Abstract
γδ T cells located near the epithelial barrier are integral components of local inflammatory and innate immune responses. We have previously reported the presence of choroidal γδ T cells in a model of chronic degeneration of the retinal pigment epithelium (RPE). The goals of the current study were to further define the functions of choroidal γδ T cells and to explore the underlying mechanisms of their action. Our data demonstrate that choroidal γδ T cells are activated by RPE injury in response to NaIO3 treatment, and that they express genes that encode immunosuppressive cytokines, such as IL-4 and IL-10. γδ–T-cell–deficient mice developed profound RPE and retinal damage at doses that caused minimal effects in wild-type mice, and adoptive transfer of γδ T cells prevented sensitization. Intravitreal injection of IL-4 and IL-10 ameliorated RPE toxicity that was induced by NaIO3. Ex vivo coculture of γδ T cells with RPE explants activated the production of anti-inflammatory cytokines via an aryl hydrocarbon receptor (AhR)–dependent mechanism. AhR deficiency abolished the protective effects of γδ T cells after adoptive transfer. Collectively, these findings define important roles for choroid γδ T cells in maintaining tissue homeostasis in the outer retina.—Zhao, Z., Liang, Y., Liu, Y., Xu, P., Flamme-Wiese, M. J., Sun, D., Sun, J., Mullins, R. F., Chen, Y., Cai, J. Choroidal γδ T cells in protection against retinal pigment epithelium and retinal injury.
Keywords: inflammation, choriocapillaris drop out, immune modulation, AhR
The epithelial barrier of skin, lung, intestine, and the reproductive tract is part of the innate immune system and separates the core of the body from the environment. Tissue-associated T cells near the epithelium are part of the first line of defense that encounters pathogens and plays major roles in immune surveillance (1–3). A majority of these cells are γδ T cells, which can display tissue-specific and unconventional functions, such as producing neurotrophic (4) and keratinocyte growth factors, to support epithelial cells and maintain the integrity of the barrier (5). Deficiency in γδ T cells has been associated with an increased vulnerability of epithelial or mucosal tissues to injury and aggravated autoimmune responses (6, 7). Retinal pigment epithelium (RPE) is part of the blood-retinal barrier that lines between the neural sensory retina and choroid. γδ T cells in the choroid have not been well characterized, but likely share some of the distinct properties of the intraepithelial lymphocytes (IELs) in other organs (8).
RPE controls the immune responses of the posterior eye in a number of ways, such as producing cytokines and complement proteins, and interacts with T lymphocytes, macrophages, and microglia (9–11). Conditions of RPE degeneration, including age-related macular degeneration (AMD) and retinitis pigmentosa, are believed to involve dysregulated immune responses (12, 13). Although extensive studies have been performed to demonstrate how activated immune cells and complement proteins contribute to the inflammatory and angiogenic responses in the outer retina (14–17), it has not been established whether the innate immune cells of the choroid can exert protective effects against RPE injury.
In our previous studies on the basis of a chronic RPE degeneration model, we identified choroidal γδ T cells that can produce IL-17 (18). The goals of the current study were to further define the functions of choroidal γδ T cells and to explore the underlying mechanism that controls their properties in genetic and chemical toxicity models of RPE injury. Our results demonstrate that RPE damage elicited the expansion and activation of protective choroidal γδ T cells. RPE modulated the functions of γδ T cells and promoted their production of immunosuppressive cytokines via mechanisms that are dependent on aryl hydrocarbon receptors (AhRs).
MATERIALS AND METHODS
Mice
Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. T-cell receptor (TCR) δ-chain-knockout (Tcrdtm1Mom) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). AhR-knockout (Ahrtm1.2Arte) mice were purchased from Taconic (Hudson, NY, USA). All mice were housed under cyclic 12-h light/dark conditions and fed ad libitum. The Rd8 mutation in Crb1 gene was routinely screened (19, 20) and was not detected. Euthanasia was performed by exposing mice to carbon dioxide that was delivered at 2 L/min via a 3-stage gas regulator. All procedures were conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. All study mice were age 3–5 mo.
Abs and chemicals
Ab against glial fibrillary acidic protein was purchased from Cell Signaling Technology (Danvers, MA, USA). All other Abs were obtained from eBioscience (San Diego, CA, USA), and their detailed information is listed in Supplemental Table 1. NaIO3 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Peanut agglutinin, DAPI and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil) were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
In vivo examination of mouse fundus
Spectral-domain optical coherence tomography was performed on a Spectralis multimodality imaging system (Heidelberg Engineering, Carlsbad, CA, USA) as described previously (18). A 20-diopter lens (Edmund Optics, Barrington, NJ, USA) was used to adapt for the refraction of the mouse eye. Color fundus images were obtained by using a Micron III mouse fundus imaging system (Phoenix Research Labs, Pleasanton, CA, USA) (18). During image acquisition, animals were anesthetized by isoflurane inhalation from a Precision vaporizer (Harvard Apparatus, Holliston, MA, USA), with the cornea kept moisturized and the pupil fully dilated by Tropicamide Ophthalmic Solution (Bausch & Lomb, Rochester, NY, USA).
Histopathology and immunofluorescence staining
Paraffin sections of eyes were prepared as described previously (18). For each mouse eye, ∼250 4-μm-thick sagittal sections were cut from the cornea to the optic nerve and stained with hematoxylin and eosin. Every section was reviewed for RPE and retina pathology. To count the number of photoreceptor nuclei in the outer nuclear layer (ONL), serial sections were cut along the horizontal meridian. Ten slides from the perioptic nerve area that spanned a distance of ∼200 μm were picked for quantification of the number of ONL nuclei (21). Cryosections of posterior eyes were used for immunostaining. Sagittal cryosections (8 μm thick) were prepared from the cornea to the optic nerve and stained for various antigens of interest (18). To block nonspecific binding, tissue sections were incubated with normal serum that was diluted in PBS with 0.5% Triton X-100. They were then incubated with primary Abs followed by Alexa Fluor–conjugated secondary Abs (Thermo Fisher Scientific). Nuclei were counterstained with DAPI. Images were obtained with a Carl Zeiss AxioVision microscope equipped with ApoTome (Zeiss, Jena, Germany). Cryosections of human donor eyes were obtained from the Iowa Lions Eye Bank (Iowa City, IA, USA) (22). Among the 10 AMD eyes that were examined, two showed lesions of choroidal neovascularization.
Quantitative RT-PCR analyses of RNA isolated from retina or RPE/choroid tissues
Eyes were enucleated from euthanized mice. After removal of the anterior segment, the neural retina and RPE/choroid tissues were harvested as described previously (20), and lysed in Trizol reagent (Thermo Fisher Scientific). Total RNA was isolated according to manufacturer instructions and treated with DNase (Thermo Fisher Scientific) to remove the trace amount of genomic DNA (23). cDNA was synthesized by using M-MLV Reverse Transcriptase and oligo(dT)15 primer (Promega, Madison, WI, USA). Primers used for gene-specific PCR amplifications were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Primer sequences are listed in Supplemental Table 2.
Adoptive γδ–T-cell transfer
Splenic γδ T cells were isolated by using paramagnetic beads according to the manufacturer’s protocol (Miltenyi Biotec, San Diego, CA, USA). In brief, cells from the spleen were liberated by collagenase digestion and filtered through a 70-μm nylon mesh. Red blood cells were removed by hypotonic lysis (Sigma-Aldrich). Remaining cells were labeled with FITC-conjugated anti-TCRγδ Ab (GL3; eBioscience), followed by a secondary labeling with bead-conjugated anti-FITC Ab. The resultant cell suspension was loaded onto a separation column within a magnetic field that trapped the bead-bound γδ T cells. Purified γδ T cells were counted, and a total of 1 × 106 cells were transferred into the TCRδ−/− mouse by intraperitoneal injection (24). For cell-tracing studies, isolated γδ T cells were labeled with 5 μM carboxyfluorescein succinimidyl ester (Thermo Fisher Scientific) before adoptive transfer (24, 25).
Optokinetic tracking
Visual function assessment was performed by using previously described methods (26). In brief, the testing mouse was placed in the center of a square box with 4 sides that were composed of LED monitors. A 3-dimensional virtual rotating drum was created to trigger the optokinetic reflex by displaying vertical sine wave grating, the frequency and direction of which were controlled by a computer program (OptoMotry; CerebralMechanics, Lethbride, AB, Canada). A positive response was regarded as a smooth pursue movement of the mouse’s head and neck. Responses were recorded when the spatial frequency of the grating was gradually increased until an optomotor movement could not be elicited.
