADPH and 40 μM FosKR2 was added. One reaction was quenched immediately by treatment with 150 mM NaOH (18 μL of 2 M NaOH) at 65 °C for 20 min. The other portion was incubated for 30 min before being quenched in the same manner with aq.with 150 mM of NaOH. After hydrolysis, each sample was cooled on ice and then acidified to pH 3.0–3.5 by addition of 54 μL of 1 M HCl. Each sample was centrifuged at 14000g for 5 min to remove precipitated protein. The supernatant was then extracted with 4 × 800 μL of CH2Cl2. The solvent was removed by rotary evaporation. The residue dissolved in 100 μL of CH2Cl2 was derivatized by treatment with 10 μL BSTFA. The samples were directly analyzed by chiral GC-MS on a ChiraSil_Dex capillary GC column using a temperature program (GC Method D) with a 1 min hold at an initial temperature of 50 °C followed by an increment of 8 °C/min to 150 °C, a 1 min hold at 150 °C, an increase of 20 °C/min to 210 °C, and a final 3 min hold at 210 °C (Figure S15).
FosDH2-Catalyzed Dehydration and Hydration of C6 Acyl-FosACP2 Substrates, (3R,4E)-18 and (2Z,4E)-21
(2Z,4E)-2,4-Hexadienoyl-CoA or (3R,4E)-3-hydroxy-4-hexenoyl-CoA (200–500 μM) was incubated with 50 μM apo-FosACP2 and 40 μM Sfp, 2 mM DTT, and 15 mM MgCl2 in reaction buffer (350 mM NaCl 50 mM phosphate pH 7.5, total vol 2.5 mL) for 30 min at 30 °C. The residual CoA substrate, MgCl2, and DTT were removed by passage of 2.5 mL of the incubation mixture through a PD-10 column that had been equilibrated with 25 mL of the reaction buffer. FosACP2-bound product, 18 or 21, was eluted by 3.5 mL of the reaction buffer. The PD-10 eluate was concentrated using an Amicon centrifuge unit 10,000 MWCO (30 min at 1,600g at 4 °C reduced the volume to 200–300 μL. The acylated FosACP2 was analyzed by LC-MS The low concentration of FosACP2 was used to avoid precipitation during the procedure. FosDH2 was added to a final concentraion of 300 μM along with 2 mM DTT and the reaction buffer was added to adjust the total volume to 500 μL. The reaction mixture was incubated at room temperature for 1–2 h. At the end of the reaction, the product was released from the FosACP2 by PICS TE (20 μL per reaction, 20 min at room temperature). The reaction mixture was acidified to pH <3 by addtion of 1 M HCl. The C6 acids were extracted with 2 × 800 μL ethyl acetate. The solvent was removed by rotary evaporation. The product was taken up in 80 μL of MeOH, 20 μL of TMS-CHN2 was added, and the solution incubated at room temperature for 5 min. The resulting methyl esters were analyzed by chiral GC-MS on a ChiraSil-Dex capillary GC column using GC Method B (Figures S16–S19).
FosDH2-Catalyzed Dehydration and Hydration of C4 Acyl-FosACP Substrates. Chiral GC-MS Analysis
The above-described samples (1 mL) of acyl-FosACP2 (7, 8, 13, and 14 were mixed with FosDH2 (final concentration 50 μM). Aliquots of 250 μL were collected at 0, 15, 30 and 45 min incubation time and added to Eppendorf tubes containing 200 μM of PICS TE and incubated for 15 min at room temperature. After hydrolysis by PICS TE, the reaction was quenched and the pH was adjusted to 3.0–3.5 by addition of ~9 μL of 1 M HCl. Samples were centrifuged at 13000g for 5 min to remove precipitated protein. The supernatant was extracted with 4 × 800 μL of CH2Cl2. The solvent was then removed by rotary evaporation and the residue was dissolved in 100 μL CH2Cl2 to which 10 μL BSTFA was added for derivatization of acids (Total 110 μl). These samples were directly analyzed by chiral GC-MS (HP GCD system) with Varian CP ChiraSil_DEX column (25 m length, 0.25 μm diameter) using a temperature program (GC Method D) with a 1 min hold at 55 °C, followed by an increment of 0.5 °C/min to 65 °C, a 1 min hold, and then an increase of 15 °C/min to 90 °C, a 1-min hold at 90 °C, an increase of 20 °C/min to 200 °C, and a final 1 min hold at this temperature (Figures S22, and S25).
