Abstract
Spermatogenesis is a highly coordinated process that requires tightly regulated gene expression programmed by transcription factors and epigenetic modifiers. In this study, we found that nuclear respiratory factor (NRF)-1, a key transcription factor for mitochondrial biogenesis, cooperated with DNA methylation to directly regulate the expression of multiple germ cell–specific genes, including Asz1. In addition, conditional ablation of NRF1 in gonocytes dramatically down-regulated these germline genes, blocked germ cell proliferation, and subsequently led to male infertility in mice. Our data highlight a precise crosstalk between transcriptional regulation by NRF1 and epigenetic modulation during germ cell development and unequivocally demonstrate a novel role of NRF1 in spermatogenesis.—Wang, J., Tang, C., Wang, Q., Su, J., Ni, T., Yang, W., Wang, Y., Chen, W., Liu, X., Wang, S., Zhang, J., Song, H., Zhu, J., Wang, Y. NRF1 coordinates with DNA methylation to regulate spermatogenesis.
Keywords: ASZ1, germline gene regulation, mitochondria
All sexually reproducing organisms arise from gametes, by which the genetic information is passed across generations (1). Soon after the formation of primordial germ cells (PGCs), the initial population of mammalian germ cell precursors migrates to the genital ridge (2). Male gonocytes then cease dividing and resume proliferation as prospermatogonia after birth and become spermatogonial stem cells (SSCs) for further differentiation (3–6). In mice, spermatocytes start to develop from SSCs via meiosis at about postnatal day (P)7 and finally mature into sperm during a process called spermatogenesis (3–6). The fate specification, proliferation, and differentiation of germ cells are tightly orchestrated by precise coordination of several key regulators, including RNA binding proteins, transcriptional factors, and signaling molecules.
Among them, ankyrin repeat, sterile α-motif and basic leucine zipper domain–containing (ASZ)-1 protein is an important germ cell–specific protein that is first detected in postmigrating PGCs ∼12.5 d postcoitum (dpc) (7) and is highly expressed in spermatocytes from adult testis (8). Previous reports have demonstrated that ASZ1 is localized at the mitochondrial outer membrane at nuage, a specialized electron-dense cytoplasmic structure, where it modulates piwi-interacting RNA biosynthesis in germ cells, probably through interaction with RNA-processing proteins, such as miwi-like (MILI) and DEAD-box 4 (DDX4) proteins (9, 10). Either deletion of ASZ1 or mutation of its mitochondrial localization signal leads to male infertility in mice (9, 10). Similarly, gene-knockout studies have revealed the essential roles and similar expression patterns of RNA-binding proteins such as deleted in azoospermia-like (DAZL), DDX25, and NANOS (Drosophila NOS homolog) family members in germ cell development (11–14). It is of great interest to understand how these key players are regulated and synchronized at the transcriptional level during the process of spermatogenesis.
Both epigenetic modifications and transcription factors play essential roles in modulating gene expression in spermatogenesis. For example, the transcriptional repressor B-lymphocyte–induced maturation protein 1 (BLIMP1) associates with protein arginine N-methyltransferase 5 (PRMT5), a histone methyltransferase, to participate in PGC maintenance (15), and the transcript levels of key germ cell–specific genes, such as Ddx4 and Dazl, may be regulated by DNA methylation (16, 17). On the other hand, transcription factors such as promyelocytic leukemia zinc finger (PLZF), neurogenin-3, and spermatogenesis and oogenesis specific basic helix-loop-helix (SOHLH) family members modulate many genes known for SSC self-renewal and differentiation (18). Nevertheless, the interplay between epigenetic regulation, such as DNA methylation and transcription factors, largely remains a fundamental unanswered question in the field of developmental biology.
Nuclear respiratory factor 1 (NRF1) is a transcription factor known for its role in mitochondrial biogenesis (19). NRF1 was first identified through its binding to the promoter of cytochrome c (20) and was subsequently shown to regulate the expression of genes involved in the mitochondrial respiratory chain (21, 22). Genome-wide chromatin immunoprecipitation (ChIP) analyses have uncovered thousands of NRF1-binding sites in somatic cells (23, 24), indicating much broader roles for NRF1 than have been originally defined. A recent report demonstrated that NRF1 regulates the expression of piwi during chicken spermatogenesis (25). However, as homologous disruption of NRF1 leads to embryonic lethality between 3.5 and 6.5 dpc (26), its physiologic function and target genes in mammalian postnatal spermatogenesis has not been determined. In this study, we found that NRF1 was bound to the cytosine-guanosine dinucleotide (CpG)–rich region of the Asz1 promoter and coordinated with DNA methylation to activate Asz1 expression. Further analyses demonstrate that NRF1 deletion results in down-regulation of multiple germ cell–related genes, which in turn leads to male infertility. These results therefore reveal an essential requirement for NRF1 in transcriptional regulation during germ cell development.
MATERIALS AND METHODS
Cell culture and establishing a NRF1 conditional-knockout mouse model
Mouse embryonic fibroblasts (MEFs), NIH3T3, 293T, and GC1 cells were cultured, transfected, or infected according to published protocols (7, 9). All were originally from American Type Culture Collection (Manassas VA, USA), except the MEFs, which were made from mouse embryos at 13.5 dpc. All were tested for the presence of bacteria, yeast, or mycoplasma and found to be free of contamination. Mouse embryonic stem cells (ESCs) were maintained in medium with 1000 U/ml of leukemia inhibitory factor (LIF; Millipore-Sigma, St. Louis, MO, USA) on γ-irradiated MEFs. To generate a NRF1 conditional-knockout ESC line, V6.5 ESCs (a kind gift from Dr. Rudolf Jaenisch, Massachusetts Institute of Technology, Cambridge, MA, USA) were electroporated with linearized NRF1 targeting construct (CSD22636, KOMP Repository) at 500 μF, 250 V on an electroporator (Bio-Rad Shanghai, Shanghai, China) and selected for G418 individual clones. Two ESC clones harboring the NRF1 floxed allele were further introduced by electroporation with an Flp-puromycin plasmid at 500 μF and 250 V to delete the LacZ-Neo insertion. The ESCs, with a normal karyotype and a NRF1 conditional-knockout allele, were injected into C57BL/6 blastocysts. Design and characterization of the conditional Nrf1 ESC and mouse model are described in detail in Supplemental Fig. S2. The Nrf1f/+ and Nrf1f/Δ-Cre progenies were obtained by crossing Nrf1f/f female mice with Nrf1f/+-Cre male animals (with Ddx4-Cre mice from Model Animal Research Center at Nanjing University). All offspring were genotyped by PCR. All animal experimental procedures (AR2013/05007) were conducted in accordance with the local Animal Welfare Act and Public Health Service Policy and approved by the Committee of Animal Experimental Ethics at East China Normal University.