Flow cytometry analyses of lymphocytes in the posterior eye
Ocular lymphocytes in single-cell suspension were prepared as described previously (18). In brief, retina and RPE/choroid tissues were collected from enucleated eyes and digested with 0.05% collagenase D at 37°C for 30 min. At the end of the incubation, tissues were gently grinded and pipetted to release cells. After passing through a 40-μm nylon cell strainer, cells were collected by centrifugation at 400 g for 4 min. The cell pellet was resuspended in 30% Percoll/RPMI 1640 solution and laid over a 30%/70% discontinuous Percoll gradient (Sigma-Aldrich), then centrifuged at 400 g for 30 min at room temperature. Enriched lymphocytes at the interphase of the lower one third of the gradient were carefully collected for additional analyses. Staining for cell-surface markers and intracellular antigens was performed according to our previously published methods (18). In brief, cells were stained with fixable viability dye efluor 506 (eBioscience), blocked with FcγR blocker (CD16/32), and stained for specific surface molecules. For intracellular staining, cells were incubated for 4 h with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (750 ng/ml) in the presence of GolgiStop (BD Bioscience, Brea, CA, USA). After surface staining, cells were fixed, permeabilized, and stained for intracellular cytokines by using intracellular fixation buffer (eBioscience). Samples were analyzed on an LSRII fluorescence-activated cell sorting Fortessa flow cytometer (Becton Dickinson, San Jose, CA, USA), and data were processed with FlowJo software (TreeStar, Ashland, OR, USA).
Indirect immunofluorescent staining of choroid/sclera flat mount
Mice were terminally anesthetized and subjected to whole-body perfusion with PBS followed by 10 ml Dil dye (0.12 mg/ml in PBS) at a rate of 1–2 ml/min (27). At the end the perfusion, eyes were enucleated, and whole-mount tissues of RPE/choroid were prepared (20). RPE was removed by a brief exposure to hypertonic saline during tissue preparation (28) and by a gentle brush with a silicon-tipped cannula needle (Becton Dickinson). Tissues were fixed in 4% paraformaldehyde. After blocking in 10% fetal bovine serum, 0.5% Triton X-100, and 1× PBS, choroid flat mount was stained with various Abs as described. To quantify TCRδ+ cells on the flat mounts, 5 images were obtained randomly in the midperipheral region using a ×40 lens for each flat mount, and 6 samples were used for each experimental group.
RNA sequencing and bioinformatics analyses
Twelve-week-old wild-type mice were injected with 20 mg/kg NaIO3 to induce eye injury. At 5 d postinjection, eyes were enucleated, and γδ T cells were isolated by using the magnetic-activated cell-sorting method described above. For each experimental condition, 16 eyes were used to collect γδ T cells for RNA isolation and sequencing. Splenic γδ T cells from the same animals were harvested as control. Altogether 6 samples were generated, with 3 biologic replicates—a total of 48 eyes—for choroidal γδ T cells and the other 3 for splenic γδ T cells. All RNA samples were processed at the same time and sequenced on an Illumina HiSeq 2500 platform (LC Sciences, Houston, TX, USA). Raw reads were trimmed and aligned to the mouse genome according to a previously described method (29). Differential gene expression was analyzed on the basis of gene counts by using DESeq2 3.4 in R (version 3.3.2; Bioconductor, Buffalo, NY, USA).
For gene ontology analysis, differentially expressed gene lists were generated by comparing choroidal γδ T cells with splenic samples. Up- or down-regulated genes were defined as those with at least a 2-fold change in either direction with Padj < 0.01. Biologic process gene ontology over-representation was analyzed with InnateDB (30), using the hypergeometric algorithm with Benjamini-Hochberg correction. Most pertinent processes were picked from the top entries. Canonical pathway analyses were carried out by using the Ingenuity pathway analysis software (winter release, December 2016; Qiagen, Germantown, MD, USA). Representative genes that were related to T-lymphocyte functions were selected and a heat map of normalized expression was drawn by pheatmap package in R (The R Foundation, Vienna, Austria).
For analyzing downstream genes of AhR, we compiled a list of target genes by using two approaches. First, by searching the mouse genome, we obtained the list of genes with promoter regions that contained AhR binding motifs that are annotated in TRANSFAC, a database of experimentally determined transcription factor binding sites (31). Second, we downloaded the literature-curated AhR target genes from the Transcriptional Regulatory Relationships Unravelled by Sentence-based Text-mining (TRRUST) database (32). Combining these two approaches, we obtained a list of 41 AhR targets. Among those, we only kept the genes with at least 1 read/million in all the samples according to our RNA sequencing data. This reduced the list to 31 genes, among which there were 11 and 2 genes significantly up- and down-regulated, respectively (false discovery rate < 0.01).
Statistics
Data were composed of at least 3 independent experiments. Statistical analyses were performed with GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Differences were determined by using a Student’s t test in 2 group comparisons or 1-way ANOVA followed by Bonferroni’s post hoc test when comparing more than 2 conditions.
RESULTS
Characterization of choroidal γδ T lymphocytes and their responses to RPE injury
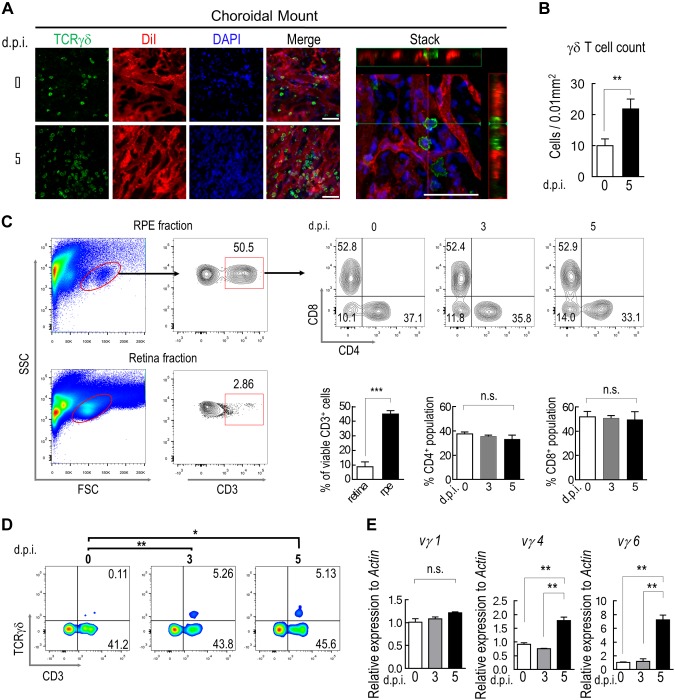
To visualize tissue-associated γδ T cells in the choroidal stroma, we performed immunofluorescence staining of the choroidal flat mount (Fig. 1A). RPE was removed by a brief exposure to hypertonic saline during tissue preparation (28) to avoid the interference of its pigment on optical imaging of the choroid. Choroidal blood vessels were labeled with a lipophilic dye, Dil (27), so that cells that were trapped in residual blood during tissue processing could be excluded. The results (Fig. 1A) displayed the distribution of TCRγδ+ cells in the extravascular space of the choroid. NaIO3 treatment is a well-established approach to induce selective lesions of the RPE and retina in mouse models without causing apparent systemic toxicities (33–35). The number of choroidal γδ T cells increased significantly at 5 d in mice that were treated with NaIO3 at 20 mg/kg body weight via tail vein injection (Fig. 1A, B).
Figure 1.
Choroidal γδ–T-cell infiltration after RPE injury. A) Immunostaining of TCRγδ (green) on whole mounts of choroid/sclera prepared from mice that were perfused with endothelial cell dye, Dil (red). Nuclei were counterstained with DAPI (blue). A 3-dimensional reconstructed image was generated from a z-stack scan to show extravascular distribution of γδ T cells (stack). B) Quantification of the average number of γδ T cells per unit area on choroidal whole mount (n = 6). C, D) Flow cytometry analyses of enriched mononuclear cells that were isolated from RPE/choroid or retina for expression of CD3, CD4, CD8 (C) and TCRγδ (D). E) RT-PCR measurement of choroidal γδ–T-cell subtype. γδ T cells were purified by Ab-conjugated magnetic beads at indicated times after NaIO3 treatment. N.s., not significant. Data presented are averages from 3 separate experiments (means ± sem). **P < 0.01. Scale bars, 20 μm.
