Abstract
Adenosine, a key extracellular signaling mediator, regulates several aspects of metabolism by activating 4 G-protein-coupled receptors, the A1, A2A, A2B, and A3 adenosine receptors (ARs). The role of A2AARs in regulating high-fat-diet (HFD)–induced metabolic derangements is unknown. To evaluate the role of A2AARs in regulating glucose and insulin homeostasis in obesity, we fed A2AAR-knockout (KO) and control mice an HFD for 16 wk to initiate HFD-induced metabolic disorder. We found that genetic deletion of A2AARs caused impaired glucose tolerance in mice fed an HFD. This impaired glucose tolerance was caused by a decrease in insulin secretion but not in insulin sensitivity. Islet size and insulin content in pancreata of A2AAR-deficient mice were decreased compared with control mice after consuming an HFD. A2AAR-KO mice had decreased expression of the β-cell-specific markers pdx1, glut2, mafA, and nkx6.1 and increased expression of the dedifferentiation markers sox2 and hes1. Ex vivo islet experiments confirmed the role of A2AARs in protecting against decreased insulin content and release caused by HFD. Other experiments with bone marrow chimeras revealed that inflammation was not the primary cause of decreased insulin secretion in A2AAR-KO mice. Altogether, our data showed that A2AARs control pancreatic dysfunction in HFD-induced obesity.—Csóka, B., Törő, G., Vindeirinho, J., Varga, Z. V., Koscsó, B., Németh, Z. H., Kókai, E., Antonioli, L., Suleiman, M., Marchetti, P., Cseri, K., Deák, Á., Virág, L., Pacher, P., Bai, P., Haskó, G. A2A adenosine receptors control pancreatic dysfunction in high-fat-diet-induced obesity.
Keywords: diabetes, islet, β-cell, β-cell dedifferentiation
Currently, 29.1 million people or 9.3% of the U.S. population have diabetes. In adults, type 2 diabetes (T2D) accounts for 90–95% of all diagnosed cases of diabetes (1). The pathophysiology of T2D comprises both peripheral insulin resistance in metabolically active organs and diminished insulin production by pancreatic β cells (2). During the progress of T2D, β-cell mass and insulin production initially increase in response to peripheral insulin resistance (3, 4). With time, however, this adaptive increase in insulin secretion becomes insufficient, insulin production cannot compensate for the increased insulin demand, and T2D develops. Later the progress of T2D, β-cell mass and function decrease, which results in an impairment of glucose-stimulated insulin secretion (GSIS) (2, 4).
Although the primary reason for β-cell deterioration is not well known, several mechanisms have been proposed as mediating the progressive loss of β-cell function in T2D. Oxidative (5, 6) and endoplasmic reticulum stress (7, 8), hypoxia (9, 10), as well as exposure to proinflammatory cytokines (11, 12) or high concentration of glucose or fatty acids (known as glucotoxicity/lipotoxicity) (13) are all well documented to be involved in β-cell dysfunction. In response to these stress signals, β cells undergo apoptosis or autophagy (14–16) or they lose their proliferative capacity (17). In addition, increasing evidence implicates β-cell dedifferentiation as a mechanism of β-cell loss. In fact, both in vitro (18) and in vivo rodent (19, 20) and primate (21) studies emphasized the importance of β-cell dedifferentiation as a general mechanism in the progress of T2D.
The extracellular levels of the purinergic signaling molecule adenosine increase in response to metabolic stress, tissue, and inflammation (22). Extracellular adenosine has been called a “retaliatory metabolite,” because its physiologic actions have a common tendency to redress the deleterious effects of stress and tissue injury and therefore preserve and restore tissue homeostasis (23). Adenosine binds to 4 specific G-protein–coupled adenosine receptors (ARs): (24) A1-, A2A-, A2B-, and A3ARs (25). ARs are expressed in metabolically active organs, such as the liver (26) and pancreas (27), and in fatty tissue (28) and the immune system (29), which indicates a crucial role for this signaling molecule in the regulation of metabolic homeostasis. In fact, a plethora of experimental evidence supports an essential function for adenosine in the regulation of glucose homeostasis and the pathophysiology of diabetes mellitus (30).
Recent in vitro and in vivo zebrafish data demonstrate that A2AARs also modulate β-cell function by promoting the proliferation and regeneration of β cells (31), in addition to maintaining their survival in an inflammatory microenvironment (32). However, the role of A2AARs in regulating β-cell function and the course of T2D is unknown. We report that A2AARs are important for preserving β-cell homeostasis in a mouse model of T2D.
MATERIALS AND METHODS
Mouse model, intraperitoneal glucose tolerance test, intraperitoneal insulin tolerance test, and GSIS
C57BL6/J wild-type (WT) and A2AAR-knockout (KO) mouse colonies were established via heterozygous breeding at our animal facility. WT and A2AAR-KO mice were kept in the same room, and animal husbandry was identical for all mice. All mice were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA), and the experiments were approved by the Rutgers New Jersey Medical School Animal Care Committee. After birth, male WT and A2AAR-KO mice were fed with regular rodent diet, and then the diet of the 8–10-wk-old mice was switched to a low-fat chow diet (CD; 10 kcal% fat; Research Diet, New Brunswick, NJ, USA) or high-fat diet (HFD; 60 kcal% fat) for 16–24 wk. After 16–24 wk of CD or HFD, intraperitoneal glucose tolerance test (ipGTT) and intraperitoneal insulin tolerance test (ipITT) were performed on WT and A2AAR-KO mice. For ipGTT and GSIS, mice were left unfed overnight, and glucose (1 g/kg body weight, i.p) was injected. Blood glucose was measured before and after glucose injection at various time points with Accu-Chek Active glucose monitoring system (Roche Diagnostic, Indianapolis, IN, USA), and plasma insulin level was measured with Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem, Downers Grove, IL, USA, USA). ipITT was conducted by injecting 0.75 U insulin/kg body weight (i.p.) and measuring glucose levels before and after the injection. One week after ipGTT and ipITT, the animals were not fed for 4–6 h, and then blood, white fat depots, brown adipose tissue, pancreas, and liver were collected, and the weight of these organs and tissues was measured. Tissue samples were stored in formalin at −70°C for further analysis.
Generation of A2AAR-KO bone marrow chimeric mice
Bone marrow chimeras were generated as described (33). In brief, male donor mice (8–10-wk-old WT or A2AAR-KO) were euthanized, and bone marrow from the femur was harvested by flushing the marrow cavity with sterile isotonic NaCl solution. The bone marrow cells were centrifuged at 400 g for 5 min, resuspended, and counted. Recipient mice (8- to 10-wk-old WT mice) were irradiated with a total dose (in 2 doses) of 12 Gy delivered from a 137Cs source. Bone marrow cells (107 per recipient mouse) were injected in 0.2 ml of physiologic saline retro-orbitally. The resulting chimeric mice were housed for at least 8 wk before experimentation and were given water containing tetracycline (100 μg/ml) in the first 2 wk after bone marrow transplantation. Chimeric mice were fed an HFD (60 kcal% fat) for 16 wk, after which, the ipGTT and ipITT were performed. One week after the tests, the animals were left unfed for 4–6 h, and then tissue was collected as previously described.