Plasmid construction
The Nrf1 gene was amplified from a cDNA library of mouse testis. DNA fragments containing Asz1, Lin28a, or Ddx25 promoter were cloned upstream of a firefly luciferase cDNA in pGL3. Mutant Asz1 promoter with deletion of NRF1 binding sites was established by PCR mutagenesis. Primers for cloning and mutagenesis are listed in Supplemental Table S4.
Gel shift assays
GST-fusion proteins were incubated with FITC- or 32P-labeled probes before separation on 6% nondenaturing polyacrylamide gel, and analysis on a Phosphoimager for 32P (Molecular Dynamics, Sunnyvale, CA, USA) or on an Image Station 400 MM Pro for FITC (Eastman Kodak, Rochester, NY, USA) . For competition assays, a 100-fold molar excess of unlabeled, mutant, or methylated probes was included in the same binding reaction. For the super-shift assay, reaction was incubated with an NRF1 antibody (Santa Cruz Biotechnology, Dallas, TX USA) after binding with the labeled probes. Probe sequences are listed in Supplemental Table S4.
Dual luciferase reporter assay
293T cells were cotransfected with firefly luciferase driven by Asz1, Ddx25, or Lin28a promoters, Renilla luciferase, and NRF1-expressing plasmids. The firefly luciferase activity relative to Renilla luciferase activity after transfection was measured with a Dual-Gloluciferase kit (Promega, Madison, WI, USA). To methylate promoter in vitro, a pAsz1-Luc plasmid was treated with M.SssI (New England Biolabs, Ipswich, MA, USA), according to the manufacturer’s instructions.
Bisulfate sequencing
Genomic DNA was treated with 6 N NaOH before the conversion solution (107 μl of 4.04 M NaHSO3, 7 μl of 10 mM hydroquinone, and 6 μl of 6N NaOH) was added. Fifteen cycles of DNA, denatured at 95°C for 30 s, was followed by bisulfate treatment for 15 min at 50°C. Nested PCR was performed to amplify the DNA methylation region, except Mov10L1, which was amplified by only 1 round of PCR. Sample treatment and processing among different samples were performed simultaneously for quality-control purposes.
ChIP-PCR and ChIP followed by sequencing
For ChIP, tissues were fixed in 1% formaldehyde for 10 min at 37°C and sonicated to get DNA fragments at 200–1000 bp. Lysates were precipitated with an NRF1 antibody (ab34682, 1:100; Abcam, Cambridge, MA, USA), and targeted DNA was analyzed by real-time PCR or prepared for ChIP followed by sequencing (ChIP-seq) libraries according to published protocols (27). All ChIP-seq libraries were sequenced on a HiSeq 2000 instrument (Illumina, San Diego, CA, USA) and analyzed (28).
Western blots, RT-PCR, real-time PCR, and methylation-sensitive restriction enzyme-PCR
Western blots, RT-PCR, and real-time PCR were performed as published (9). For methylation-sensitive restriction enzyme-PCR (MSRE-PCR), genomic DNA was treated with MspI or HpaII at 37°C for 2 h before PCR analysis. Antibodies against the following proteins were used in this study: ASZ1 (a kind gift from Dr. Martin Matzuk (Baylor College of Medicine, Houston, TX, USA), 1:50) (10), MILI (5940, 1:500), and LIN28a (8641, 1:500) from Cell Signaling Technology (Danvers, MA, USA), β-ACTIN (A5441, 1:1000; Millipore-Sigma), DAZL (ab34139, 1:1000), cytochrome c (ab13575, 1:1000), NRF1 (ab34682, 1:1000), and cytochrome c oxidase (COX) subunit 4 (ab33985, 1:1000) from Abcam. Primers are listed in Supplemental Table S4.
Flow cytometry
Dissociated cells from testis were stained with antibodies against CD9 (1:50; eBioscience, San Diego, CA, USA), C-KIT (1:200; BD Biosciences, San Jose, CA, USA), and Hoechst33342 (Thermo Fisher Scientific, Waltham, MA, USA), before sorting with FACSAria II (BD Biosciences).
Histology, immunohistofluorescence, immunohistochemistry, and TUNEL assay
Mouse testis and epididymis were fixed in Bouin’s fixative at 4°C overnight before paraffin embedding. Testicle sections at 5 μm thick were stained with hematoxylin and eosin (Millipore-Sigma) or with appropriate antibodies for immunohistofluorescence (IHF) and immunohistochemistry (IHC). Apoptotic cells were detected by a TUNEL-FITC-Apoptosis Detection Kit (Vazyme Biotech, Nanjing, China). Testicle sections were visualized under a confocal TCS SP5 microscope orDM4000B (Leica, Wetzlar, Germany). Primary antibodies used in IHF and IHC were the same ones for Western blot analyses except for PLZF (sc-28319, 1:50; Santa Cruz Biotechnology), PCNA (ab29, 1:400), DDX4 (ab27591, 1:400), antigerm cell-specific antigen antibody produced from hybridoma clone 98 (TRA98) (ab82527, 1:500), and heat-shock protein-60 (ab46978, 1:500) from Abcam.
Histochemistry of COX/NADH dehydrogenase/succinate dehydrogenase
Cryostat-cut testicle sections (12 μm) were prepared from freshly frozen testis samples, and the activities of COX/NADH dehydrogenase/succinate dehydrogenase (SDH) on testicle sections were measured according to published protocols (29) and on-line procedures from the Washington University Neuromuscular Disease Center (St. Louis, MO, USA; http://neuromuscular.wustl.edu/index.html). Slides were incubated with corresponding COX (40 min), SDH (60 min), and NADH dehydrogenase (30 min) reaction buffer at 37°C separately before visualization.
Intracellular ROS measurement
The ROS levels were measured with the ROS detection kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instruction. In brief, the dissociated cells from testis were incubated with 10 µM H2DCFDA in DMEM for 30 min at 37°C, and the CD9+ cells were analyzed for the fluorescent derivative of H2DCFDA upon reactive oxygen species oxidation with BD FACSCalibur (BD Biosciences).
Bioinformatics prediction and statistical analysis
The prediction of binding motifs for transcription factors was performed with online software: rVista 2.0 (http://rvista.dcode.org/) and Jaspar (http://jaspar.genereg.net/). All statistical analyses were performed with the F test (to estimate the variance within each group of data) and the unpaired Student’s t test (for comparison between groups) with Prism software (GraphPad, La Jolla, CA, USA).