We next analyzed the lineage-specific markers of mononuclear cells from both retina and RPE/choroid fractions by using flow cytometry. Under normal conditions, CD3+ lymphocytes in the retina were barely detectable (Fig. 1C), and the number remained low after NaIO3 injection. The percentage of lymphocytes in total mononuclear cells that were isolated from the RPE/choroidal fraction was approximately 50% (Fig. 1C). Consistent with data from flat-mount staining (Fig. 1A), RPE damage that was caused by NaIO3 treatment led to an increase in the number of choroidal γδ T cells (Fig. 1D), whereas the numbers of choroidal CD8+ or CD4+ lymphocytes were not altered by NaIO3 (Fig. 1C). We then used the RT-PCR approach to define the subsets of choroidal γδ T cells (Fig. 1E). After NaIO3 treatment, both Vγ4 and Vγ6 cells were markedly increased after 5 d, and both subsets are likely to be tissue residential (36–40). The amount of γδ T cells in the Vγ1 group was not changed.
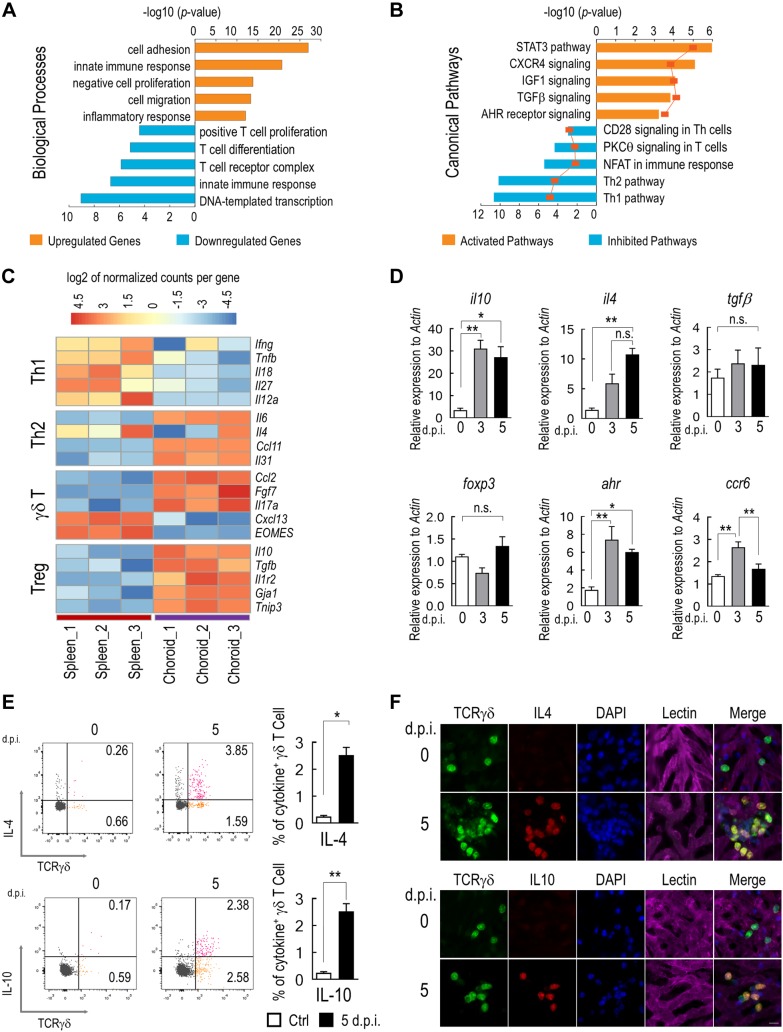
Gene expression profiles of choroidal γδ T cells were further analyzed by RNA sequencing. γδ T cells were isolated by magnetic bead–conjugated anti-TCRγδ Ab from the RPE/choroid of mice that were treated with NaIO3. The purity of cells was approximately 94% as determined by flow cytometry (Supplemental Fig. 1). Comparisons of the transcriptomes of choroidal and splenic γδ T cells revealed more than 2900 gene transcripts with highly significant differences in either up- or down-regulation (fold change >2; Padj <0.01, Benjamini-Hochberg procedure). Gene ontology analyses revealed key biologic processes and signaling pathways that are likely involved in the recruitment, migration, and activation of choroidal γδ T cells (Fig. 2A, B). mRNA levels of IFN-γ and other proinflammatory cytokine genes were markedly lower in choroidal γδ T cells than in splenic γδ T cells (Fig. 2C). Expression of the IL-17a transcript was consistent with the enrichment of the Vγ4 and Vγ6 subsets in the choroid (8).
Figure 2.
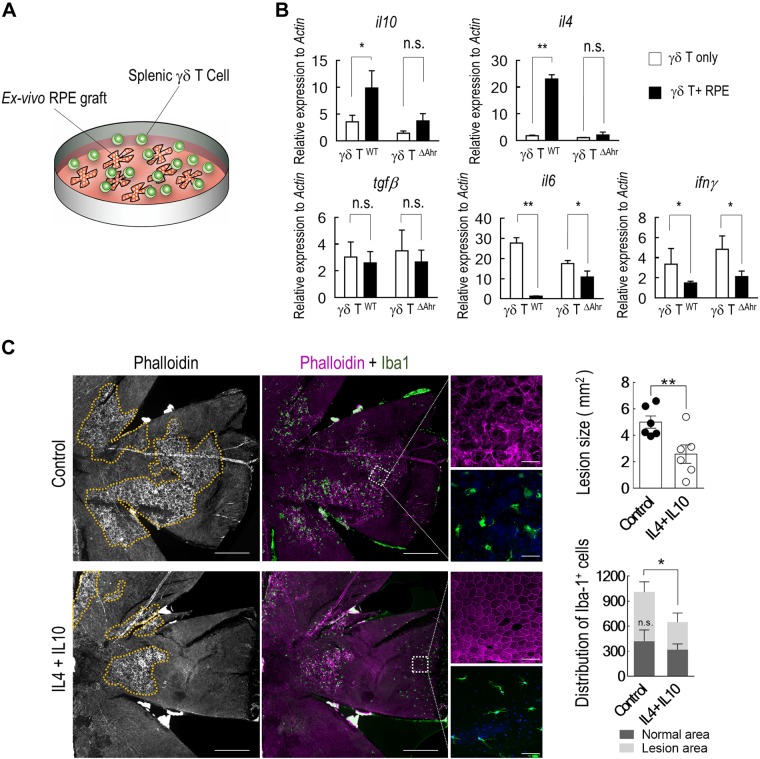
Production of immunosuppressive cytokines by choroidal γδ T cells. A) Top over-represented biologic processes, as determined by gene ontology analysis, among differentially expressed genes (fold change >2; Padj < 0.01, Benjamini-Hochberg procedure) between choroidal and splenic γδ T cells. B) Significantly enriched immunology canonical pathways (P < 0.01; Fisher’s exact test) were illustrated with respective percentages of overlapped genes shown on the line graph. Pathway activation and inhibition were determined by z score. C) Heat map of representative genes related to distinct T-cell functions. D) Quantitative RT-PCR analyses of genes that are indicative of γδ–T-cell function. γδ T cells were purified from mice that were treated with NaIO3 at indicated times (n = 5, 1-way ANOVA with Bonferroni test). E) Flow cytometry analyses and quantification of IL-4 and IL-10 expression by choroidal γδ T cells (n = 3, Student’s t test). F) Representative images of costaining of IL-4 or IL-10 with TCRγδ on choroidal flat mounts. Data are presented as mean ± sem. *P < 0.05, **P < 0.01. Scale bar, 20 μm.
Selected sets of RNA sequencing data were further validated by quantitative RT-PCR. Sequencing data suggested that choroidal γδ T cells expressed immunosuppressive cytokines, including IL-10 and IL-4, but not IFN-γ or TNF-α. Increased expression of IL-10 and IL-4 in isolated γδ T cells was evident 3 d after NaIO3 treatment and remained high at 5 d (Fig. 2D). CCR6 is a marker of IL-17–producing γδ T cells (36, 41) and functions as a chemokine receptor that mediates γδ–T-cell migration (42). Its expression was up-regulated after 3 d. Furthermore, we observed a significantly elevated expression of AhR at both 3 and 5 d after NaIO3 challenge, which was consistent with in silicon pathway analyses (Fig. 2B, D). No significant changes were detected for the expression of TGF-β and Foxop3 in choroidal γδ T cells that were prepared from NaIO3-treated mice. Cytokine profiles of choroidal γδ T cells were further verified by immunostaining and flow cytometry. On choroidal flat mounts, IL-10– and IL-4–positive γδ T cells were readily detectable at 5 d after NaIO3 treatment, whereas their expression in control samples was low (Fig. 2F). Flow cytometry analyses (Fig. 2E) also confirmed the increase of both IL-4+ and IL-10+ γδ T cells in the choroid of NaIO3-treated animals. Taken together, these data suggest that choroidal γδ T cells are activated by RPE injury.