Measurement of plasma free fatty acids and triglycerides
Plasma levels of free fatty acids (FFAs) and triglycerides (TGs) were measured with commercially available kits from Bioassays Systems (Hayward, CA, USA).
Determination of pancreatic insulin content
The weight of approximately one-quarter of the whole pancreas was measured, and the tissue was placed into 5 ml of 1.5% HCl in 70% ethanol. After an overnight incubation at −20°C, the pancreas was homogenized with a Dounce homogenizer (Bellco Glass, Vineland, NJ, USA). Homogenates were then incubated overnight at −20°C. After centrifugation at 500 g for 15 min at 4°C, the supernatants were neutralized with an equal volume of 1 M Tris (pH 7.5). Insulin concentrations were determined with commercially available Ultra Sensitive Insulin Mouse ELISA Kit (CrystalChem, Downers Grove, IL, USA), and insulin contents were normalized to the weight of the pancreas tissue.
Islet isolation and GSIS
Murine pancreatic islets were isolated (34–36). In brief, HFD-fed WT and A2AAR-KO mice were euthanized by cervical dislocation. Under a dissection microscope, the abdominal cavity was opened, and the common bile duct was localized. Pancreases were inflated via the common bile duct with 4 ml of 1× HBSS containing 0.8 mg/ml collagenase P (Roche, Mannheim, Germany) using a 30-guage needle attached to a 5-ml syringe. Inflated pancreata were removed and placed in a 50-ml conical tube containing 5 ml HBSS and were placed into 37°C water bath for 17 min, after which the tubes were swirled 15 times to promote tissue dissociation. HBSS (25 ml) containing 0.3% bovine serum albumin [BSA (HBSS/BSA)] was added to the tubes, and digested pancreata were centrifuged at 290 g for 1 min. Supernatants were removed, and pellets were aspirated up and down twice using a 14-guage needle attached to a 30-ml syringe containing 10 ml HBSS/BSA. The tissue suspensions were filtered through a tea strainer and centrifuged at 330 g for 2 min. The supernatants were decanted, and the pellets were resuspended in 10 ml cold 1100 Histopaque (Sigma-Millipore) and were overlaid with 10 ml HBSS/BSA and centrifuged at 900 g for 18 min. After centrifugation, the supernatant was passed through an inverted 70 μm filter, and islets from the filter were rinsed into a Petri dish with islet medium (1640 RPMI containing 11.1 mM glucose and 10% FBS).
For GSIS assessment, islets were picked up under a light microscope using a 20-μl tip attached to a pipette. Eight islets from each mouse were transferred into microcentrifuge tubes containing 1 ml of low glucose (1.1 mM) RPMI-1640. After an incubation at 37°C for 90 min, islets were centrifuged at 50 g for 2 min, and then the supernatants were discarded and replaced with 300 μl of RPMI-1640 containing 11.1 or 25 mM glucose. Islets were incubated for 20 min at 37°C and the supernatants were saved for insulin ELISA.
Insulin, Ki67, sox2, and TUNEL staining
Immunohistochemical staining for insulin and Ki67 was performed as described, with minor modifications (37, 38). The slides were deparaffinized, endogenous peroxidase activity was blocked (3% H2O2 in methanol on 5-µm paraffin-embedded sections for 15 min at room temperature), and the slides were rinsed with PBS briefly. Nonspecific binding was blocked by incubating the slides for 1 h in 1% BSA diluted in PBS. To detect insulin protein, guinea pig polyclonal insulin antibody (Dako, Glostrup, Denmark) was applied overnight at 4°C. For the detection of Ki67, mouse monoclonal Ki67 antibody (Dako) was used overnight at 4°C. Thereafter, the slides were stained with Envision 1-step polymer horseradish peroxidase (HRP) (BioGenex, Fremont, CA, USA) for 90 min. Color reaction was developed with nickel 3,3′-diaminobenzidine tetrachloride (Ni-DAB) as a substrate for antibody-coupled HRP [1.6 mM DAB, 140 mM NaCl, 90 mM NiSO4, 100 mM Na-acetate, and 3 mM H2O2 (pH 6.6)] for 2 min. After rinsing the sections in 50 mM Tris-HCl (pH 7.4), the color was enhanced by incubating the sections for 2 min in 2% cobalt chloride (in 50 mM Tris-HCl; pH 7.2). Then, the sections were rinsed in distilled water and counterstained with 1% methyl green solution. Islet size was determined on insulin-stained slides. To detect sox2 protein, polyclonal anti-sox2 Ab from Cell Signaling Technology (Danvers, MA, USA) was applied overnight at 4°C. Thereafter, secondary Alexa Fluor594-conjugated anti-rabbit IgG Ab was incubated with the slides. The presence of apoptotic cells was assessed with the In Situ Cell Death Detection Kit (TUNEL; Roche).
RNA extraction, cDNA synthesis, and real-time quantitative PCR
Total RNA was extracted from epididymal adipose tissue, pancreas, and islets using Tri reagent (Molecular Research Center, Cincinnati, OH, USA) and reverse transcribed. For detecting various mRNA transcripts, a commercially available real-time PCR kit was used (Thermo Fisher Scientific, Waltham, MA, USA), and all data were normalized to constitutive rRNA (18S) values. The ABI 7700 sequence detector was used for amplification of target sequences, and quantitation of differences between treatment groups was performed with the Ct method.
Protein extraction and Western blot analysis
Epididymal fat tissue, pancreatic tissue, and islets for Western blot analysis and ELISA measurements were homogenized in modified RIPA buffer. The lysates were centrifuged at 15,000 g for 15 min, and the supernatant was recovered. The Bio-Rad Laboratories (Hercules, CA, USA) protein assay kit was used to determine the protein concentrations. Protein samples (20 μg) were separated on 8–12% Tris-glycine gel (Thermo Fisher Scientific) and transferred to nitrocellulose membrane. The membranes were probed with polyclonal anti-cleaved PARP Ab from Cell Signaling Technology, with polyclonal anti-nkx6.1 Ab from Santa Cruz Biotechnology (Dallas TX, USA), with polyclonal anti-pdx1 Ab from Abcam (Cambridge, MA, USA) or with polyclonal anti-A2AAR Ab from Thermo Fisher Scientific. After several wash cycles, the nitrocellulose membranes were incubated with a secondary HRP-conjugated anti-rabbit Ab (Santa Cruz Biotechnology). To assess equal protein loading, we used HRP-conjugated polyclonal goat anti-β-actin Ab from Santa Cruz Biotechnology. Bands were detected with ECL Western Blot Detection Reagent (GE Healthcare, Piscataway, NJ, USA).
Adipocyte morphometry and adipose tissue immunohistochemistry
Hematoxylin and eosin (H&E) staining, F4/80 immunohistochemistry, and analysis of paraffin-embedded epididymal adipose tissue were performed by IHC World (Woodstock, MD, USA) (39).