RESULTS
The expression of Asz1 is regulated by DNA methylation
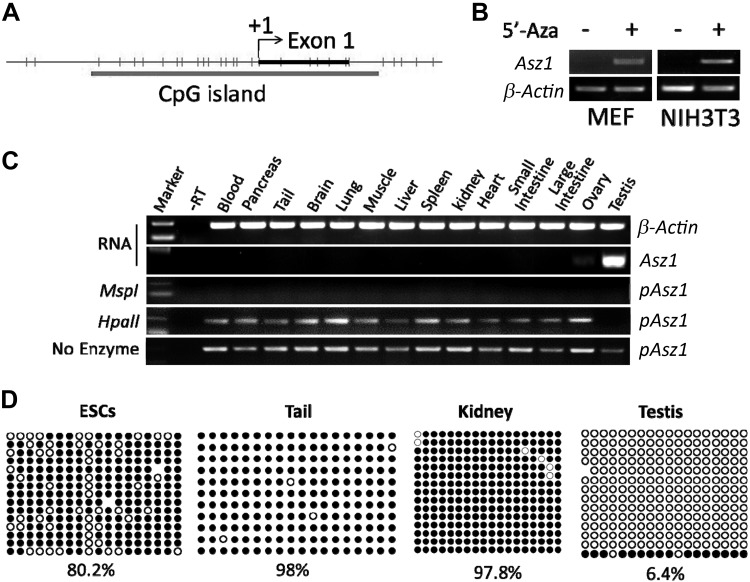
When exploring the upstream regulators of ASZ1, a key player in spermatogenesis, we uncovered a CpG-rich region at its promoter (Fig. 1A), indicating that ASZ1 expression was regulated by DNA methylation. Asz1 is a germ cell–specific gene that is not expressed in somatic cells such as MEFs or NIH3T3 cells. Upon treatment by 5-aza-2′-deoxycytidine (5′-Aza), an inhibitor of DNA methyltransferase, Asz1 was significantly induced in these somatic cells (Fig. 1B), suggesting that DNA methylation is responsible for suppressing Asz1 transcription in non–germ cell populations. This result was confirmed in adult mouse tissues by MSRE-PCR (30). In this assay, genomic DNA from various tissues was treated with a methylation-sensitive restriction enzyme HpaII, after which the Asz1 promoter was amplified by PCR. No PCR product from testis was detected with prior HpaII treatment, but was present in other tissues that had little or no Asz1 expression (Fig. 1C and Table 1). This result indicates that the Asz1 promoter is hypermethylated in somatic tissues, but not in testis. As a control, no visible band of PCR product from any tissue was observed in the presence of MspI, a methylation insensitive isoenzyme of HpaII.
Figure 1.
The expression of ASZ1 is regulated by DNA methylation. A) Asz1 promoter contains multiple CpG-rich sites. B) Transcript levels of ASZ1 in MEF and NIH3T3 cells in the absence or presence of 5′-Aza by RT-PCR. C) RT-PCR of Asz1 and β-actin on total RNAs or MSRE-PCR on the promoter region of Asz1 (pAsz1) from various tissues. The deduction of methylation status and expression level of Asz1 was summarized in Table 1. No expression of Asz1 was detected when its promoter was methylated, which was indicated by a band present with prior treatment with HpaII. D) Bisulfate sequencing of the Asz1 promoter on genomic DNA. White circles: unmethylated CpG; black circles: methylated CpGs.
TABLE 1.
Interpretation of DNA methylation by MSRE-PCR
| PCR Product |
Expression | Methylation | ||
|---|---|---|---|---|
| No enzyme | HpaII | MspI | ||
| Present | Present | Absent | No/low | Yes |
| Present | Absent | Absent | High | No |
We further examined the methylation status of the CpG-rich region of Asz1 by bisulfate sequencing. We observed that this region was highly methylated in cells or tissues having little or no Asz1 transcripts (e.g., ESCs, tail, and kidney), but the methylation level was dramatically lower in testis when Asz1 was highly expressed (Fig. 1D and Supplemental Fig. S1A). Taken together, these data suggest that Asz1 expression is regulated by DNA methylation.
NRF1 coordinates with DNA methylation to modulate the expression of Asz1
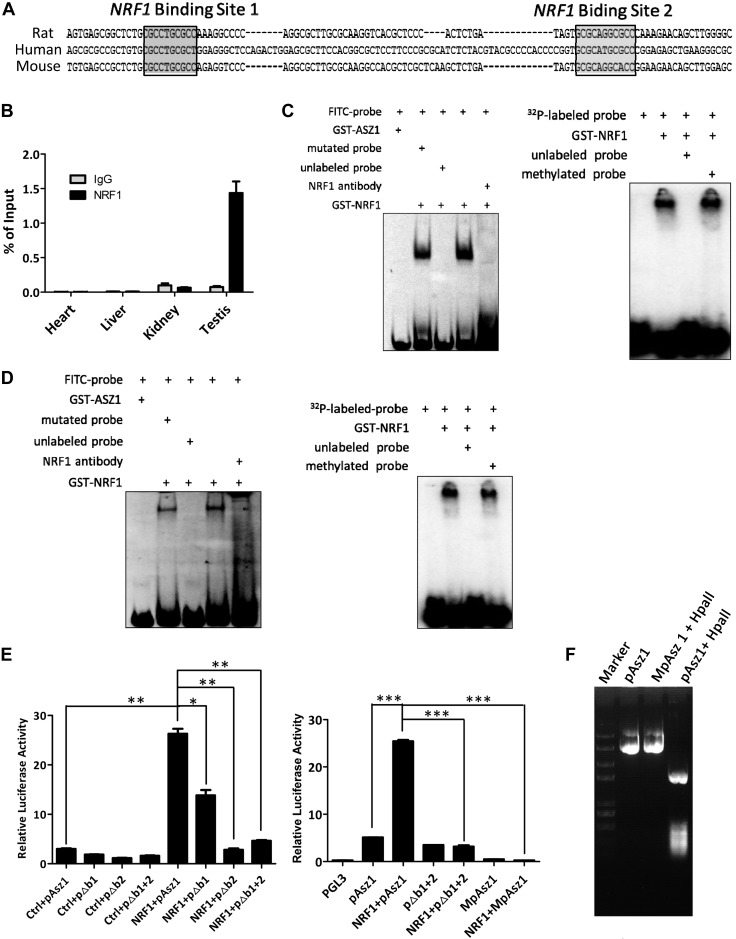
At the CpG-rich region of the Asz1 promoter, we also found 2 NRF1-binding motifs via bioinformatics prediction (Fig. 2A). We thus determined whether NRF1 could be recruited to these sites in various tissues. ChIP-PCR analyses demonstrated that NRF1 was mainly bound to the promoter of Asz1 in testis (Fig. 2B and Supplemental Fig. S1C). As the NRF1 binding motif contains CpG sequence, these results prompted us to elucidate the relationship of NRF1 binding and DNA methylation at the Asz1 promoter. In gel shift assays, NRF1 was bound to both predicted targeting sequences (labeled with FITC or 32P), and this binding could be disrupted by unlabeled probes or an NRF1 antibody, but not by methylated probes (Fig. 2C, D), suggesting that NRF1 selectively associates with unmethylated DNA sequences. We further determined the influence of DNA methylation and NRF1 on transcription using luciferase reporter assays. Notably, NRF1 efficiently up-regulated the luciferase activity driven by the Asz1 promoter (Fig. 2E), yet this up-regulation was significantly decreased by deleting either of the 2 NRF1 binding motifs, or by methylation of Asz1 promoter with a CpG methyltransferase (M.SssI) (Fig. 2E, F). Thus, Asz1 expression appears to be regulated by both DNA methylation and NRF1 transactivation.