FosDH2-Catalyzed Isotope Exchange of 3-Hydroxybutyryl-FosACP2
(3R)- and (3S)-3-Hydroxybutyryl-FosACP2 (7 and 8) were generated by incubation of 800–1000 μM (3R)- or (3S)-3-hydroxybutyryl-CoA and 200 μM apo-FosACP2 with 5 μM Sfp in the presence of 10 mM MgCl2 and 1 mM TCEP in 50 mM sodium phosphate, pH 7.5 in a total volume of 100 μL. After 15 min incubation at room temperature to form (3R)- or (3S)-3-hydroxybutyryl-FosACP2 (7 or 8), the reaction mixture was passed through a Millipore 3 KDa MWCO 500 μL filter with centrifugation at 14000g to remove unreacted acyl-CoA by buffer exchange. The retentate (100 μL) containing 7 or 8 was mixed with 300 μL of [18O]-H2O-based buffer (final 18O enrichment 75 atom%.) To this solution, 50 μM (final concentration) FosDH2 was added. Samples of 95 μL were withdrawn after 0, 30 and 90 min for (3S)-3-hydroxybutyryl-FosACP2 (8) and 0, 30, 90 and 300 min for (3R)-3-hydroxybutyryl-FosACP2 (7). Each sample was added to an Eppendorf tube containing 3.5% formic acid (final concentration) and then directly analyzed by LC-ESI(+)-MS/MS with monitoring of the pPant ejection fragment from only the 3-hydroxybutyryl-FosACP and measurement of the relative abundance of the 347 Da (16O) and 349 Da (18O) species (Figure S26 and S31.
Supplementary Material
Acknowledgments
This work was supported by a grant from the U. S. National Institutes of Health, GM022172, to D.E.C. We thank Will Furuyama and Colin Gould for valuable experimental assistance.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI:10.1021/jacs.******* Sequence alignments and protein structure comparisons, PKS domain boundaries, SDS-PAGE and LC-MS analysis of recombinant proteins, LC-MS/MS and chiral GC-MS analysis.
References
- 1.Lewy DS, Gauss CM, Soenen DR, Boger DL. Curr Med Chem. 2002;9:2005–2032. doi: 10.2174/0929867023368809. [DOI] [PubMed] [Google Scholar]
- 2.a) Amemiya M, Someno T, Sawa R, Naganawa H, Ishizuka M, Takeuchi T. J Antibiot (Tokyo) 1994;47:541–544. doi: 10.7164/antibiotics.47.541. [DOI] [PubMed] [Google Scholar]; b) Fushimi S, Furihata K, Seto H. J Antibiot. 1989;42:1026–1036. doi: 10.7164/antibiotics.42.1026. [DOI] [PubMed] [Google Scholar]; c) Palaniappan N, Kim BS, Sekiyama Y, Osada H, Reynolds KA. J Biol Chem. 2003;278:35552–35557. doi: 10.1074/jbc.M305082200. [DOI] [PubMed] [Google Scholar]
- 3.Mamber SW, Okasinski WG, Pinter CD, Tunac JB. J Antibiot (Tokyo) 1986;39:1467–1472. doi: 10.7164/antibiotics.39.1467. [DOI] [PubMed] [Google Scholar]
- 4.Kong R, Liu X, Su C, Ma C, Qiu R, Tang L. Chem Biol. 2013;20:45–54. doi: 10.1016/j.chembiol.2012.10.018.Palaniappan N, Alhamadsheh MM, Reynolds KA. J Am Chem Soc. 2008;130:12236–12237. doi: 10.1021/ja8044162.. c) FosER1, the enoyl reductase domain of module 1, is apparently catalytically silent.