Liver immunohistochemistry
H&E and Oil Red O staining of liver sections
Livers were fixed with 10% neutral formalin, embedded in paraffin, and stained with H&E for histologic examination. Liver steatosis was graded on the basis of a semiquantitative estimation of the percentage of lipid-laden hepatocytes, according to the following criteria: grade 0, no hepatocytes involved; grade 1, 1–25% of hepatocytes involved; grade 2, 26–50% of hepatocytes involved; grade 3, 51–75% of hepatocytes involved; and grade 4, 76–100% of hepatocytes involved. At least 5 different high-power fields (original magnification, ×200) were graded in a blinded procedure. For Oil Red O (Sigma-Millipore) staining, liver sections were flash frozen and embedded in frozen medium. The 5-μm sections were cut and fixed on microscope slides, allowed to air dry overnight at room temperature, stained with fresh Oil Red O for 15 min, rinsed in water, and counterstained with hematoxylin.
Determination of adipokine, cytokine, and chemokine levels
Concentrations of adiponectin, leptin, resistin, chemokine (C-C motif) ligand 2, IL-6, IL-1β, TNF-α, IFN-γ, IL-12p40, and IL-10 were determined in blood and protein extracts of epididymal fat and pancreas. Levels of adipokines, cytokines, and chemokines were determined with commercially available ELISA duosets (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.
Statistics
Data in the figures are means ± sem of n observations. Statistical analysis of the data was performed by Student’s t test or 1-way analysis of variance followed by Dunnett’s test. A value of P < 0.05 indicated significance.
RESULTS
Role of A2AARs in regulating fat accumulation in HFD-fed mice
First, we addressed the impact of A2AAR deficiency on weight gain of mice fed a CD or HFD. The weight of A2AAR-KO mice and WT littermates on CD was comparable (Fig. 1A) and A2AAR-KO mice gained less weight than WT mice during 16 wk of HFD (Fig. 1B). We then studied the food consumption of A2AAR-KO and WT animals, and we found that A2AAR-KO mice consumed less food than their WT littermates (Fig. 1C). Despite gaining less weight, HFD-fed A2AAR-KO mice displayed increased epididymal fat pad weight when compared to WT animals (Fig. 1D). Furthermore, there was no difference in the size of epididymal fat tissue in CD-fed WT vs. A2AAR-KO mice (Fig. 1D). The size of other fat depots including subcutaneous, mesenteric, retroperitoneal, and brown adipose tissue was similar in WT and A2AAR-KO mice, irrespective of whether they were CD or HFD fed (Fig. 1E–H). Because white adipose tissue accumulates fat mostly through adipocyte hypertrophy, we assessed adipocyte size in H&E-stained epididymal fat tissue. In agreement with the increase of epididymal fat tissue, quantitative analysis showed augmented adipocyte size in the epididymal tissue of HFD-fed A2AAR-KO vs. WT mice (Fig. 1I). In addition, we found that A2AAR deficiency prevented the HFD-induced enlargement of metabolically important organs, such as pancreas and liver (Fig. 1J, K). In agreement with the smaller liver size, mice lacking A2AARs were protected from developing a fatty liver after consuming an HFD (Fig. 1L).
Figure 1.
A2AARs regulate fat accumulation in HFD-fed mice. A, B) Body weight of WT and A2AAR-KO mice fed CD (A) or HFD (B) (n = 7–10 mice/group). C) Food consumption of WT and A2AAR-KO mice (n = 7–8). D–H) Weight of epididymal fat (D), subcutaneous fat (E), mesenteric fat (F), retroperitoneal fat (G), and brown adipose tissue (H) depots from CD- or HFD-fed WT and A2AAR-KO mice (n = 7, 8, 10, 10, respectively). I) Representative images and quantitative analysis of H&E-stained epididymal adipose tissue from HFD-fed A2AAR-KO and WT animals (n = 3). J, K) Weight of pancreas (J) and liver (K) from WT and A2AAR-KO mice fed a CD or an HFD (n = 7, 8, 10, 10, respectively). L) Images of liver sections from HFD-fed WT and A2AAR-KO mice, with steatosis grades in the liver sections of WT and A2AAR-KO mice after HFD (n = 5, 5). Original magnification, ×200. Results are representative of 3 experiments. Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT littermates or CD; #P < 0.05, ##P < 0.01 vs. WT littermates.
HFD-fed A2AAR-KO mice display impaired glucose tolerance
We next examined the role of A2AARs in regulating glucose and insulin homeostasis in CD- and HFD-fed animals. Fasting blood glucose levels were elevated in A2AAR-KO vs. WT animals fed an HFD (Fig. 2A), which indicates impaired glucose homeostasis. We next performed ipGTT. HFD-fed A2AAR-KO animals showed impaired glucose clearance when compared with WT mice (Fig. 2B). There was no difference in fasting blood glucose levels and glucose clearance between CD-fed WT and A2AAR-KO mice (Supplemental Fig. S1A, B). In addition, HFD-fed A2AAR-KO mice displayed elevated FFA levels compared with those of WT mice (Fig. 2C), but TG, leptin, adiponectin, and resistin concentrations were comparable between WT and A2AAR-KO mice fed an HFD (Fig. 2D–G). Furthermore, the levels of FFA, liver TG, leptin, adiponectin, and resistin were similar in A2A-KO and WT mice that were fed a CD (Supplemental Fig. S1C–G).
Figure 2.
HFD-fed A2AAR-KO mice display impaired glucose tolerance. A) Plasma glucose levels from WT and A2AAR-KO mice left unfed for 16 h. Results are representative of 3 experiments (n = 7–10 mice/group). B) Glucose levels during the ipGTT with the HFD-fed A2AAR-KO and WT mice after a 16 wk HFD. Results are representative of 3 experiments (n = 7–10 mice/group). C) FFA levels in the plasma of A2AAR-KO and WT mice after 16 wk of HFD. D) TG concentrations in the livers of A2AAR-KO and WT mice after 16 wk of HFD. E–G) Plasma levels of leptin (E), adiponectin (F), and resistin (G) in A2AAR-KO and WT mice after 16 wk of HFD. Results are representative of 3 experiments (n = 10 mice/group). Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT littermates.