Figure 2.
NRF1 coordinates with DNA methylation to modulate ASZ1 expression. A) Asz1 core promoter contains 2 NRF1 binding sites. B) ChIP assays followed by real-time PCR analyses with a NRF1 antibody from various tissues on the Asz1 promoter containing predicated NRF1 binding sites. C, D) Gel shift assays of a GST-NRF1 fusion protein with synthesized FITC- or 32P-labeled probe containing NRF1 binding sites 1 (C) and 2 (D), respectively. The GST-ASZ1 fusion protein was used as a negative control. E) Activities of luciferase driven by wild-type, mutated (with 1 or both NRF1 binding site deleted: e.g., pΔb1), or methylated promoter of Asz1 were measured upon cotransfection of a NRF1 transgene or an empty vector control (ctrl). The pGL3 is an additional vector control without Asz1 promoter. Relative firefly luciferase activity is normalized to the Renilla and represented as mean ± 1 sem from 6 biologic replicates. *P < 0.05, **P < 0.01, ***P < 0.001. F) Vector containing the Asz1 promoter (pASZ1 in E) was treated with or without CpG methyltransferase M.SssI (MpAsz1) and methylation status was confirmed by digestion of the methylation-sensitive restriction enzyme HpaII.
NRF1 is enriched at the promoters of multiple germline genes to regulate their expression
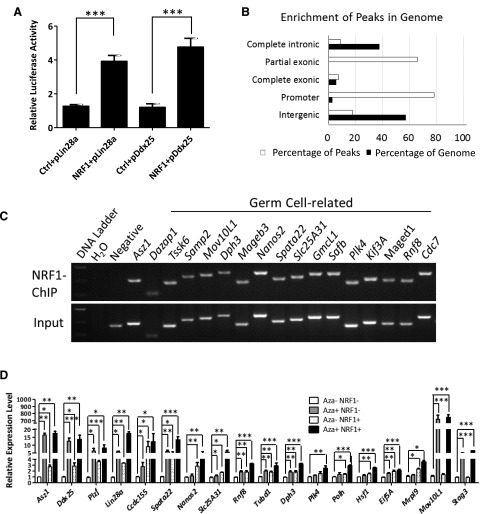
To determine whether ASZ1 is the sole target of NRF1 during germ cell development, we searched for the NRF1 binding sites in other germ cell–related genes. We found that promoters of multiple genes contained NRF1 binding sequences, including Lin28a and Ddx25 (Supplemental Table S1). In addition, NRF1 increased the luciferase activities driven by the promoter of either Lin28a or Ddx25 (Fig. 3A). These results indicate a broad role of NRF1 in mammalian reproduction. To fully identify genes that were directly regulated by NRF1 in the male reproductive organ, we performed genome-wide ChIP-seq analyses in mouse testes, using hearts from the same animals for comparison. In total, 457 genes from testes were identified to be bound by NRF1 (Supplemental Fig. 1D and Supplemental Table S2), and out of these NRF1 binding peaks, 78.1% resided in the promoter regions, showing a 30-fold enrichment over the percentage of promoter occupancy at the whole-genome level (Fig. 3B). This observation is consistent with the role of NRF1 as a transcription factor. NRF1 target genes identified by ChIP-seq were further validated by independent ChIP-PCR analyses (Fig. 3C and Supplemental Fig. 1E).
Figure 3.
NRF1 targets multiple germ cell–specific genes in testes. A) Luciferase activities driven by Lin28a or Ddx25 promoters containing NRF1 binding sites were examined upon cotransfection of an NRF1 transgene or a vector control (ctrl). Relative firefly luciferase activity was normalized to Renilla activity and represented as means ± 1 sem from 6 biologic replicates. ***P < 0.001. B) NRF1 is mainly enriched at promoter regions of its targeted genes. C) Confirmation of NRF1 target sites by ChIP-PCR with an antibody against NRF1 from wild-type adult testes. D) GC1 cells were treated with or without 5′-Aza and transfected with a vector control or a NRF1-expressing plasmid. Transcript levels of NRF1 target genes were measured by real-time RT-PCR and represented as mean ± 1 sem from 6 biologic replicates. *P < 0.05, **P < 0.01, ***P < 0.001. Minus indicates without; plus denotes in the presence of 5′-Aza or the NRF1 transgene.
A total of 161 NRF1 target genes were found in both testes and hearts (Supplemental Fig. S1D), some of which are involved in metabolism (Supplemental Fig. S1D, F), consistent with the established role of NRF1 in regulating mitochondrial activities. Gene ontology analyses further demonstrated the NRF1-bound genes from testis were highly enriched in the functional category with relevance to male infertility (Fig. 3C and Supplemental Fig. S1E, G). These genes included Asz1, Tssk6, Mov10L1, Stag3, and Nanos2, among others (Supplemental Table S2). In addition, genes involved in DNA stability and DNA repair (including Xrcc2, Lig4, Nhej1, and Rad23) were overrepresented in NRF1 target genes, implying that NRF1 also participates in cellular processes in response to DNA damage (Supplemental Fig. 1G and Supplemental Table S2).
As NRF1 binding to Asz1 promoter was shown to be methylation sensitive (Fig. 2), we investigated whether NRF1 coordinated with DNA methylation to regulate the expression of these newly identified target genes. We used GC1, a type B spermatogonial cell line immortalized by simian virus 40 large T antigen (31). We treated GC1 with the DNA methyltransferase inhibitor 5′-Aza, ectopic NRF1 expression, or both, and examined the target gene expression by using real-time RT-PCR. Indeed, either suppression of DNA methylation or enforced NRF1 expression significantly up-regulated the NRF1 target genes identified by ChIP-seq (Fig. 3D). Notably, because the promoters of germline genes were not completely methylated in GC1 cells (Supplemental Fig. S2A), an increase in NRF1 expression activated its target genes in the absence of 5′-Aza treatment. In addition, many of the NRF1 target genes (e.g., Lin28a, Spata22, Slc25A31, and Rnf8) exhibited a synergistic increase in expression upon 5′-Aza treatment and NRF1 stimulation, thus suggesting a coordinated regulation of gene transcription between DNA methylation and NRF1 transactivation. Because the level of endogenous NRF1 expression is high, an additional exogenous NRF1 transgene did not further augment the transcript levels of some of its target genes from 5′-Aza treatment.