- 5.Shiomi K, Omura S. In: Macrolide Antibiotics. Chemistry, Biology, and Practice. 2. Omura S, editor. Academic Press; San Diego, CA: 2002. pp. 1–56. [Google Scholar]
- 6.Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, Julien B. Science. 2000;287:640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- 7.a) Akey DL, Razelun JR, Tehranisa J, Sherman DH, Gerwick WH, Smith JL. Structure. 2010;18:94–105. doi: 10.1016/j.str.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fiers WD, Dodge GJ, Sherman DH, Smith JL, Aldrich CC. J Am Chem Soc. 2016;138:16024–16036. doi: 10.1021/jacs.6b09748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Olano C, Wilkinson B, Sanchez C, Moss SJ, Sheridan R, Math V, Weston AJ, Brana AF, Martin CJ, Oliynyk M, Mendez C, Leadlay PF, Salas JA. Chem Biol. 2004;11:87–97. doi: 10.1016/j.chembiol.2003.12.018. [DOI] [PubMed] [Google Scholar]; b) Vergnolle O, Hahn F, Baerga-Ortiz A, Leadlay PF, Andexer JN. Chembiochem. 2011;12:1011–1014. doi: 10.1002/cbic.201100011. [DOI] [PubMed] [Google Scholar]; c) Hahn F, Kandziora N, Friedrich S, Leadlay PF. Beilstein J Org Chem. 2014;10:634–640. doi: 10.3762/bjoc.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Schupp T, Toupet C, Engel N, Goff S. FEMS Microbiol Lett. 1998;159:201–207. doi: 10.1111/j.1574-6968.1998.tb12861.x. [DOI] [PubMed] [Google Scholar]; b) August PR, Tang L, Yoon YJ, Ning S, Muller R, Yu TW, Taylor M, Hoffmann D, Kim CG, Zhang X, Hutchinson CR, Floss HG. Chem Biol. 1998;5:69–79. doi: 10.1016/s1074-5521(98)90141-7. [DOI] [PubMed] [Google Scholar]; c) Tang L, Yoon YJ, Choi CY, Hutchinson CR. Gene. 1998;216:255–265. doi: 10.1016/s0378-1119(98)00338-2. [DOI] [PubMed] [Google Scholar]; d) Gay D, You YO, Keatinge-Clay A, Cane DE. Biochemistry. 2013;52:8916–8928. doi: 10.1021/bi400988t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Alhamadsheh MM, Palaniappan N, Daschouduri S, Reynolds KA. J Am Chem Soc. 2007;129:1910–1911. doi: 10.1021/ja068818t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bonnett SA, Whicher JR, Papireddy K, Florova G, Smith JL, Reynolds KA. Chem Biol. 2013;20:772–783. doi: 10.1016/j.chembiol.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(3R,4E)-3-Hydroxy-4-hexenoic acid (17) has the same configuration (L) at C-3 as the corresponding (3S)-3-hydroxybutyric acid (10), reflecting the difference in priority of the respective propenyl and methyl substituents in the Cahn-Ingold-Prelog nomenclature.
- 12.To prepare 17 and it (3S)-enantiomer, synthetic (±)-ethyl-(3RS,4E)-3-hydroxy-4-hexenoate was kinetically resolved by incubation with Amano lipase P and vinyl acetate to give a mixture consisting predominantly of ethyl (3R,4E)-3-acetoxy-4-hexenoate and ethyl (3S,4E)-3-hydroxy-4-hexenoate, which were readily separated by SiO2 chromatography (Scheme S1). Chiral GC-MS analysis of the derived methyl 3-hydroxy-4-hexenoate esters established that (3R,4E) alcohol had ~80% e.e., while the (3S,4E) enantiomer had ~90% e.e. The absolute configuration of 17 was assigned based on the well-established preference of Amano lipase for (S)/[L] alcohols. Cf. Brem J, Paizs C, Toşa MI, Vass E, Irimie FD. Tetrahedron: Asymmetry. 2009;20:489–496.Ghanem A, Aboul-Enein HY. Chirality. 2005;17:1–15. doi: 10.1002/chir.20089.
- 13.Schmidt B, Kunz O. Eur J Org Chem. 2012;2012:1008–1018. [Google Scholar]
- 14.a) Reid R, Piagentini M, Rodriguez E, Ashley G, Viswanathan N, Carney J, Santi DV, Hutchinson CR, McDaniel R. Biochemistry. 2003;42:72–79. doi: 10.1021/bi0268706. [DOI] [PubMed] [Google Scholar]; b) Caffrey P. Chem-BioChem. 2003;4:654–657. doi: 10.1002/cbic.200300581. [DOI] [PubMed] [Google Scholar]; c) Keatinge-Clay AT. Chem Biol. 2007;14:898–908. doi: 10.1016/j.chembiol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 15.a) Dorrestein PC, Bumpus SB, Calderone CT, Garneau-Tsodikova S, Aron ZD, Straight PD, Kolter R, Walsh CT, Kelleher NL. Biochemistry. 2006;45:12756–12766. doi: 10.1021/bi061169d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Meluzzi D, Zheng WH, Hensler M, Nizet V, Dorrestein PC. Bioorg Med Chem Lett. 2008;18:3107–3111. doi: 10.1016/j.bmcl.2007.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Analysis of the stereochemistry of the FosDH2-catalyzed dehydration of (3R,4E)-3-hydroxy-4-hexenoyl-FosACP2 (18) was complicated by competing buffer-catalyzed dehydration of (18) to the isomeric (2E,4E)-22, as well buffer-catalyzed isomerization of initially formed (2Z,4E)-21 to 22, as established by control incubations carried out in the absence of added FosDH2 (cf Figure S17C). Thus the formation of (2Z,4E)-21 was accompanied by a time-dependent increase in the proportion of the isomeric dienoic ester (2E,4E)-22.