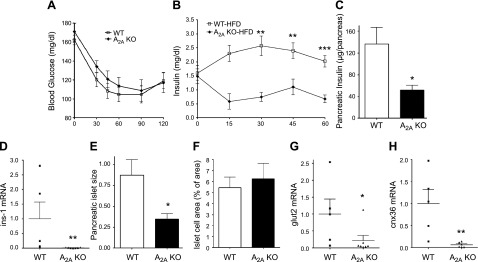
HFD-fed A2AAR-KO mice have decreased in vivo insulin secretion, which is caused by impaired pancreatic function
Next, we explored further the role of A2AARs in regulating metabolic function during HFD conditions. An ipITT showed that there was no difference in insulin responsiveness between HFD-fed WT and A2AAR-KO mice (Fig. 3A). These data, together with the suppressed glucose clearance in HFD-fed A2A-KO mice (Fig. 2B), indicated that A2AAR-KO mice had a deficient insulin response to glucose. Indeed, this was the case, in that, although WT animals responded to glucose injection with an increase in insulin secretion, A2AAR-KO mice failed to do so (Fig. 3B). Given that A2AAR-KO mice had decreased pancreas size after HFD feeding (Fig. 1J), we hypothesized that the decreased insulin response was related to the decreased pancreatic insulin content (2, 4). In fact, we found that HFD-fed A2AAR-KO mice had decreased insulin content at both the protein (Fig. 3C) and mRNA (Fig. 3D) levels. In addition, islet size was reduced (Fig. 3E) in HFD-fed A2AAR-KO vs. WT mice. Moreover, the mRNA expression of key β-cell markers, such as glut2 and cnx36 was diminished in the pancreata of HFD-fed A2AAR-KO vs. WT mice (Fig. 3F, G). There was no difference in pancreatic insulin content and islet size between WT and A2AAR-KO mice fed with CD (Supplemental Fig. S2A, D). These data, taken together, suggest impaired pancreatic function in A2AAR-KO mice after HFD.
Figure 3.
A2AAR deficiency exacerbates HFD-induced β-cell dysfunction without impairing insulin sensitivity. A) Glucose levels during an ipITT of HFD-fed A2AAR-KO and WT mice (n = 10 mice/group). B) GSIS of HFD-fed WT and A2AAR-KO mice (n = 6–7 mice/group). C, D) Protein levels (C) and mRNA expression (D) of insulin from pancreatic tissue obtained from HFD-fed WT and A2AAR-KO mice. E, F) Islet size (E) and area (F) of HFD-fed A2AAR-KO and WT mice. G, H) mRNA levels of glut2 (G) and cnx36 (H) from pancreata of A2AAR-KO and WT mice after HFD. Results are representative of 3 experiments (n = 5–7 mice/group). Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT littermates.
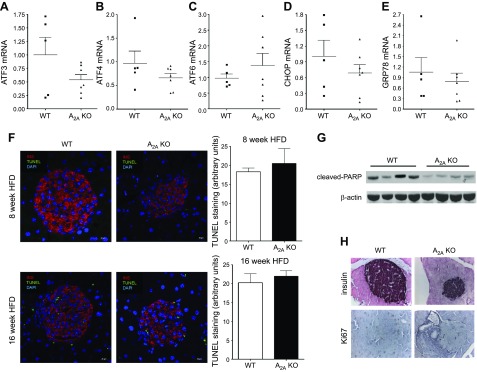
ER stress, apoptosis, and decreased proliferation are not responsible for impaired pancreatic function in A2AAR-KO mice after HFD
Exacerbated apoptosis as a result of increased ER stress in response to elevated levels of toxic metabolic compounds, such as FFAs is responsible for augmented β-cell death after HFD feeding (7, 8, 14, 15). Therefore, we next examined the role of A2AARs in regulating pancreatic ER stress and apoptosis in HFD-fed mice. There was no difference in the expression of the ER stress markers ATF3, ATF4, ATF6, CHOP, and GRP78 in A2A-KO vs. WT mice (Fig. 4A–E). In addition, TUNEL staining of islets, an indicator of apoptosis, was comparable in A2AAR-KO and WT mice after both 8 and 16 wk of HFD (Fig. 4F). Moreover, proteolytic cleavage of PARP, a further apoptotic marker (40), did not increase but actually decreased in A2AAR-KO mice when compared to WT mice after HFD feeding (Fig. 4G). These data together indicate that ER stress and apoptosis are not responsible for the reduced pancreatic β-cell function of HFD-fed A2AAR-KO mice.
Figure 4.
ER stress, apoptosis, and altered proliferation are not responsible for pancreatic β-cell dysfunction in A2AAR-KO mice after HFD. A–E) mRNA expression of ATF3 (A), ATF4 (B), ATF6 (C), CHOP (D), and GRP78 (E) from pancreatic tissue of A2AAR-KO and WT mice that were fed an HFD (n = 5–7). F) Representative images and quantitative analysis of TUNEL-stained pancreas obtained from HFD-fed A2AAR-KO and WT mice after 8 or 16 wk of HFD (n = 3/group). G) Levels of cleaved-PARP and β-actin, as detected by Western blot, from protein extract of pancreas from A2AAR-KO and WT mice after 16 wk of HFD (n = 4/group). H) Representative images from insulin and Ki67 staining of pancreas obtained from HFD-fed A2AAR-KO and WT mice. Each result is representative of 3 experiments. Data are means ± sem.
In response to weight gain and obesity, β-cell proliferation is enhanced to meet the increased demand for insulin production (41), and, in response to excessive insulin demand, β cells lose their proliferative capacity (17). Recent data demonstrated that A2AARs promotes proliferation of β cells in an acute model of type 1 diabetes (T1D) (31). Therefore, we examined the level of the proliferation marker Ki67 in the pancreas. Islet-associated Ki67 staining was low and was comparable in HFD-fed WT and A2AAR-KO mice (Fig. 4H).
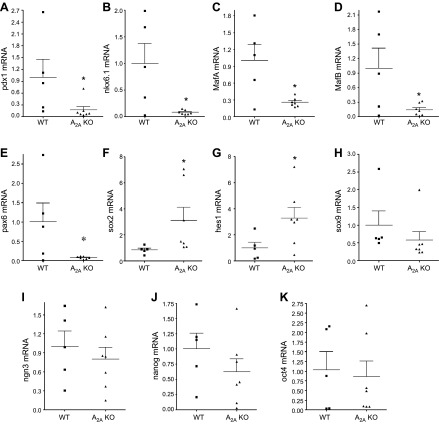
A2AARs decrease HFD-induced expression of β-cell dedifferentiation markers
Growing evidence suggests that β-cell dedifferentiation is a mechanism of β-cell failure in T2D (18–21). Because we could not detect differences in ER stress, apoptosis, and proliferation of β cells between A2AAR-KO and WT mice, we examined the expression of specific markers of β-cell differentiation/dedifferentiation in the pancreas. Pancreatic tissue of A2AAR-KO mice had decreased expression of the β-cell-specific markers pdx1, nkx6.1 MafA, MafB, and pax6 compared with WT pancreata (Fig. 5A–E). At the same time, the expression of the dedifferentiation markers sox2 and hes1 increased in the pancreas of A2AAR-KO vs. WT animals (Fig. 5F, G). However, not all dedifferentiation markers were increased, as mRNA levels of sox9, neurogenin 3 (ngn3), nanog, and oct4 were similar in HFD-fed WT and A2AAR-KO cohorts (Fig. 5H–K).
Figure 5.
A2AARs protect against HFD-induced β-cell dedifferentiation. mRNA expression of pdx1 (A), nkx6.1 (B), MafA (C), MafB (D), pax6 (E), sox2 (F), hes1 (G), sox9 (H), ngn3 (I), nanog (J), and oct4 (K) from pancreas of HFD-fed A2AAR-KO and WT mice. Results are representative of 3 experiments (n = 5–7 mice/group). Data are means ± sem. *P < 0.05 vs. WT littermates.