Conditional deletion of NRF1 in germ cells leads to male infertility
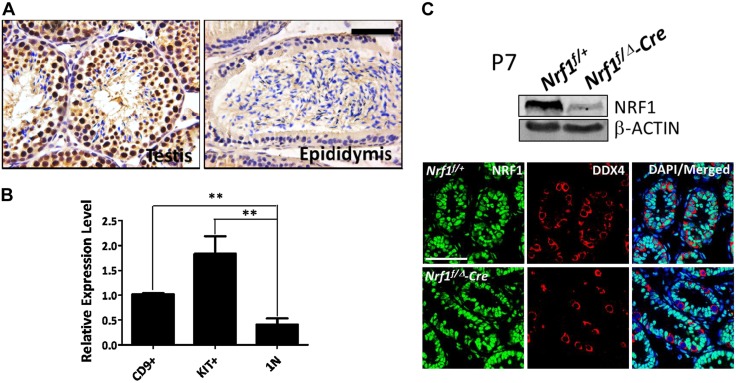
Given that NRF1 modulated the expression of multiple germ cell–related genes, we next investigated the physiologic role of NRF1 in spermatogenesis. IHC showed that NRF1 was highly expressed in both spermatogonia and spermatocytes (Fig. 4A). Notably, although mitochondrial metabolism is necessary for round spermatids and spermatozoa, very low level of NRF1 expression was found in spermatids from testes, and no NRF1 protein was detected in sperm from epididymis. IHF analyses yielded similar results. Costaining of NRF1 and PLZF (a protein expressed in spermatogonial stem cells) or of NRF1 and DDX4 (in both spermatogonia and spermatocytes) was clearly observed (Supplemental Fig. S2B). The differential expression of NRF1 in testis was further confirmed by real-time RT-PCR, in which NRF1 was expressed at a much lower level in sorted haploid spermatids compared with CD9+/c-Kit− spermatogonia and CD9−/c-Kit+ spermatocytes (Fig. 4B).
Figure 4.
Establishing Nrf1 conditional-knockout ESCs and mouse model. A) NRF1 protein was examined by IHC. B) Transcript level of NRF1 was measured by real-time RT-PCR in CD9+/C-KIT− spermatogonia, C-KIT+ spermatocytes, and haploid spermatids from wild-type adult testes. Data are means ± 1 sem from 4 biologic replicates. **P < 0.01. C) The NRF1 protein level was measured by Western blot and IHF in testes with conditional deletion of Nrf1 at P7, compared with their wild-type littermates. Arrowheads: the DDX4+ germ cells that were devoid of NRF1 expression in Nrf1f/Δ-Cre testicle sections. Scale bars, 50 μm.
To bypass the problem of embryonic lethality caused by the Nrf1-null mutation, we established Nrf1 conditional-knockout (Nrf1f/f) mice. We crossed these mice with a Ddx4-Cre line to obtain Nrf1 deletion, specifically in germ cells (Fig. 3A and Supplemental Fig. S2C–F), as Cre recombinase under the promoter of Ddx4 starts to express only in gonocytes at ∼15.5 dpc (32). The specific ablation of NRF1 in germ cells from testis was confirmed by Western blot and IHF (Fig. 4C). Nrf1f/Δ-Cre mice were born at appropriate mendelian frequencies and appeared to be physiologically normal, and we observed no obvious alteration in the reproductive organs of heterozygous (Nrf1f/Δ) mice (Supplemental Fig. S3B).
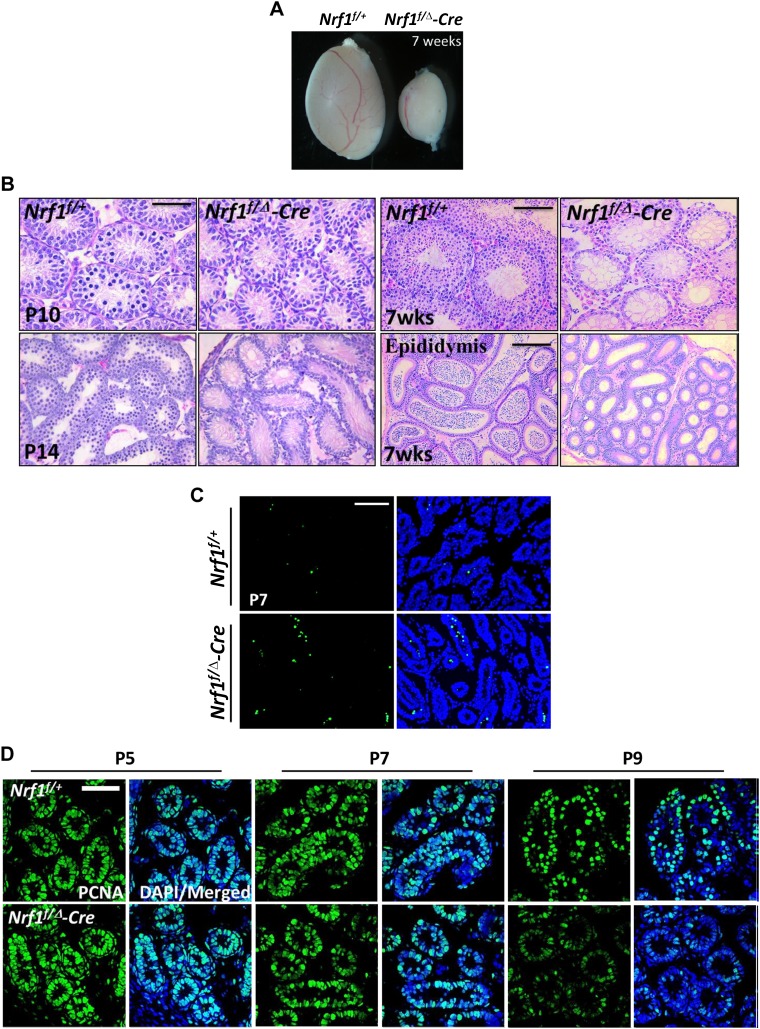
We dissected the testes of Nrf1f/Δ-Cre and Nrf1f/+ mice at different time points, and found significantly smaller testes in Nrf1-null adult mice (Fig. 5A), but no obvious changes were observed at P7 (Supplemental Fig. S3C). Blocked development of Nrf1f/Δ-Cre testes with fewer spermatocytes was first observed at P10 and progressed rapidly (Fig. 5B). By P14, no spermatocytes in Nrf1f/Δ-Cre testes were detected, whereas most Nrf1f/+ tubules had advanced to the midpachytene and zygotene stages. At 7 wk after birth, few germ cells remained in Nrf1f/Δ-Cre testes, along with a complete absence of mature sperm in the epididymis (Fig. 5B and Supplemental Fig. 3C). In addition, increased apoptosis was observed in NRF1-deficient testes at P7 compared with wild-type littermates (Fig. 5C). Markedly reduced expression of proliferating cell nuclear antigen at P9 was also detected, indicating an arrest of germ cell proliferation upon Nrf1 knockout (Fig. 5D). As expected, Nrf1f/Δ-Cre male mice were infertile, thereby demonstrating an essential requirement of NRF1 in spermatogenesis.