γδ–T-cell deficiency exacerbated NaIO3-induced retinal inflammation
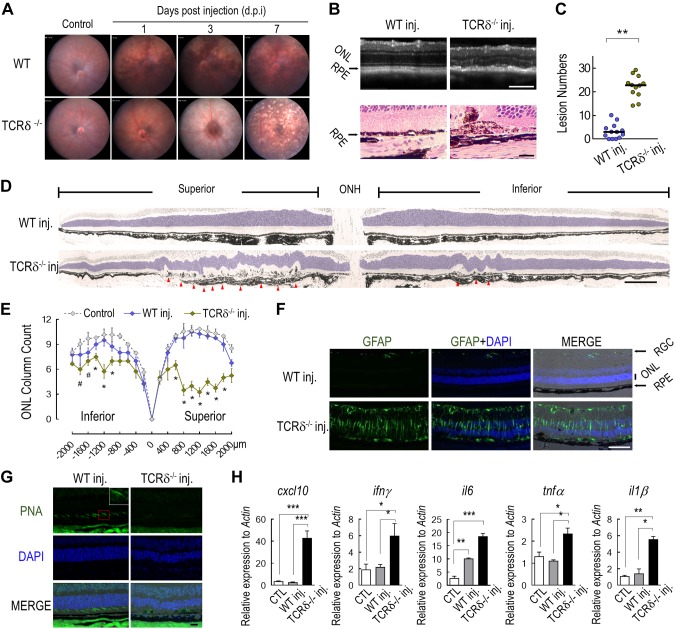
To characterize the function of choroidal γδ T cells, we measured the responses of wild-type and TCRδ−/− mice to RPE injury in response to NaIO3 treatment. Consistent with previous reports (33), NaIO3 caused minimal retinal pathology in wild-type mice at the dose of 15 mg/kg body weight via tail vein injection. In contrast, TCRδ−/− mice that received this dose demonstrated extensive fundus changes at 3 d after NaIO3 injection, and the lesions progressed to diffused pigment loss at d 7 (Fig. 3A). RPE injury was confirmed by both optical coherence tomography and histopathology (Fig. 3B). Quantification of the atrophic RPE sites (Fig. 3D, arrowheads) revealed significantly more RPE lesions in TCRδ−/− mice than in wild-type animals (Fig. 3C). Undulating lesions in the ONL (Fig. 3D, blue-tinted area) of the neural retina were noted in areas with RPE pathology. Substantial losses of ONL nuclei and cone photoreceptor cells were observed only in γδ–T-cell–deficient mice and were more prominent in the superior retina (Fig. 3E, G). Widespread Müller cell activation was observed in the retina of knockout mice (Fig. 3F), which indicated aggravated neuronal injury. Expression levels of genes that encode proinflammatory cytokines and chemokines, including IFN-γ, TNF-α, IL-6, IL-1β, and CXCL10, were found to be markedly elevated in the retina of TCRδ−/− mice that were treated with NaIO3 compared with wild-type mice that received the same treatment (Fig. 3H).
Figure 3.
Ocular phenotype of TCRδ−/− mice to NaIO3. A) Fundus photos of wild-type (WT) and TCRδ−/− mice at indicated times after NaIO3 treatment. B) Optical coherence tomography scan (top) and hematoxylin and eosin staining of posterior eye sections (bottom) of mice 5 d after NaIO3 injection. C) Quantification of the number of RPE and subretinal lesions on histology sections from NaIO3-injected mice. **P < 0.01, Student’s t test. D) Panoramic images of posterior eye sections form mice that were treated with NaIO3 for 5 d. Sections were stained with toluidine blue. Arrowheads indicate RPE lesion sites. E) Numbers of rows of photoreceptor nuclei in mice with or without NaIO3 treatment. F) Immunostaining of glial fibrillary acidic protein on frozen sections of posterior eyes. G) Peanut agglutinin (PNA) staining for cone cells in WT or γδ-T-deficient mice that were treated with NaIO3. H) Quantitative RT-PCR analyses of retinal proinflammatory cytokine gene expression. Data were from 3 independent experiments and are presented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 200 μm (B, upper; D; F); 20 μm (B, lower; G).
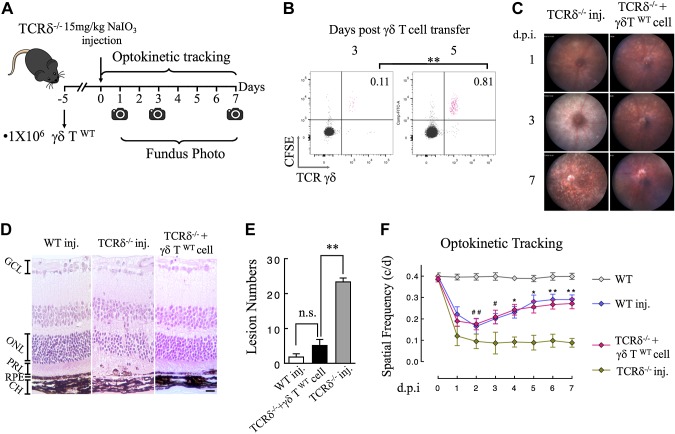
To confirm that the susceptibility of TCRδ−/− mice to NaIO3 treatment was a result of deficiency in γδ T cells, we measured whether replenishing the ocular γδ–T-cell pool would restore the resistance to damage by NaIO3 via transferring purified splenic γδ T cells from wild-type mice to TCRδ−/− mice (Fig. 4A). To verify the presence of transferred cells in the eye, we labeled purified γδ T cells with carboxyfluorescein succinimidyl ester and examined the homing of labeled cells by using flow cytometry. Results (Fig. 4B) show that transferred γδ T cells started to appear in the eyes of recipient animals 3 d after the procedure, and the population was further increased after 5 d; therefore, in subsequent functional studies, we waited for 5 d after adoptive transfer before NaIO3 treatment (Fig. 4A). Adoptive transfer of wild-type γδ T cells conferred marked protection in TCRδ−/− mice. As shown on fundus images, mice that received γδ–T-cell transfer displayed mitigated retinal swelling 3 d after NaIO3 treatment and RPE lesions were mostly recovered at day 7 (Fig. 4C). Consistent with clinical examinations, histopathology data demonstrated that γδ T-cell transfer induced TCRδ−/− mice to respond to NaIO3 in a way that was similar to wild-type animals (Fig. 4D), whereas the knockout mice without transfer exhibited significantly more RPE lesions than the other 2 groups (Fig. 4E).
Figure 4.
Responses of TCRδ−/− mice to NaIO3 after adoptive transfer of γδ T cells. A) Schematic illustration of the experimental protocol for γδ–T-cell adoptive transfer and NaIO3 treatment. B) Flow cytometry analysis of γδ T cells in the RPE/choroid tissues of recipient mice after homing of transferred cells. Purified splenic γδ T cells were prelabeled with fluorescent dye, carboxyfluorescein succinimidyl ester (y axis). C, D) Representative fundus images and histopathology of NaIO3-treated mice with or without γδ–T-cell transfer. E) Quantification of the number of RPE and subretinal lesions on histology sections (n = 6 for each group). F) Time-dependent changes of visual acuity after NaIO3 treatment. Daily measurements of optokinetic tracking were performed in different groups of mice for up to 7 d (n = 6 for each group). CH, choroid; PRL, photoreceptor layer. Data are presented as means ± sem. *P < 0.05, **P < 0.01 (1-way ANOVA and Tukey’s post hoc test). Scale bar, 20 μm.
We further tested the visual functional changes by optokinetic tracking. Similar to the previous report (43), low-dose NaIO3 caused a transient decrease of visual acuity in wild-type mice, followed by a recovery phase 3 d after injection (Fig. 4F, blue line). TCRδ−/− mice demonstrated progressive deterioration of visual function, which became significantly different from wild-type animals at 4 d postinjection. Adoptive transfer of γδ T cells before NaIO3 treatment facilitated the recovery of visual acuity in a pattern that was similar to wild-type mice (Fig. 4F). Collectively, our data demonstrate a previously unidentified role for γδ T cells in protecting against structural and functional damages to the RPE and retina.