A2AARs have no effect on HFD-induced adipose tissue inflammation
Adipose tissue inflammation driven by macrophages and other immune cell types is a hallmark of metabolic syndrome and T2D (39, 42). Because A2AARs are major regulators of inflammation and macrophages (29, 43–48), we next examined whether deletion of the A2AAR has any impact on inflammation and adipose tissue macrophages. We first enumerated F4/80+ macrophages in the epididymal adipose tissue of HFD-fed A2AAR-KO and WT animals. We found that macrophage numbers were the not different in the adipose tissue of WT and A2AAR-KO mice (Fig. 6A). Macrophages surrounding dying or dead adipocytes form crown-like structures in the adipose tissue of mice fed HFD (49). Therefore, we next analyzed the distribution of macrophages in the adipose tissue of WT and A2AAR-KO mice. The quantity of crown-like structures was similar in the two cohorts (Fig. 6B). We then compared plasma and adipose tissue levels of inflammatory cytokines and chemokines in WT mice with levels of these mediators in A2AAR-KO mice. There were no differences in the level of CCL2, IL-6, IL-1β, TNF-α, IFN-γ, IL-12p40, and IL-10, either in the plasma or in adipose tissue between WT and A2AAR-KO animals (Fig. 6C–L).
Figure 6.
A2AARs have no effect on HFD-induced adipose tissue inflammation. A, B) Quantitative analysis of the number of F4/80+ cells (A) and number of crown-like structures (B) in epididymal adipose tissue obtained from A2AAR-KO and WT animals after 16 wk of HFD. Data are means ± sem (n = 3 mice/group). C–E) Plasma levels of CCL2 (C), IL-6 (D), and IL-1β (E) of HFD-fed A2AAR-KO and WT mice. F–L) Levels of CCL2 (F), IL-6 (G), IL-1β (H), TNF-α (I), IFN-γ (J), IL-12p40 (K), and IL-10 (L) from protein extracts of epididymal fat obtained from HFD-fed WT and A2AAR-KO mice (n = 10 mice/group). Data are means ± sem. M) Glucose levels during an ipGTT of HFD-fed A2AAR-KO→WT and WT→WT bone marrow chimeric mice after 16 wk of HFD (n = 5–6 mice/group). Data are means ± sem. N) Glucose levels during ipITT of HFD-fed A2AAR-KO→WT and WT→WT bone marrow chimeric mice after HFD (n = 5–6 mice/group). O) Weight of pancreas from HFD-fed A2AAR-KO→WT and WT→WT bone marrow chimeric mice (n = 5–7). P, Q) Protein levels of insulin in pancreas (P) and islet size (Q) of HFD-fed A2AAR-KO→WT and WT→WT bone marrow chimeric mice after HFD (n = 5–7). All results are representative of 3 experiments.
A2AARs on nonmyeloid cells are responsible for maintaining glucose homeostasis and pancreatic insulin production after HFD
A2AARs are expressed at the highest level on hematopoietic cells, where they suppress inflammation (29, 47). In addition, there is a plethora of evidence that HFD-induced β-cell impairment is a result of islet inflammation triggered by infiltrating T cells and macrophages (11, 12). To study whether A2AAR signaling on hematopoietic cells was primarily responsible for A2AAR-mediated protection against impaired glucose homeostasis and islet function, we generated A2AAR bone marrow-chimeric mice by transferring A2AAR-KO bone marrow into irradiated WT mice (33, 50). A2AAR-KO→WT chimeras that have KO bone marrow and WT parenchyma had weight and fat tissue depots similar to those of WT→WT mice with WT parenchyma and WT bone marrow after HFD (Supplemental Fig. S3A–F). Furthermore, A2A-KO→WT chimeras displayed similar glucose tolerance (Fig. 6M) and response to insulin (Fig. 6N) to the WT→WT mice. Furthermore, the weight of pancreas, pancreatic insulin content, and pancreatic islet size were similar in A2AAR-KO→WT and WT→WT mice after HFD (Fig. 6O–Q). These observations suggest that A2AAR signaling on hematopoietic cells is not necessary for the protective effect of A2AARs after HFD.
A2AARs signaling preserves β-cell phenotype and GSIS in islets after HFD
In addition to immune cells, both neuronal and endocrine cells express high levels of A2AARs, and therefore it is possible that A2AAR signaling on neurons or endocrine cells indirectly imparts its protective effect on islet β-cell function (51). Because insulin secretion in vivo is under neuronal and endocrine control, GSIS experiments in vitro vs. in vivo can help distinguish between islet-extrinsic (neuronal and endocrine) and intrinsic roles of A2AAR signaling in protecting islet function after HFD.
Thus, we isolated islets from WT and A2AAR-KO mice after HFD and performed ex vivo GSIS. We found decreased insulin secretion in the islets of HFD-fed A2AAR-deficient mice in response to elevated glucose concentrations (11.1 or 25 mM) when compared to islets of WT mice fed an HFD (Fig. 7A). Furthermore, mRNA expression of insulin was lower, and mRNA levels of glucagon were increased in the islets of HFD-fed A2AAR-KO vs. WT mice (Fig. 7B, C). In addition, we found a decreased level of pdx1 and nkx6.1 (Fig. 7D–G) and an augmented level of sox2 protein in the islets of A2AAR-KO vs. WT mice after HFD (Fig. 7H).
Figure 7.
A2AARs signaling in islets preserve β-cell phenotype and GSIS in mice fed an HFD. A) GSIS of islets obtained from HFD-fed WT and A2AAR-KO mice (n = 7/group). mRNA levels of insulin (B), glucagon (C), pdx1 (D), and nkx6.1 (E) of the islets of HFD-fed WT and A2AAR-KO mice (n = 4–10/group). Western blot and densitometric analyses of protein levels of pdx1 (F) and nkx6.1 (G) from protein extract of islets obtained from WT and A2AAR-KO mice after a 16 wk HFD (n = 3/group). H) Representative images and quantitative analysis of sox2/insulin/DAPI-stained pancreas obtained from HFD-fed A2AAR-KO and WT mice after a 16 wk HFD (n = 3/group). I) mRNA expression of ARs in the islets of A2AAR-KO and WT mice (n = 4). J) Levels of A2AAR and β-actin as detected by Western blot from protein extract of pancreatic islets from A2AAR-KO and WT mice. All results are representative of 3 experiments. Data are means ± sem. *P < 0.05; **P < 0.01; ***P < 0.001 vs. WT littermates.
Because it is unclear whether A2AARs are expressed in islets, we assessed the presence of A2AARs in islets isolated from WT and A2AAR-KO mice. Both A2AAR mRNA transcript and protein were present in WT but not in A2AAR-KO islets (Fig. 7I, J). Altogether, these data suggest that A2AAR signaling preserves the β-cell phenotype and GSIS in islets after HFD.