Figure 5.
Conditional deletion of Nrf1 leads to male infertility. A) Gross morphology of testes from mice at 7 wk after birth. B) Histologic images of testicle sections or epididymis from wild-type mice or mice with Nrf1 deletion were obtained at different time points after birth. C, D) TUNEL assays (C) or PCNA staining (D) were performed on testes from wild-type control and mice with conditional Nrf1 deletion at various postnatal time points. Scale bars, 50 μm, testicle sections; 100 μm, epididymis sections.
NRF1 deficiency down-regulates the expression of germ cell–related genes
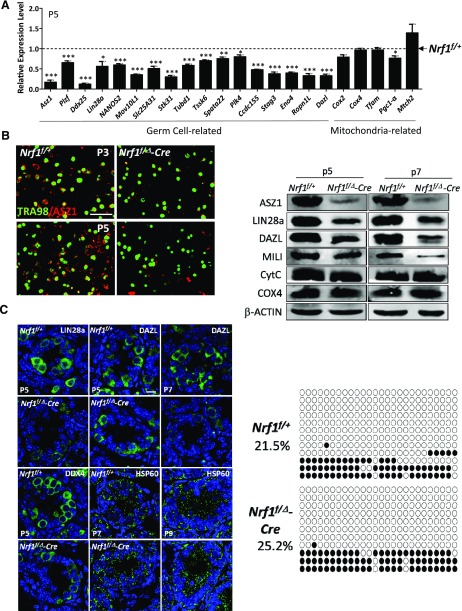
We further used the NRF1-knockout mouse model to evaluate whether NRF1 regulates the expression of its target genes in vivo. Indeed, quantitative RT-PCR analyses demonstrated that germ cell–specific genes, such as Asz1, Lin28a, and Ddx25, were significantly down-regulated in the NRF1-null testes (Fig. 6A and Supplemental Fig. S3D). In addition, whereas ASZ1 protein level was significantly down-regulated in NRF1-ablated germ cells at as early as P3 and P5, no obvious difference was detected with TRA98. (Fig. 6B). Thus, it is unlikely that the reduced expression of germline genes at early spermatogenesis is caused by a decreased number of germ cells.
Figure 6.
NRF1 deficiency leads to reduced expression of germ cell–specific genes. A) Real-time RT-PCR analyses on the testes with conditional deletion of Nrf1 in germ cells at P5, compared to their wild-type littermates. Data are the mean of relative expression from 6 replicates ± 1 sem. **P < 0.01, *P < 0.05, ***P < 0.001. B) IHF of ASZ1 and TRA98 in testes from wild-type mice or mice with Nrf1 conditional deletion. Scale bar, 50 μm. C) IHF of germ cell–related proteins (DDX4, Lin28a, and DAZL) and HSP60 (a mitochondrial protein) in testes from wild-type mice or mice with Nrf1 conditional deletion. Scale bar, 10 μm. D) Protein levels of NRF1 target genes and genes involved mitochondrial activities measured by Western blots on the testes. E) Bisulfate sequencing of the Asz1 promoter from wild-type control and mice with Nrf1 deletion at P8. White circles, unmethylated CpGs; black circles, methylated CpGs.
To discern whether the mitochondrial defects were responsible for the decreased transcription of germ cell–related factors, we examined the expression of key genes involved in mitochondrial activity including Cox2, Cox4, and transcription factor A, mitochondrial (Tfam). Notably, the transcript levels of these mitochondrial genes were not altered at early stages (P3–P7) of spermatogenesis upon Nrf1 ablation (Fig. 6A and Supplemental Fig. S3D). Similarly, the protein levels of DAZL, LIN28a, DDX4, and PLZF were decreased dramatically at various postnatal time points, whereas the expression of genes reflecting mitochondrial activities, such as HSP60, cytochrome c (CytC), and COX4 remained unaffected, whether examined by IHF (Fig. 6C and Supplemental Fig. S3E) or by Western blots (Fig. 6D). In addition, we did not observe any significant changes in the activities of mitochondrial respiratory enzyme complex in P7 NRF1-knockout testes (Supplemental Fig. S4A), including COX (cytochrome c oxidase in mitochondrial electron transport chain complex IV), SDH (succinate dehydrogenase in complex II), and NADH dehydrogenase (a component of complex I). We further examined the level of ROS in P7 testes as an indicator for mitochondrial activity, because ROS is mainly produced during mitochondrial oxidative phosphorylation. To minimize potential contamination of somatic cells, CD9+ spermatogonia from P7 testes were analyzed. We did not detect any obvious alteration in the CD9+ spermatogonia at P7 upon NRF1 deletion (Supplemental Fig. S4B). Collectively, these data essentially rule out the possibility of dysfunctional mitochondrial metabolism as a cause for the decreased expression of these germline genes in spermatogonia and thus are consistent with our hypothesis that NRF1 is directly involved in the transcriptional regulation during early spermatogenesis.
Because NRF1 coordinates with DNA methylation to regulate its target genes, we next examined whether DNA methylation status is affected by Nrf1 conditional deletion in isolated CD9+ spermatogonia (Supplemental Fig. S4C) by bisulfate sequencing. We chose to assess 6 NRF1 target genes that were highly expressed in germ cells and were down-regulated by NRF1 deficiency (Fig. 5A and Supplemental Fig. S4D, E). Compared to their wild-type controls, Nrf1-ablated germ cells showed no significant up-regulation of DNA methylation in the promoters of all 6 target genes (Fig. 6E and Supplemental Fig. 4D). Therefore, these data confirm that NRF1 deletion, but not increased DNA methylation, is responsible for reduced gene expression in testis.
DISCUSSION
DNA methylation is an epigenetic modification that regulates gene expression in mammalian development and in a wide range of cellular processes. Yet our data suggest that it plays a unique role in transcriptional modulation of germline genes. For instance, although the CpG-rich promoters in many lineage-specific genes are not methylated in somatic tissues, regardless of the low expression levels of their associated genes (33–35), we found that the transcription of germline genes (including Asz1, Lin28a, Nanos2, and Ddx25) were indeed inhibited by hypermethylation. As such, in somatic cells, the transcription factor NRF1 was excluded from its binding motifs with high methylation levels and thus was unable to activate gene expression. Therefore, the current evidence reinforces the claim that DNA methylation is a primary player in repressing germ cell–specific genes in somatic lineages (16, 36, 37).