Functional interactions between γδ T cells and the RPE
The RPE has strong immunomodulatory functions. We assessed interactions between the RPE and γδ T cells in an ex vivo coculture system (Fig. 5A). Naive γδ T cells were isolated from the mouse spleen and incubated with eye cups after removal of the retina. Consistent with in vivo cytokine profiles (Fig. 2D), RPE explants activated IL-4 and IL-10 expression in cocultured splenic γδ T cells. Proinflammatory cytokines, such as IL-6 and IFN-γ, were down-regulated (Fig. 5B).
Figure 5.
RPE promotes the suppressive phenotype of γδ T cells in an AhR-dependent manner. A) Schematic illustration of cocultured splenic γδ T cells with RPE explants. B) Cytokine gene expressions of γδ T cells were measured from either wild-type or AhR−/− mice after coculturing with RPE explants for 16 h. C) RPE flat mounts of NaIO3-injected mice with intravitreal delivery of either IL-4 plus IL-10 cocktail or vehicle control. RPE lesions are outlined in yellow. Subretinal microglia infiltration were measured by ionized calcium-binding adapter molecule 1 staining (n = 6). Scale bars: 200 μm; insert, 20 μm.
We next tested the potential therapeutic effects of IL-4 and IL-10 against NaIO3-induced injury. TCRδ−/− mice first received an intravenous injection of NaIO3 at 15 mg/kg per body weight. Immediately after the procedure, animals were treated with 1 μl of a recombinant protein cocktail of IL-4 (100 ng) and IL-10 (500 ng) via intravitreal injection (44). RPE injury was assessed 5 d thereafter. As shown by immunostaining of the RPE/choroid flat mount (Fig. 5C), IL-4 and IL-10 treatment decreased the lesion size by NaIO3. The number of ionized calcium-binding adapter molecule 1 microglia in the subretinal space was also reduced. It was noticeable that the distribution of activated microglia was mainly located in areas with RPE lesions.
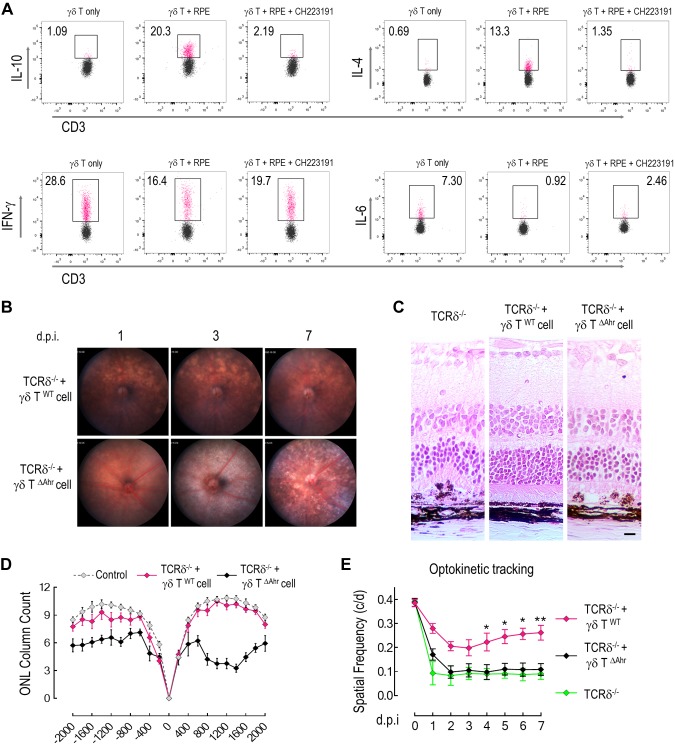
All γδ T cells express AhR, which is a key transcription factor that controls their tissue-specific properties (8, 45). RNA sequencing data revealed that a number of AhR downstream genes were differentially expressed between choroidal and splenic γδ T cells (Supplemental Fig. 2), which indicated that the transcriptional regulation by AhR is tissue specific. In ex vivo coculture, the induction of IL-4 and IL-10 production was abolished in γδ T cells that were isolated from AhR-deficient mice (Fig. 5B), and was suppressed by an AhR antagonist, CH223191 (Fig. 6A), which indicated the importance of AhR in controlling γδ T-cell function. Levels of IFN-γ or IL-6 were not influenced by CH223191.
Figure 6.
AhR is required for the protective function of γδ T cells against RPE injury. A) Flow cytometry analysis of cytokine expression from γδ T cells that were cocultured with RPE explants in the presence or absence of AhR inhibitor, CH223191. B) Fundus photos from γδ–T-cell–deficient mice that were treated with NaIO3 at indicated times after adoptive transfer of purified γδ T cells from either wild-type or AhR-deficient mice. C) Histopathology of γδ–T-cell–deficient mice after receiving adoptive transfer of wild-type or AhR-deficient γδ T cells and being treated with NaIO3 for 7 d. D, E) Counting of photoreceptor outer nuclei layers in γδ–T-cell–transferred mice (n = 3; D) and visual function examination by optokinetic tracking (n = 5; E). Data are presented as means ± sem. Statistical differences are determined by either 1-way ANOVA with Bonferroni test (D) or Student’s t test (E). *P < 0.05, **P < 0.01. Scale bars, 20 μm.
To determine whether the protective effects of choroidal γδ T cells were dependent on AhR, we used γδ T cells from AhR-knockout mice for adoptive transfer into TCRδ−/− mice and measured the response of recipient animals to NaIO3. Results show that AhR-knockout γδ T (γδ TΔAhr) cells that lacked AhR expression failed to protect the RPE and retina from NaIO3-induced injury (Fig. 6B). After adoptive transfer, recipient mice exhibited severe RPE hypertrophy and degeneration 7 d after injection (Fig. 6B, C), to the same extent as that observed in TCRδ−/− mice. Significant loss of photoreceptor nuclei (Fig. 6D) and decreased optokinetic responses (Fig. 6E) were observed in TCRδ−/− mice that received γδ T∆Ahr cells in response to NaIO3 injection. Taken together, our data suggest that the microenvironment of the outer retina, RPE, and choroid exhibited a general anti-inflammatory tone to maintain ocular immune homeostasis via γδ-T- and AhR-dependent mechanisms.
Choroidal γδ T lymphocytes in human samples
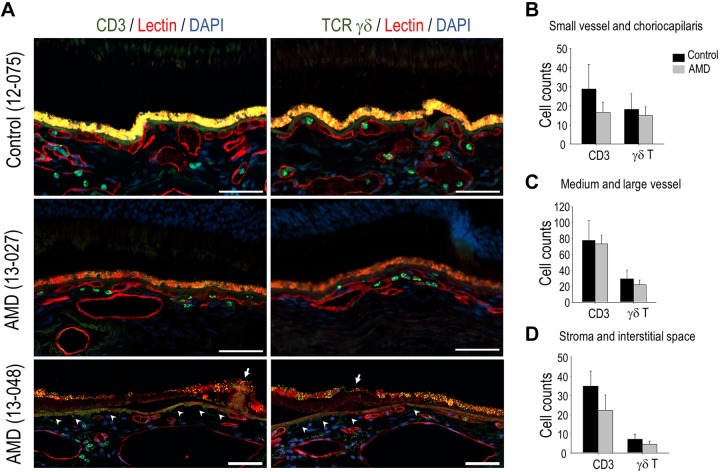
To assess γδ T lymphocytes in human choroid, we performed immunostaining of CD3 or TCRδ antigens on macular sections that were prepared from 10 eyes with AMD and 6 age-matched control eyes. Mean age was 85.6 yr (95% CI, 80–91) for the AMD group, and 85.2 yr (95% CI, 80–90) for the control group. In the choroid of control eyes, CD3+ T lymphocytes and γδ T cells were observed in the choriocapillaris, larger blood vessels, and the stroma, with focal areas of dense distribution of CD3+ T cells in the stroma (Fig. 7A). Choroid of AMD eyes displayed the same pattern of T-cell distribution (Fig. 7A), with the exception that γδ T cells in the choriocapillaris frequently showed local expansion. γδ T cells accounted for more than 50% of total T cells in the choriocapillaris and small choroidal vessels (Fig. 7B–D). Over-representation of γδ T cells, which is a feature of IELs (46), suggested that their presence on tissue sections was not merely caused by the residual blood of postmortem tissue. We did not find statistical differences in the number of CD3+ lymphocytes of γδ T cells between AMD and age-matched control groups (Fig. 7B–D); however, in areas with choriocapillaris dropout (47, 48), there was an apparent loss of interaction between the RPE and γδ T cells (Fig. 7A).
Figure 7.