- 17.a) Wu WJ, Feng Y, He X, Hofstein HA, Raleigh DP, Tonge PJ. J Am Chem Soc. 2000;122:3987–3994. [Google Scholar]; b) Fausto R, Tonge PJ, Carey PR. J Chem Soc Faraday Trans. 1994;90:3491–3503. [Google Scholar]
- 18.a) Schwab JM, Habib A, Klassen JB. J Am Chem Soc. 1986;108:5304–5308. [Google Scholar]; b) Sedgwick B, Morris C, French SJ. J C S Chem Commun. 1978:193–194. [Google Scholar]
- 19.Kimber MS, Martin F, Lu Y, Houston S, Vedadi M, Dharamsi A, Fiebig KM, Schmid M, Rock CO. J Biol Chem. 2004;279:52593–52602. doi: 10.1074/jbc.M408105200. [DOI] [PubMed] [Google Scholar]
- 20.Keatinge-Clay A. J Mol Biol. 2008;384:941–953. doi: 10.1016/j.jmb.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab JM, Henderson BS. Chem Rev. 1990;90:1203–1245. [Google Scholar]
- 22.An alternative two-base mechanism, also discussed by Schwab, and that has been widely invoked, posits that the Asp residue serve as the active site acid for the cleavage of the C–O bond. This mechanism, however, would require that the pKa of the active site Asp anomalously exceed that of the catalytic His.
- 23.Bahnson BJ, Anderson VE, Petsko GA. Biochemistry. 2002;41:2621–2629. doi: 10.1021/bi015844p. [DOI] [PubMed] [Google Scholar]
- 24.a) Valenzano CR, You YO, Garg A, Keatinge-Clay A, Khosla C, Cane DE. J Am Chem Soc. 2010;132:14697–14699. doi: 10.1021/ja107344h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guo X, Liu T, Valenzano CR, Deng Z, Cane DE. J Am Chem Soc. 2010;132:14694–14696. doi: 10.1021/ja1073432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He HY, Tang MC, Zhang F, Tang GL. J Am Chem Soc. 2014;136:4488–4491. doi: 10.1021/ja500942y. [DOI] [PubMed] [Google Scholar]
- 26.Tang L, Ward S, Chung L, Carney JR, Li Y, Reid R, Katz L. J Am Chem Soc. 2004;126:46–47. doi: 10.1021/ja030503f. [DOI] [PubMed] [Google Scholar]
- 27.a) Albery WJ, Knowles JR. Biochemistry. 1976;15:5627–5631. doi: 10.1021/bi00670a031. [DOI] [PubMed] [Google Scholar]; b) Albery WJ, Knowles JR. Biochemistry. 1976;15:5631–5640. doi: 10.1021/bi00670a032. [DOI] [PubMed] [Google Scholar]
- 28.Rappe C. Org Syn. 1973;53:123–127. [Google Scholar]
- 29.Lu H, Tsai SC, Khosla C, Cane DE. Biochemistry. 2002;41:12590–12597. doi: 10.1021/bi026006d. [DOI] [PubMed] [Google Scholar]
- 30.a) Valenzano CR, Lawson RJ, Chen AY, Khosla C, Cane DE. J Am Chem Soc. 2009;131:18501–18511. doi: 10.1021/ja908296m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xie X, Garg A, Khosla C, Cane DE. J Am Chem Soc. 2017;139:3283–3292. doi: 10.1021/jacs.7b00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2001. [Google Scholar]
- 32.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Garg A, Khosla C, Cane DE. J Am Chem Soc. 2013;135:16324–16327. doi: 10.1021/ja408944s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins I, Nadin A, Holmes AB, Long ME, Man J, Baker R. J Chem Soc Perkin Trans 1. 1994:2205–2215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.