Whereas DNA methylation can lead to inhibited transcription, hypomethylation in promoters alone does not necessarily correlate with high gene expression in germ cells (38). In addition, it has been reported that the level of DNA methylation does not show appreciable changes during spermatogenesis (38). Thus, the activation or the down-regulation of germline genes during the process of spermatogenesis requires participation of additional regulators. To this end, we observed that NRF1 was highly expressed in SSCs and spermatocytes, coincidental with the high expression of its target genes in early spermatogenesis. Further, the levels of these germline genes (including Asz1, Dazl, Lin28a, Ddx25, and Nonos2) in testis with the Nrf1-null mutation were dramatically decreased, even when their methylation status was not obviously altered. Therefore, our data clearly illuminate a novel role of NRF1 in early spermatogenesis as a crucial master regulator in the transcription of germline genes.
A recent study suggests that the association of DNA methylation–sensitive transcription factors, such as NRF1, to its targeted promoters depended on additional determinants to induce local hypomethylation (24). Notably, of 30 germ cell–related genes we examined, all contained more than 1 binding site for 11-zinc finger protein or CCCTC-binding factor (25 of 30 genes), regulatory factor X-1 (26 of 30), and RE1-silencing transcription factor (3 of 30), mostly located within 1 kb of the NRF1 targeting motifs (Supplemental Fig. S4D). These observations suggest that these superordinate factors act to maintain local hypomethylation that allows for NRF1 binding. Together with the observation of no significant difference in DNA methylation levels between Nrf1f/Δ-Cre and Nrf1f/+ testes, our data provide a biologic explanation of why loss of NRF1 binding alone is unable to alter DNA methylation of its target genes.
The infertility caused by NRF1 ablation in germ cells is likely a direct consequence of failure, to activate multiple crucial factors for SSC self-renewal and differentiation. The level of ASZ1 protein is dramatically reduced in testes at P3, before any notable changes in expression of the pan–germ-cell marker TRA98 or before the detection of increased apoptosis and blocked proliferation. These observations suggest that germ cells are intact in NRF1-null testes during early spermatogenesis and that the decreased expression of germline genes upon NRF1 deletion is not likely to be caused by a reduced number of germ cells at a later stage. These germline genes are down-regulated upon NRF1 ablation at different time points during spermatogenesis. The expression of ASZ1 decreases first, as early as at P3; DDX4 and Lin28a decrease at P5; followed by reduction of the DAZL and PLZF proteins at P7–P9. The phenomenon is possibly related to the distinct half-life or different post-transcriptional regulation of these genes, or alternatively, ASZ1 may be a critical target of NRF1 during germ cell development. In further support of our claim, a previous report demonstrated that a single-nucleotide polymorphism in the DAZL promoter was associated with decreased binding of NRF1 to this region and may confer susceptibility to male infertility in humans (39), thus indicating a conserved function of NRF1 in mammalian germ cell development.
Although the NRF1 was first described as a transcriptional activator for mitochondrial biogenesis, early embryonic death (before 6.5 dpc) in mice because of the Nrf1-null mutation (26) cannot be completely explained by the loss of mitochondrial DNA. For example, embryos with mutation of TFAM, another key transcription factor in mitochondrial DNA regulation, survive until 8.5–10.5 dpc (40), indicating that NRF1 participates in multiple biologic functions beyond mitochondrial biogenesis. Indeed, our data revealed a host of NRF1 target genes that are involved in the cell cycle (e.g., Cdc7, Cdc34, and Plk4), DNA repair (e.g., Xrcc2, Lig4, Nhej1, and Rad23), and germ cell development and therefore established a much broader spectrum for NRF1 in transcription regulation. Notably, spermatogonia rely more on glycolysis rather than on mitochondrial metabolism for energy production (41). Down-regulation of germ cell–related genes upon NRF1 deletion occurs before any significant alteration of mitochondrial metabolism, but it does not exclude the functional relevance of NRF1 to mitochondrial activities in late spermatogonial differentiation. Rather, it is possible that NRF1 plays active roles in both modulation of mitochondrial activities and direct regulation of germline genes in germ cell development. Our recent report demonstrated that ASZ1, the direct target of NRF1, is actually involved in regulating mitochondrial dynamics and metabolism in germ cells (9). Further investigation is warranted to unveil the biologic significance of NRF1 as a critical and multifunctional player in both metabolism and spermatogenesis.
ACKNOWLEDGMENTS
The authors thank Dr. Richard Mailman from Penn State College of Medicine (State College, PA, USA) for help in manuscript preparation. This work was supported by Grants 2016YFA0100300 and 2014CB964800 (to Yu.W.) from the Ministry of Science and Technology of China, Grants 31471347 and 31271589 (to Yu.W.) from the National Natural Science Foundation of China, Grants 16JC1404200 (to Yu.W.) and 11DZ2260300 from the Science and Technology Commission of Shanghai Municipality, China, and Grant 1ZICHL006058 (to J.Z.) from the Intramural Research Program of the U.S. National Institutes of Health, National Heart Lung Blood Institute. The authors declare no conflicts of interest.
Glossary
- 5′-Aza
5-aza-2′-deoxycytidine
- ASZ1
ankyrin repeat, sterile α-motif and basic leucine zipper domain–containing 1
- DAZL
deleted in azoospermia-like
- ChIP
chromatin immunoprecipitation
- ChIP-seq
chromatin immunoprecipitation followed by sequencing
- COX
cytochrome c oxidase
- CpG
cytosine-guanosine dinucleotide
- DDX4/25
DEAD-box 4/25
- dpc
day post coitum
- ESC
embryonic stem cell
- IHC
immunohistochemistry
- IHF
immunohistofluorescence
- MEF
mouse embryonic fibroblast
- MILI
miwi-like
- MSRE-PCR
methylation sensitive restriction enzyme-PCR
- NRF1
nuclear regulatory factor 1
- PGC
primordial germ cell
- PLZF
promyelocytic leukemia zinc finger
- ROS
reactive oxygen species
- SDH
succinate dehydrogenase
- SSC
spermatogonial stem cell
- TFAM
transcription factor A, mitochondrial
- TRA98
antigerm cell-specific antigen antibody produced from hybridoma clone 98
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Wang, Q. Wang, and Yu. Wang designed the research; J. Wang, C. Tang, Q. Wang, J. Su, T. Ni, W. Yang, Yo. Wang, W. Chen, X. Liu, S. Wang, J. Zhang, and H. Song performed experiments; J. Wang, J. Zhu, and Yu. Wang analyzed the data; and J. Wang, J. Zhu, and Yu. Wang wrote the paper.