Choroidal γδ T cells in human donor eyes. A) Representative images of immunolabeling of CD3 and γδ TCR on adjacent sections that were prepared from human donor eyes from either patients with AMD or age-matched controls. Vascular endothelium was labeled with lectin (red fluorescence). Arrow indicates drusen; arrowhead indicates areas with choriocapillaris dropout. B–D) Quantification data showing the number of CD3+ cells and γδ T cells in small (B) and larger (C) choroidal vessels and stoma (D) on either control or AMD sections. Scale bars, 50 μm.
DISCUSSION
IELs in epidermis, lung, and intestinal epithelium perform immune surveillance functions to protect against pathogen invasion. A majority of IELs are γδ T cells (8). Cytokines that are produced by IELs and surrounding cells in the local microenvironment can facilitate the recovery and repair of damaged epithelium (49, 50). The RPE is part of the blood-retinal barrier, which separates neuronal tissue from the circulation and limits the accessibility of immune cells to the intraocular site (51). Similar to their topology in other organs (52), γδ T cells reside on the mesenchymal side with choroidal blood vessels (Fig. 1). We found that choroidal γδ T cells produce various modulatory cytokines to suppress inflammation and maintain epithelial cell integrity and support their recovery (Figs. 2 and 4). These effects are consistent with the roles of γδ T cells in skin wound healing (4) and protection against experimental colitis (53).
Our findings from imaging, histopathologic, and functional studies demonstrate that mice that are deficient in γδ T cells are more susceptible to RPE and retinal degeneration induced by NaIO3 (Figs. 3 and 4). Adoptive transfer of wild-type γδ T cells conferred protection, and these cells demonstrated homing to the choroid (Fig. 4). γδ T cells in the choroidal tissue may directly promote tissue repair by secreting growth factors and activating cell survival pathways, as reported in skin tissue (4). Conversely, choroidal γδ T cells produce immunosuppressive cytokines, IL-4 and IL-10 (Fig. 2), which may act on subretinal microglia to augment the resolution of inflammation and tissue repair (54–56). Previous studies have reported critical roles for IL-10 in the regulation of immune senescence of the retinal microglia and infiltrated macrophages, as well as in controlling their functions in choroidal neovascularization (57, 58). Our results suggest that γδ T cells can be a source of IL-10 production near sites of RPE injury and degeneration. As a proof of concept, our data (Fig. 5C) demonstrate the in vivo protective effects of IL-4 and IL-10 against NaIO3 toxicity. Detailed molecular mechanisms of their activation and protection remain to be explored in future studies and likely extend beyond IL-4 and IL-10.
We observed γδ T cells on human posterior pole sections. Although their presence in large choroidal blood vessels can be a result of residual blood, γδ T cells in the choriocapillaris and surrounding areas have close interactions with the RPE (Fig. 7). One of the functional consequences of choriocapillaris dropout is the loss of support from protective γδ T cells. These findings are consistent with a recent report on choroidal γδ T cells in eyes with geographic atrophy (59). Compared with age-matched controls, there seemed to be a decrease in the percentage of γδ T cells in the total number of CD3+ T lymphocytes in the choroid of AMD eyes, although the difference was not statistically significant (Fig. 7). Future studies are needed to further quantify the changes in both the number and properties of choroidal γδ T cells during different stages of RPE degeneration in AMD. In a skin wound healing model, epidermal γδ T cells in aged mice show decreased activation and supportive functions for re-epithelialization (60). Similar mechanisms and signaling pathways can be applicable to choroidal γδ T cells.
The RPE is known to modulate T-cell function. RPE-conditioned medium can differentiate naive T cells to regulatory T (Treg) cells (61, 62). Cocultured RPE can suppress T-cell activation (11) by producing both soluble and membrane-associated immunomodulatory factors (63–66). Results from our current study show that the ex vivo coculture of RPE with splenic γδ T cells directed the differentiation toward regulatory γδ T cells that produced suppressive cytokines, such as IL-4 and IL-10 (Fig. 5). The transcriptome of choroidal γδ T cells that were isolated from NaIO3-treated mice has similarities to Treg cells, although they do not express Foxp3 (Fig. 2C, D). The RPE-mediated induction of γδ T cells was dependent on AhR (Fig. 5). Recent studies with AhR-knockout mice observed age-dependent RPE degeneration and retinal inflammation (67, 68), and AhR deficiency potentiated experimental choroidal neovascularization (69). Future studies can be performed to further explore the functional changes of γδ T cells and their roles in the degeneration phenotype in AhR-deficient mice. AhR is a nuclear receptor protein that can be activated by a variety of ligands, including tryptophan derivatives (70). A major tryptophan-metabolizing enzyme, indoleamine 2,3-dioxygenase, is expressed by the RPE and is likely involved in the immune modulation of RPE on lymphocytes (71). AhR-mediated gene expression can be stimulated by IL-1β (72), which can be produced by the RPE inflammasome under conditions of stress (73–75). These previously reported findings further support the role of choroidal γδ T cells as an integral component of the innate immune system at the RPE barrier.
Ocular γδ T cells have important regulatory roles during experimental autoimmune uveitis (24, 76, 77) and anterior chamber–associated immune deviation (78, 79). Experimental autoimmune uveitis and anterior chamber–associated immune deviation are adaptive immune responses that are experimentally induced by specific antigens. Activation of choroidal γδ T cells in the RPE injury model that we used in the current study is an innate response that occurred within 3 d after NaIO3 injection in nonprimed mice. The number of Vγ4 and Vγ6 cells markedly increased after 5 d (Fig. 1E). Both subsets are likely to be tissue residential (36–40). γδ T cells have moderate effects in laser-induced choroidal neovascularization (14, 80). Depending on the interactions with other immune cells and the conditions of activation, they can either enhance or suppress the inflammatory and angiogenic responses. Thus, deciphering the mechanisms of their activation and the condition-specific downstream effects will be essential for future translational applications.
There are several limitations in our current study. The number of human eye samples examined was small and we did not have sufficient statistical power to distinguish between dry and wet forms of AMD. Splenic γδ T cells were used for adoptive transfer, and they do not represent the tissue specificity and unique subsets of choroidal γδ T cells. Intravitreal injection of IL-4 and IL-10 was performed as a preventive treatment that was delivered as a single shot at a high dose. Other cytokines that were produced by choroidal γδ T cells, such as IL-17, will likely contribute to their interactions with RPE.
In summary, choroidal γδ T cells are a special group of immune cells with properties that resemble those of IELs, and that exert protective functions under conditions of sterile inflammation and RPE degeneration. γδ T cells produce an optimized combination of protective and immunosuppressive cytokines. γδ–T-cell therapy is potentially a novel interventional strategy for treating retinal diseases that involve RPE degeneration.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Eye Institute Grants EY021937, EY026999, EY022403, and EY024605; the Ted Nash Long Life Foundation; Carl Marshall Reeves and Mildred Almen Reeves Foundation; BrightFocus Foundation; and International Retina Research Foundation. The authors declare no conflicts of interest.
Glossary
- AhR
aryl hydrocarbon receptor
- AMD
age-related macular degeneration
- Dil
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- IEL
intraepithelial lymphocyte
- ONL
outer nuclear layer
- RPE
retinal pigment epithelium
- TCR
T-cell receptor
- Treg
regulatory T
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. Sun, J. Sun, R. F. Mullins, Y. Chen, and J. Cai designed research; Z. Zhao, Y. Liang, P. Xu, M. J. Flamme-Wiess, Y. Chen, and J. Cai performed the experiments; Z. Zhao, Y. Liu, Y. Chen and J. Cai analyzed data; and Z. Zhao, Y. Liu, Y. Chen, and J. Cai wrote the manuscript.