DISCUSSION
The major new finding of our study is that HFD-fed A2AAR-KO animals show impaired glucose disposal and elevated blood glucose levels that are related to defective insulin secretion. This finding indicates that endogenous adenosine protects β-cell homeostasis through A2AARs. It has been shown in isolated mouse islets that adenosine augments glucose-induced insulin secretion, which can be inhibited by the A2AAR antagonist SCH58261 (52). Our in vivo data, however, suggest a different mechanism behind the beneficial effect of A2AARs. Since we found impaired β-cell function and decreased insulin content in the pancreas of A2AAR-KO mice vs. WT mice, we conclude that A2AARs protect by preserving the β-cell phenotype during HFD. Numerous mechanisms have been shown to lead to the loss of β-cell function in T2D, including augmented ER stress and apoptosis (7, 8, 14, 15), decreased proliferative capacity (17), and dedifferentiation (18). Our data exclude the possibility that A2AARs protect by targeting ER stress and apoptosis. Recently, Talchai et al. (19) proposed that the pathogenesis of T2D involved β-cell dedifferentiation, and they reported that the transcription factor FoxO1 is important in maintaining the β-cell phenotype. In fact, β-cell-specific loss of FoxO1 launched a dedifferentiation program in β cells with increased expression of ngn3, oct4, and nanog and decreased expression of pdx1, MafA, and nkx6.1. Later, additional studies confirmed the role of these and other transcription factors, including pdx1 (53), sox9 (54), MafA, MafB, and nkx6.1 (55), in maintaining β-cell identity. Our data demonstrating diminished expression of pdx1, MafA, MafB, nkx6.1, and pax6 and augmented levels of sox2 and hes1 suggest that β-cell dedifferentiation contributed to the underlying impaired insulin production in HFD-fed A2AAR-KO mice. However, the expression of other β-cell dedifferentiation markers, such as ngn3, sox9, nanog, and oct4, as well as FoxO1, was comparable in pancreatic tissue of WT and A2AAR-KO mice, indicating that A2AARs may govern a specific expression program that maintains β-cell phenotype after long-term high-fat consumption. In the future, the question of how A2AAR signaling protects against HFD-induced β-cell dedifferentiation should be addressed.
In addition to the protective roles of A2AARs in the pancreas and islets, A2AARs have various roles in regulating the metabolic function of other organs. For example, it appears that A2AARs are also important in the liver, as we found that A2AAR-KO mice had decreased liver size with lower fat content (Fig. 1L). In this context, it is interesting to note that A2AARs had no role in regulating the development of ethanol-induced fatty liver disease (56). These discrepancies between our data showing that A2AARs protect the liver in response to HFD feeding but that they have no role in regulating ethanol-induced fatty liver development (56) indicate that the role of A2AARs depends on the context.
Our data showing the reduced food intake of A2AAR-KO mice also implicate the brain and point to dysregulation in the arcuate nucleus region of the hypothalamus, which is responsible for appetite control (57). Dysregulation by A2AAR-KO in the arcuate nucleus may compromise the compensatory expansion of the pancreas and islets during HFD and may account for the reduced expression of several β-cell markers in HFD-fed A2AAR-KO mice. Altogether, further studies are needed to delineate the cells and tissues, which mediate the protective effect of A2AARs.
Recent data reveal that A2AAR signaling can also modulate β-cell function by influencing the proliferation and regeneration of β cells (31). In a zebrafish model of T1D, the nonselective AR agonist 5′-N-ethylcarboxamidoadenosine increased proliferation of β cells, and ablation of A2AARs reversed the effect of 5′-N-ethylcarboxamidoadenosine, implicating A2AARs in promoting proliferation. Furthermore, the proliferation-stimulating effect of A2AARs on β cells was confirmed in streptozotocin-induced T1D in mice. However, our results suggest that A2AARs affect the β-cell phenotype independent of regulating proliferation in HFD-induced T2D, as pancreatic Ki67 levels were comparable in A2AAR-KO and WT mice consuming an HFD.
In a recent study, Gnad et al. (58) demonstrated that A2AAR activation increases thermogenesis through increasing the mass of brown adipose tissue via “browning” of white adipocytes. In contrast, we found that HFD-fed A2AAR-KO mice displayed similar brown adipose tissue mass compared with WT mice, indicating a lack of a role for A2AARs in mediating white adipocyte browning. The discordance between our result and the data presented by Gnad et al. may be the consequence of the different approach that was used in the two studies: we employed A2AAR-KO and WT mice, while they used pharmacologic activation of A2AARs.
HFD can induce inflammation in various tissues including adipose tissue, liver, and pancreas. Hypoxia at the site of tissue injury and inflammation frequently disrupts tissue homeostasis. HIF-1 is a central regulator of hypoxia and the role of hypoxia, and HIF-1 in pancreatic homeostasis is well demonstrated (59). The relationship between adenosine and hypoxia is also well known, where, although A2BARs are dominant in regulating tissue responses during hypoxia (60–63), the role of A2AARs have also been confirmed (64, 65). Because A2AARs, similar to A2BARs, which we and others have shown to regulate glucose homeostasis in T2D through immune-mediated mechanisms (39, 42), are primarily expressed by immune cells (47), it was plausible that A2AARs regulate glucose homeostasis through a primarily immune-mediated mode of action. However, our studies show that this is unlikely, given that we did not find exacerbated inflammation or increased macrophage infiltration in the adipose tissue of A2AAR-KO vs. WT mice after HFD, and glucose homeostasis was similar in HFD-fed A2AAR-KO→WT and WT→WT bone marrow chimeras. Together, these results indicate that A2AAR signaling on nonmyeloid cells is responsible for regulating glucose homeostasis in HFD.
In summary, in this study we implicated A2AARs as crucial components in maintaining β-cell identity and insulin production during consumption of an HFD through restricting β-cell dedifferentiation in a murine model of T2D. In addition, we stress that our study has translational relevance, as any drug targeting A2AARs would likely be administered systematically and would affect A2A receptors in all tissues. Therefore, we propose that A2AARs represent a valuable target for preventing or reversing T2D-associated β-cell failure.
ACKNOWLEDGMENTS
The authors thank Dr. Katalin Erdelyi (University of Debrecen) for technical assistance. This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grant R01GM66189 (to G.H.), NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK113790, U.S. Army Medical Research and Materiel Command Grant 09065004 (to G.H.), Hungarian Scientific Research Fund (OTKA) Grants CK 78275 (to G.H.) and PD83473 (to P.B.), the Intramural Research Program of the NIH National Institute on Alcohol Abuse and Alcoholism (to P.P.), a Bolyai fellowship (to P.B.), the Hungarian National Innovation Office (Baross Program Seahorse Grants TéT_09-2010-0023), TÁMOP-4.2.2. A-11/1/KONV-2012-0025, TÁMOP-4.2.1./B-09/KONV-2010-0007, TÁMOP-4.2.2/B-10/1-2010-0024), and the Medical and Health Science Center (Mecenatura Mec-8/2011). L.V. is supported by National Research, Development and Innovation Office Grants GINOP-2.3.2-15-2016-00020 TUMORDNS, GINOP-2.3.2-15-2016-00048-STAYALIVE, and OTKA K112336. The authors declare no conflicts of Interest.