REFERENCES
- 1.O’Brien R. L., Born W. K. (2010) Gammadelta T cell subsets: a link between TCR and function? Semin. Immunol. 22, 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayday A., Tigelaar R. (2003) Immunoregulation in the tissues by gammadelta T cells. Nat. Rev. Immunol. 3, 233–242 [DOI] [PubMed] [Google Scholar]
- 3.Chien Y. H., Meyer C., Bonneville M. (2014) γδ T cells: first line of defense and beyond. Annu. Rev. Immunol. 32, 121–155 [DOI] [PubMed] [Google Scholar]
- 4.Gay D., Kwon O., Zhang Z., Spata M., Plikus M. V., Holler P. D., Ito M., Yang Z., Treffeisen E., Kim C. D., Nace A., Zhang X., Baratono S., Wang F., Ornitz D. M., Millar S. E., Cotsarelis G. (2013) Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 19, 916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havran W. L., Chien Y. H., Allison J. P. (1991) Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science 252, 1430–1432 [DOI] [PubMed] [Google Scholar]
- 6.Born W. K., Lahn M., Takeda K., Kanehiro A., O’Brien R. L., Gelfand E. W. (2000) Role of gammadelta T cells in protecting normal airway function. Respir. Res. 1, 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kühl A. A., Pawlowski N. N., Grollich K., Loddenkemper C., Zeitz M., Hoffmann J. C. (2007) Aggravation of intestinal inflammation by depletion/deficiency of gammadelta T cells in different types of IBD animal models. J. Leukoc. Biol. 81, 168–175 [DOI] [PubMed] [Google Scholar]
- 8.Stange J., Veldhoen M. (2013) The aryl hydrocarbon receptor in innate T cell immunity. Semin. Immunopathol. 35, 645–655 [DOI] [PubMed] [Google Scholar]
- 9.Detrick B., Hooks J. J. (2010) Immune regulation in the retina. Immunol. Res. 47, 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R., Horai R., Silver P. B., Mattapallil M. J., Zárate-Bladés C. R., Chong W. P., Chen J., Rigden R. C., Villasmil R., Caspi R. R. (2012) The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3+ regulatory cells following local antigen recognition. J. Immunol. 188, 1742–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregerson D. S., Heuss N. D., Lew K. L., McPherson S. W., Ferrington D. A. (2007) Interaction of retinal pigmented epithelial cells and CD4 T cells leads to T-cell anergy. Invest. Ophthalmol. Vis. Sci. 48, 4654–4663 [DOI] [PubMed] [Google Scholar]
- 12.Patel M., Chan C. C. (2008) Immunopathological aspects of age-related macular degeneration. Semin. Immunopathol. 30, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambati J., Atkinson J. P., Gelfand B. D. (2013) Immunology of age-related macular degeneration. Nat. Rev. Immunol. 13, 438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coughlin B., Schnabolk G., Joseph K., Raikwar H., Kunchithapautham K., Johnson K., Moore K., Wang Y., Rohrer B. (2016) Connecting the innate and adaptive immune responses in mouse choroidal neovascularization via the anaphylatoxin C5a and γδT-cells. Sci. Rep. 6, 23794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S., Yu N., Zhang R., Zhang S., Wu J. (2016) Interleukin-17A induces IL-1β secretion from RPE cells via the NLRP3 inflammasome. Invest. Ophthalmol. Vis. Sci. 57, 312–319 [DOI] [PubMed] [Google Scholar]
- 16.Chen S., Shen D., Popp N. A., Ogilvy A. J., Tuo J., Abu-Asab M., Xie T., Chan C. C. (2015) Responses of multipotent retinal stem cells to IL-1β, IL-18, or IL-17. J. Ophthalmol. 2015, 369312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y., Tan W., Demetriades A. M., Cai Y., Gao Y., Sui A., Lu Q., Shen X., Jiang C., Xie B., Sun X. (2016) Interleukin-17A neutralization alleviated ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization. Immunology 147, 414–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z., Xu P., Jie Z., Zuo Y., Yu B., Soong L., Sun J., Chen Y., Cai J. (2014) γδ T cells as a major source of IL-17 production during age-dependent RPE degeneration. Invest. Ophthalmol. Vis. Sci. 55, 6580–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattapallil M. J., Wawrousek E. F., Chan C. C., Zhao H., Roychoudhury J., Ferguson T. A., Caspi R. R. (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest. Ophthalmol. Vis. Sci. 53, 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu B., Xu P., Zhao Z., Cai J., Sternberg P., Chen Y. (2014) Subcellular distribution and activity of mechanistic target of rapamycin in aged retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 55, 8638–8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollyfield J. G., Bonilha V. L., Rayborn M. E., Yang X., Shadrach K. G., Lu L., Ufret R. L., Salomon R. G., Perez V. L. (2008) Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 14, 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirco K. R., Tucker B. A., Stone E. M., Mullins R. F. (2016) Selective accumulation of the complement membrane attack complex in aging choriocapillaris. Exp. Eye Res. 146, 393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Cai J., Murphy T. J., Jones D. P. (2002) Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J. Biol. Chem. 277, 33242–33248 [DOI] [PubMed] [Google Scholar]
- 24.Liang D., Zuo A., Zhao R., Shao H., Born W. K., O’Brien R. L., Kaplan H. J., Sun D. (2016) CD73 expressed on γδ T cells shapes their regulatory effect in experimental autoimmune uveitis. PLoS One 11, e0150078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cong Y., Feng T., Fujihashi K., Schoeb T. R., Elson C. O. (2009) A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. USA 106, 19256–19261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prusky G. T., Alam N. M., Beekman S., Douglas R. M. (2004) Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci. 45, 4611–4616 [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Song Y., Zhao L., Gaidosh G., Laties A. M., Wen R. (2008) Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat. Protoc. 3, 1703–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheruvu N. P., Kompella U. B. (2006) Bovine and porcine transscleral solute transport: influence of lipophilicity and the Choroid-Bruch’s layer. Invest. Ophthalmol. Vis. Sci. 47, 4513–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S., McCarthy D. J., Chen Y., Okoniewski M., Smyth G. K., Huber W., Robinson M. D. (2013) Count-based differential expression analysis of RNA sequencing data using R and bioconductor. Nat. Protoc. 8, 1765–1786 [DOI] [PubMed] [Google Scholar]
- 30.Lynn D. J., Winsor G. L., Chan C., Richard N., Laird M. R., Barsky A., Gardy J. L., Roche F. M., Chan T. H., Shah N., Lo R., Naseer M., Que J., Yau M., Acab M., Tulpan D., Whiteside M. D., Chikatamarla A., Mah B., Munzner T., Hokamp K., Hancock R. E., Brinkman F. S. (2008) InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Mol. Syst. Biol. 4, 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matys V., Kel-Margoulis O. V., Fricke E., Liebich I., Land S., Barre-Dirrie A., Reuter I., Chekmenev D., Krull M., Hornischer K., Voss N., Stegmaier P., Lewicki-Potapov B., Saxel H., Kel A. E., Wingender E. (2006) TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 34, D108–D110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han H., Shim H., Shin D., Shim J. E., Ko Y., Shin J., Kim H., Cho A., Kim E., Lee T., Kim H., Kim K., Yang S., Bae D., Yun A., Kim S., Kim C. Y., Cho H. J., Kang B., Shin S., Lee I. (2015) TRRUST: a reference database of human transcriptional regulatory interactions. Sci. Rep. 5, 11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W., Noel J., Kaplan H. J., Dean D. C. (2011) Circulating reactive oxidant causes apoptosis of retinal pigment epithelium and cone photoreceptors in the mouse central retina. Ophthalmol. Eye Dis. 3, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannan R., Hinton D. R. (2014) Sodium iodate induced retinal degeneration: new insights from an old model. Neural Regen. Res. 9, 2044–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balmer J., Zulliger R., Roberti S., Enzmann V. (2015) Retinal cell death caused by sodium iodate involves multiple caspase-dependent and caspase-independent cell-death pathways. Int. J. Mol. Sci. 16, 15086–15103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas J. D., Ravens S., Düber S., Sandrock I., Oberdörfer L., Kashani E., Chennupati V., Föhse L., Naumann R., Weiss S., Krueger A., Förster R., Prinz I. (2012) Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 37, 48–59 [DOI] [PubMed] [Google Scholar]
- 37.Sumaria N., Roediger B., Ng L. G., Qin J., Pinto R., Cavanagh L. L., Shklovskaya E., Fazekas de St Groth B., Triccas J. A., Weninger W. (2011) Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J. Exp. Med. 208, 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim G. K., Rajaserkar R., Dessing M., Augustin A. (1994) Homing and in situ differentiation of resident pulmonary lymphocytes. Int. Immunol. 6, 1287–1295 [DOI] [PubMed] [Google Scholar]
- 39.Misiak A., Wilk M. M., Raverdeau M., Mills K. H. (2017) IL-17-producing innate and pathogen-specific tissue resident memory γδ T cells expand in the lungs of bordetella pertussis-infected mice. J. Immunol. 198, 363–374 [DOI] [PubMed] [Google Scholar]
- 40.Akitsu A., Ishigame H., Kakuta S., Chung S. H., Ikeda S., Shimizu K., Kubo S., Liu Y., Umemura M., Matsuzaki G., Yoshikai Y., Saijo S., Iwakura Y. (2015) IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2+Vγ6+γδ T cells. Nat. Commun. 6, 7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas J. D., González F. H., Schmitz S., Chennupati V., Föhse L., Kremmer E., Förster R., Prinz I. (2009) CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur. J. Immunol. 39, 3488–3497 [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki T., Yang X. O., Chung Y., Fukunaga A., Nurieva R., Pappu B., Martin-Orozco N., Kang H. S., Ma L., Panopoulos A. D., Craig S., Watowich S. S., Jetten A. M., Tian Q., Dong C. (2008) CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 181, 8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carido M., Zhu Y., Postel K., Benkner B., Cimalla P., Karl M. O., Kurth T., Paquet-Durand F., Koch E., Münch T. A., Tanaka E. M., Ader M. (2014) Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation. Invest. Ophthalmol. Vis. Sci. 55, 5431–5444 [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum J. T., Angell E. (1995) Paradoxical effects of IL-10 in endotoxin-induced uveitis. J. Immunol. 155, 4090–4094 [PubMed] [Google Scholar]
- 45.Li Y., Innocentin S., Withers D. R., Roberts N. A., Gallagher A. R., Grigorieva E. F., Wilhelm C., Veldhoen M. (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 [DOI] [PubMed] [Google Scholar]
- 46.Cheroutre H., Lambolez F., Mucida D. (2011) The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLeod D. S., Grebe R., Bhutto I., Merges C., Baba T., Lutty G. A. (2009) Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 50, 4982–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullins R. F., Johnson M. N., Faidley E. A., Skeie J. M., Huang J. (2011) Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 1606–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song X., Dai D., He X., Zhu S., Yao Y., Gao H., Wang J., Qu F., Qiu J., Wang H., Li X., Shen N., Qian Y. (2015) Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity 43, 488–501 [DOI] [PubMed] [Google Scholar]
- 50.Maxwell J. R., Zhang Y., Brown W. A., Smith C. L., Byrne F. R., Fiorino M., Stevens E., Bigler J., Davis J. A., Rottman J. B., Budelsky A. L., Symons A., Towne J. E. (2015) Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 43, 739–750 [DOI] [PubMed] [Google Scholar]
- 51.Wenkel H., Streilein J. W. (2000) Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Invest. Ophthalmol. Vis. Sci. 41, 3467–3473 [PubMed] [Google Scholar]
- 52.Nowarski R., Jackson R., Flavell R. A. (2017) The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell 168, 362–375 [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Chou K., Fuchs E., Havran W. L., Boismenu R. (2002) Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA 99, 14338–14343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dace D. S., Khan A. A., Kelly J., Apte R. S. (2008) Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS One 3, e3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wohl S. G., Schmeer C. W., Friese T., Witte O. W., Isenmann S. (2011) In situ dividing and phagocytosing retinal microglia express nestin, vimentin, and NG2 in vivo. PLoS One 6, e22408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 57.Kelly J., Ali Khan A., Yin J., Ferguson T. A., Apte R. S. (2007) Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J. Clin. Invest. 117, 3421–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Apte R. S., Richter J., Herndon J., Ferguson T. A. (2006) Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 3, e310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camelo S., Lavelette S., Guillonneau X., Raoul W., Sennlaub F. (2016) Association of choroidal interleukin-17-producing T lymphocytes and macrophages with geographic atrophy. Ophthalmologica 236, 53–58 [DOI] [PubMed] [Google Scholar]
- 60.Keyes B. E., Liu S., Asare A., Naik S., Levorse J., Polak L., Lu C. P., Nikolova M., Pasolli H. A., Fuchs E. (2016) Impaired epidermal to dendritic T cell signaling slows wound repair in aged skin. Cell 167, 1323–1338.e1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawazoe Y., Sugita S., Keino H., Yamada Y., Imai A., Horie S., Mochizuki M. (2012) Retinoic acid from retinal pigment epithelium induces T regulatory cells. Exp. Eye Res. 94, 32–40 [DOI] [PubMed] [Google Scholar]
- 62.Imai A., Sugita S., Kawazoe Y., Horie S., Yamada Y., Keino H., Maruyama K., Mochizuki M. (2012) Immunosuppressive properties of regulatory T cells generated by incubation of peripheral blood mononuclear cells with supernatants of human RPE cells. Invest. Ophthalmol. Vis. Sci. 53, 7299–7309 [DOI] [PubMed] [Google Scholar]
- 63.Kaestel C. G., Lovato P., Ødum N., Nissen M. H., Röpke C. (2005) The immune privilege of the eye: human retinal pigment epithelial cells selectively modulate T-cell activation in vitro. Curr. Eye Res. 30, 375–383 [DOI] [PubMed] [Google Scholar]
- 64.Wallace C. A., Moir G., Malone D. F., Duncan L., Devarajan G., Crane I. J. (2013) Regulation of T-lymphocyte CCL3 and CCL4 production by retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 54, 722–730 [DOI] [PubMed] [Google Scholar]
- 65.Clemson C. M., Yost J., Taylor A. W. (2017) The role of alpha-MSH as a modulator of ocular immunobiology exemplifies mechanistic differences between melanocortins and steroids. Ocul. Immunol. Inflamm. 25, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green D. R., Ferguson T. A. (2001) The role of Fas ligand in immune privilege. Nat. Rev. Mol. Cell Biol. 2, 917–924 [DOI] [PubMed] [Google Scholar]
- 67.Hu P., Herrmann R., Bednar A., Saloupis P., Dwyer M. A., Yang P., Qi X., Thomas R. S., Jaffe G. J., Boulton M. E., McDonnell D. P., Malek G. (2013) Aryl hydrocarbon receptor deficiency causes dysregulated cellular matrix metabolism and age-related macular degeneration-like pathology. Proc. Natl. Acad. Sci. USA 110, E4069–E4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S. Y., Yang H. J., Chang Y. S., Kim J. W., Brooks M., Chew E. Y., Wong W. T., Fariss R. N., Rachel R. A., Cogliati T., Qian H., Swaroop A. (2014) Deletion of aryl hydrocarbon receptor AHR in mice leads to subretinal accumulation of microglia and RPE atrophy. Invest. Ophthalmol. Vis. Sci. 55, 6031–6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choudhary M., Kazmin D., Hu P., Thomas R. S., McDonnell D. P., Malek G. (2015) Aryl hydrocarbon receptor knock-out exacerbates choroidal neovascularization via multiple pathogenic pathways. J. Pathol. 235, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen L. P., Bradfield C. A. (2008) The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 21, 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park C. Y., Yang S. H., Chuck R. S., Gehlbach P. L., Park C. G. (2010) The role of indoleamine 2,3-dioxygenase in retinal pigment epithelial cell-mediated immune modulation. Ocul. Immunol. Inflamm. 18, 24–31 [DOI] [PubMed] [Google Scholar]
- 72.Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 [DOI] [PubMed] [Google Scholar]
- 73.Tseng W. A., Thein T., Kinnunen K., Lashkari K., Gregory M. S., D’Amore P. A., Ksander B. R. (2013) NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 54, 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kauppinen A., Niskanen H., Suuronen T., Kinnunen K., Salminen A., Kaarniranta K. (2012) Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells--implications for age-related macular degeneration (AMD). Immunol. Lett. 147, 29–33 [DOI] [PubMed] [Google Scholar]
- 75.Doyle S. L., Campbell M., Ozaki E., Salomon R. G., Mori A., Kenna P. F., Farrar G. J., Kiang A. S., Humphries M. M., Lavelle E. C., O’Neill L. A., Hollyfield J. G., Humphries P. (2012) NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat. Med. 18, 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang D., Zuo A., Shao H., Born W. K., O’Brien R. L., Kaplan H. J., Sun D. (2013) IL-23 receptor expression on γδ T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J. Immunol. 191, 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nian H., Shao H., O’Brien R. L., Born W. K., Kaplan H. J., Sun D. (2011) Activated gammadelta T cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest. Ophthalmol. Vis. Sci. 52, 5920–5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skelsey M. E., Mellon J., Niederkorn J. Y. (2001) Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J. Immunol. 166, 4327–4333 [DOI] [PubMed] [Google Scholar]
- 79.Ashour H. M., Niederkorn J. Y. (2006) Gammadelta T cells promote anterior chamber-associated immune deviation and immune privilege through their production of IL-10. J. Immunol. 177, 8331–8337 [DOI] [PubMed] [Google Scholar]
- 80.Hasegawa E., Sonoda K. H., Shichita T., Morita R., Sekiya T., Kimura A., Oshima Y., Takeda A., Yoshimura T., Yoshida S., Ishibashi T., Yoshimura A. (2013) IL-23-independent induction of IL-17 from γδT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J. Immunol. 190, 1778–1787 [DOI] [PubMed] [Google Scholar]