Glossary
- AR
adenosine receptor
- BSA
bovine serum albumin
- CD
chow diet
- FFA
free fatty acid
- GSIS
glucose-stimulated insulin secretion
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- HRP
horseradish peroxidase
- ipGTT
intraperitoneal glucose tolerance test
- ipITT
intraperitoneal insulin tolerance test
- KO
knockout
- ngn3
neurogenin 3
- PARP
poly (ADP-ribose) polymerase
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TG
triglyceride
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. Csóka and P. Bai designed the research and analyzed the data; Z. H. Németh, L. Virág, P. Pacher, P. Marcheti, and P. Bai contributed vital new reagents; B. Csóka, B. Koscsó, G. Törő, J. Vindeirinho, Z. V. Varga, E. Kókai, K. Cseri, L. Antonioli, A. Deák, M. Suleiman, and Z. H. Németh performed the research; B. Csóka and G. Haskó wrote the paper; P. Bai and G. Haskó reviewed and edited the manuscript; and B. Csóka is the guarantor of the manuscript.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). (2014) National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014, U.S. Department of Health and Human Services, CDC, Atlanta, GA. Retrieved July 14, 2017, from https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf [Google Scholar]
- 2.Prentki M., Nolan C. J. (2006) Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 116, 1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heit J. J., Apelqvist A. A., Gu X., Winslow M. M., Neilson J. R., Crabtree G. R., Kim S. K. (2006) Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 443, 345–349 [DOI] [PubMed] [Google Scholar]
- 4.Ahrén B. (2005) Type 2 diabetes, insulin secretion and beta-cell mass. Curr. Mol. Med. 5, 275–286 [DOI] [PubMed] [Google Scholar]
- 5.Robertson R. P. (2004) Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 279, 42351–42354 [DOI] [PubMed] [Google Scholar]
- 6.Robertson R. P., Harmon J., Tran P. O., Poitout V. (2004) Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53(Suppl 1), S119–S124 [DOI] [PubMed] [Google Scholar]
- 7.Kaneto H., Nakatani Y., Kawamori D., Miyatsuka T., Matsuoka T. A., Matsuhisa M., Yamasaki Y. (2005) Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance. Int. J. Biochem. Cell Biol. 37, 1595–1608 [DOI] [PubMed] [Google Scholar]
- 8.Eizirik D. L., Cardozo A. K., Cnop M. (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 [DOI] [PubMed] [Google Scholar]
- 9.Bensellam M., Duvillié B., Rybachuk G., Laybutt D. R., Magnan C., Guiot Y., Pouysségur J., Jonas J. C. (2012) Glucose-induced O2 consumption activates hypoxia inducible factors 1 and 2 in rat insulin-secreting pancreatic beta-cells. PLoS One 7, e29807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zehetner J., Danzer C., Collins S., Eckhardt K., Gerber P. A., Ballschmieter P., Galvanovskis J., Shimomura K., Ashcroft F. M., Thorens B., Rorsman P., Krek W. (2008) PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev. 22, 3135–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehses J. A., Perren A., Eppler E., Ribaux P., Pospisilik J. A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M. K., Biollaz G., Fontana A., Reinecke M., Homo-Delarche F., Donath M. Y. (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 [DOI] [PubMed] [Google Scholar]
- 12.Jourdan T., Godlewski G., Cinar R., Bertola A., Szanda G., Liu J., Tam J., Han T., Mukhopadhyay B., Skarulis M. C., Ju C., Aouadi M., Czech M. P., Kunos G. (2013) Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 19, 1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poitout V., Robertson R. P. (2008) Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr. Rev. 29, 351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 15.Butler A. E., Janson J., Soeller W. C., Butler P. C. (2003) Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52, 2304–2314 [DOI] [PubMed] [Google Scholar]
- 16.Masini M., Bugliani M., Lupi R., del Guerra S., Boggi U., Filipponi F., Marselli L., Masiello P., Marchetti P. (2009) Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 52, 1083–1086 [DOI] [PubMed] [Google Scholar]
- 17.Hull R. L., Kodama K., Utzschneider K. M., Carr D. B., Prigeon R. L., Kahn S. E. (2005) Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia 48, 1350–1358 [DOI] [PubMed] [Google Scholar]
- 18.Weinberg N., Ouziel-Yahalom L., Knoller S., Efrat S., Dor Y. (2007) Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes 56, 1299–1304 [DOI] [PubMed] [Google Scholar]
- 19.Talchai C., Xuan S., Lin H. V., Sussel L., Accili D. (2012) Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z., York N. W., Nichols C. G., Remedi M. S. (2014) Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19, 872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiori J. L., Shin Y. K., Kim W., Krzysik-Walker S. M., González-Mariscal I., Carlson O. D., Sanghvi M., Moaddel R., Farhang K., Gadkaree S. K., Doyle M. E., Pearson K. J., Mattison J. A., de Cabo R., Egan J. M. (2013) Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes 62, 3500–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonioli L., Pacher P., Vizi E. S., Haskó G. (2013) CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 19, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonioli L., Blandizzi C., Pacher P., Haskó G. (2013) Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer 13, 842–857 [DOI] [PubMed] [Google Scholar]
- 24.Fredholm B. B., IJzerman A. P., Jacobson K. A., Linden J., Müller C. E. (2011) International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors: an update. Pharmacol. Rev. 63, 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fredholm B. B., Arslan G., Halldner L., Kull B., Schulte G., Wasserman W. (2000) Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol. 362, 364–374 [DOI] [PubMed] [Google Scholar]
- 26.Grden M., Podgorska M., Szutowicz A., Pawelczyk T. (2007) Diabetes-induced alterations of adenosine receptors expression level in rat liver. Exp. Mol. Pathol. 83, 392–398 [DOI] [PubMed] [Google Scholar]
- 27.Koupenova M., Ravid K. (2013) Adenosine, adenosine receptors and their role in glucose homeostasis and lipid metabolism [Epub ahead of print]. J. Cell. Physiol. 10.1002/jcp.24352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenstein A., Ravid K. (2014) G protein-coupled receptors and adipogenesis: a focus on adenosine receptors. J. Cell. Physiol. 229, 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haskó G., Linden J., Cronstein B., Pacher P. (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonioli L., Blandizzi C., Csóka B., Pacher P., Haskó G. (2015) Adenosine signalling in diabetes mellitus: pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 11, 228–241 [DOI] [PubMed] [Google Scholar]
- 31.Andersson O., Adams B. A., Yoo D., Ellis G. C., Gut P., Anderson R. M., German M. S., Stainier D. Y. (2012) Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab. 15, 885–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson O. (2014) Role of adenosine signalling and metabolism in β-cell regeneration. Exp. Cell Res. 321, 3–10 [DOI] [PubMed] [Google Scholar]
- 33.Csóka B., Németh Z. H., Rosenberger P., Eltzschig H. K., Spolarics Z., Pacher P., Selmeczy Z., Koscsó B., Himer L., Vizi E. S., Blackburn M. R., Deitch E. A., Haskó G. (2010) A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J. Immunol. 185, 542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D. S., Yuan Y. H., Tu H. J., Liang Q. L., Dai L. J. (2009) A protocol for islet isolation from mouse pancreas. Nat. Protoc. 4, 1649–1652 [DOI] [PubMed] [Google Scholar]
- 35.Stull N. D., Breite A., McCarthy R., Tersey S. A., Mirmira R. G. (2012) Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J. Vis. Exp. 7:4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szot G. L., Koudria P., Bluestone J. A. (2007) Murine pancreatic islet isolation. J. Vis. Exp., 25, (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Géhl Z., Bai P., Bodnár E., Emri G., Remenyik É., Németh J., Gergely P., Virág L., Szabó É. (2012) Poly(ADP-ribose) in the skin and in melanomas. Histol. Histopathol. 27, 651–659 [DOI] [PubMed] [Google Scholar]
- 38.Bai P., Canto C., Brunyánszki A., Huber A., Szántó M., Cen Y., Yamamoto H., Houten S. M., Kiss B., Oudart H., Gergely P., Menissier-de Murcia J., Schreiber V., Sauve A. A., Auwerx J. (2011) PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 13, 450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csóka B., Koscsó B., Töro G., Kókai E., Virág L., Németh Z. H., Pacher P., Bai P., Haskó G. (2014) A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes 63, 850–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burkart V., Wang Z. Q., Radons J., Heller B., Herceg Z., Stingl L., Wagner E. F., Kolb H. (1999) Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat. Med. 5, 314–319 [DOI] [PubMed] [Google Scholar]
- 41.Linnemann A. K., Baan M., Davis D. B. (2014) Pancreatic β-cell proliferation in obesity. Adv. Nutr. 5, 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston-Cox H., Eisenstein A. S., Koupenova M., Carroll S., Ravid K. (2014) The macrophage A2B adenosine receptor regulates tissue insulin sensitivity. PLoS One 9, e98775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haskó G., Kuhel D. G., Chen J. F., Schwarzschild M. A., Deitch E. A., Mabley J. G., Marton A., Szabó C. (2000) Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 14, 2065–2074 [DOI] [PubMed] [Google Scholar]
- 44.Pinhal-Enfield G., Ramanathan M., Hasko G., Vogel S. N., Salzman A. L., Boons G. J., Leibovich S. J. (2003) An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am. J. Pathol. 163, 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Németh Z. H., Csóka B., Wilmanski J., Xu D., Lu Q., Ledent C., Deitch E. A., Pacher P., Spolarics Z., Haskó G. (2006) Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J. Immunol. 176, 5616–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Csóka B., Németh Z. H., Virág L., Gergely P., Leibovich S. J., Pacher P., Sun C. X., Blackburn M. R., Vizi E. S., Deitch E. A., Haskó G. (2007) A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 110, 2685–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haskó G., Pacher P. (2008) A2A receptors in inflammation and injury: lessons learned from transgenic animals. J. Leukoc. Biol. 83, 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Csóka B., Selmeczy Z., Koscsó B., Németh Z. H., Pacher P., Murray P. J., Kepka-Lenhart D., Morris S. M. Jr., Gause W. C., Leibovich S. J., Haskó G. (2012) Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 26, 376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A. S., Obin M. S. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 [DOI] [PubMed] [Google Scholar]
- 50.Csóka B., Németh Z. H., Törő G., Koscsó B., Kókai E., Robson S. C., Enjyoji K., Rolandelli R. H., Erdélyi K., Pacher P., Haskó G. (2015) CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J. 29, 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J. F., Lee C. F., Chern Y. (2014) Adenosine receptor neurobiology: overview. Int. Rev. Neurobiol. 119, 1–49 [DOI] [PubMed] [Google Scholar]
- 52.Ohtani M., Oka T., Ohura K. (2013) Possible involvement of A2A and A3 receptors in modulation of insulin secretion and β-cell survival in mouse pancreatic islets. Gen. Comp. Endocrinol. 187, 86–94 [DOI] [PubMed] [Google Scholar]
- 53.Gao T., McKenna B., Li C., Reichert M., Nguyen J., Singh T., Yang C., Pannikar A., Doliba N., Zhang T., Stoffers D. A., Edlund H., Matschinsky F., Stein R., Stanger B. Z. (2014) Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 19, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puri S., Akiyama H., Hebrok M. (2013) VHL-mediated disruption of Sox9 activity compromises β-cell identity and results in diabetes mellitus. Genes Dev. 27, 2563–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo S., Dai C., Guo M., Taylor B., Harmon J. S., Sander M., Robertson R. P., Powers A. C., Stein R. (2013) Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 123, 3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng Z., Borea P. A., Varani K., Wilder T., Yee H., Chiriboga L., Blackburn M. R., Azzena G., Resta G., Cronstein B. N. (2009) Adenosine signaling contributes to ethanol-induced fatty liver in mice. J. Clin. Invest. 119, 582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anand B. K., Brobeck J. R. (1951) Localization of a “feeding center” in the hypothalamus of the rat. Proc. Soc. Exp. Biol. Med. 77, 323–324 [DOI] [PubMed] [Google Scholar]
- 58.Gnad T., Scheibler S., von Kügelgen I., Scheele C., Kilić A., Glöde A., Hoffmann L. S., Reverte-Salisa L., Horn P., Mutlu S., El-Tayeb A., Kranz M., Deuther-Conrad W., Brust P., Lidell M. E., Betz M. J., Enerbäck S., Schrader J., Yegutkin G. G., Müller C. E., Pfeifer A. (2014) Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 [DOI] [PubMed] [Google Scholar]
- 59.Girgis C. M., Cheng K., Scott C. H., Gunton J. E. (2012) Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol. Metab. 23, 372–380 [DOI] [PubMed] [Google Scholar]
- 60.Grenz A., Clambey E., Eltzschig H. K. (2012) Hypoxia signaling during intestinal ischemia and inflammation. Curr. Opin. Crit. Care 18, 178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hart M. L., Grenz A., Gorzolla I. C., Schittenhelm J., Dalton J. H., Eltzschig H. K. (2011) Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J. Immunol. 186, 4367–4374 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Eckle T., Kewley E. M., Brodsky K. S., Tak E., Bonney S., Gobel M., Anderson D., Glover L. E., Riegel A. K., Colgan S. P., Eltzschig H. K. (2014) Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J. Immunol. 192, 1249–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eltzschig H. K., Rivera-Nieves J., Colgan S. P. (2009) Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin. Ther. Targets 13, 1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sitkovsky M. V. (2009) T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 30, 102–108 [DOI] [PubMed] [Google Scholar]
- 65.Sitkovsky M. V., Hatfield S., Abbott R., Belikoff B., Lukashev D., Ohta A. (2014) Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol. Res. 2, 598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]