REFERENCES
- 1.Wylie C. (1999) Germ cells. Cell 96, 165–174 [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg M., Snow M. H., McLaren A. (1990) Primordial germ cells in the mouse embryo during gastrulation. Development 110, 521–528 [DOI] [PubMed] [Google Scholar]
- 3.Ewen K. A., Koopman P. (2010) Mouse germ cell development: from specification to sex determination. Mol. Cell Endocrinol. 323, 76–93 [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K., de Sousa Lopes S. M., Surani M. A. (2007) Germ cell specification in mice. Science 316, 394–396 [DOI] [PubMed] [Google Scholar]
- 5.Saga Y. (2008) Mouse germ cell development during embryogenesis. Curr. Opin. Genet. Dev. 18, 337–341 [DOI] [PubMed] [Google Scholar]
- 6.Saitou M. (2009) Germ cell specification in mice. Curr. Opin. Genet. Dev. 19, 386–395 [DOI] [PubMed] [Google Scholar]
- 7.Wang Q., Liu X., Tang N., Archambeault D. R., Li J., Song H., Tang C., He B., Matzuk M. M., Wang Y. (2013) GASZ promotes germ cell derivation from embryonic stem cells. Stem Cell Res. 11, 845–860 [DOI] [PubMed] [Google Scholar]
- 8.Yan W., Rajkovic A., Viveiros M. M., Burns K. H., Eppig J. J., Matzuk M. M. (2002) Identification of Gasz, an evolutionarily conserved gene expressed exclusively in germ cells and encoding a protein with four ankyrin repeats, a sterile-alpha motif, and a basic leucine zipper. Mol. Endocrinol. 16, 1168–1184 [DOI] [PubMed] [Google Scholar]
- 9.Zhang J., Wang Q., Wang M., Jiang M., Wang Y., Sun Y., Wang J., Xie T., Tang C., Tang N., Song H., Cui D., Chao R., Ding S., Ni B., Chen X., Wang Y. (2016) GASZ and mitofusin-mediated mitochondrial functions are crucial for spermatogenesis. EMBO Rep. 17, 220–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L., Buchold G. M., Greenbaum M. P., Roy A., Burns K. H., Zhu H., Han D. Y., Harris R. A., Coarfa C., Gunaratne P. H., Yan W., Matzuk M. M. (2009) GASZ is essential for male meiosis and suppression of retrotransposon expression in the male germline. PLoS Genet. 5, e1000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggiu M., Speed R., Taggart M., McKay S. J., Kilanowski F., Saunders P., Dorin J., Cooke H. J. (1997) The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389, 73–77 [DOI] [PubMed] [Google Scholar]
- 12.Sada A., Suzuki A., Suzuki H., Saga Y. (2009) The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 325, 1394–1398 [DOI] [PubMed] [Google Scholar]
- 13.Tsai-Morris C. H., Sheng Y., Lee E., Lei K. J., Dufau M. L. (2004) Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc. Natl. Acad. Sci. USA 101, 6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuda M., Sasaoka Y., Kiso M., Abe K., Haraguchi S., Kobayashi S., Saga Y. (2003) Conserved role of nanos proteins in germ cell development. Science 301, 1239–1241 [DOI] [PubMed] [Google Scholar]
- 15.Ancelin K., Lange U. C., Hajkova P., Schneider R., Bannister A. J., Kouzarides T., Surani M. A. (2006) Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat. Cell Biol. 8, 623–630 [DOI] [PubMed] [Google Scholar]
- 16.Maatouk D. M., Kellam L. D., Mann M. R., Lei H., Li E., Bartolomei M. S., Resnick J. L. (2006) DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development 133, 3411–3418 [DOI] [PubMed] [Google Scholar]
- 17.Shen L., Kondo Y., Guo Y., Zhang J., Zhang L., Ahmed S., Shu J., Chen X., Waterland R. A., Issa J. P. (2007) Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet. 3, e181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song H. W., Wilkinson M. F. (2014) Transcriptional control of spermatogonial maintenance and differentiation. Semin. Cell Dev. Biol. 30, 14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarpulla R. C. (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta. 1813, 1269–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans M. J., Scarpulla R. C. (1989) Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter: characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. J. Biol. Chem. 264, 14361–14368 [PubMed] [Google Scholar]
- 21.Kelly D. P., Scarpulla R. C. (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 [DOI] [PubMed] [Google Scholar]
- 22.Scarpulla R. C. (2002) Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim. Biophys. Acta. 1576, 1–14 [DOI] [PubMed] [Google Scholar]
- 23.Cam H., Balciunaite E., Blais A., Spektor A., Scarpulla R. C., Young R., Kluger Y., Dynlacht B. D. (2004) A common set of gene regulatory networks links metabolism and growth inhibition. Mol. Cell 16, 399–411 [DOI] [PubMed] [Google Scholar]
- 24.Domcke S., Bardet A. F., Adrian Ginno P., Hartl D., Burger L., Schübeler D. (2015) Competition between DNA methylation and transcription factors determines binding of NRF1. Nature 528, 575–579 [DOI] [PubMed] [Google Scholar]
- 25.Qiu L., Xu L., Chang G., Guo Q., Liu X., Bi Y., Zhang Y., Wang H., Wang K., Lu W., Ren L., Zhu P., Wu Y., Zhang Y., Xu Q., Chen G. (2016) DNA methylation-mediated transcription factors regulate Piwil1 expression during chicken spermatogenesis. J. Reprod. Dev. 62, 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huo L., Scarpulla R. C. (2001) Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol. Cell. Biol. 21, 644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. (2007) High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Li B., Li W., Ma L., Zheng D., Li L., Yang W., Chu M., Chen W., Mailman R. B., Zhu J., Fan G., Archer T. K., Wang Y. (2014) Transcriptional repression by the BRG1-SWI/SNF complex affects the pluripotency of human embryonic stem cells. Stem Cell Rep. 3, 460–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H., McCaffery J. M., Chan D. C. (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130, 548–562 [DOI] [PubMed] [Google Scholar]
- 30.Azhikina T. L., Sverdlov E. D. (2005) Study of tissue-specific CpG methylation of DNA in extended genomic loci. Biochemistry 70, 596–603 [DOI] [PubMed] [Google Scholar]
- 31.Hofmann M. C., Narisawa S., Hess R. A., Millán J. L. (1992) Immortalization of germ cells and somatic testicular cells using the SV40 large T antigen. Exp. Cell Res. 201, 417–435 [DOI] [PubMed] [Google Scholar]
- 32.Gallardo T., Shirley L., John G. B., Castrillon D. H. (2007) Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 45, 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bird A. (2002) DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 [DOI] [PubMed] [Google Scholar]
- 34.Fazzari M. J., Greally J. M. (2004) Epigenomics: beyond CpG islands. Nat. Rev. Genet. 5, 446–455 [DOI] [PubMed] [Google Scholar]
- 35.Miranda T. B., Jones P. A. (2007) DNA methylation: the nuts and bolts of repression. J. Cell Physiol. 213, 384–390 [DOI] [PubMed] [Google Scholar]
- 36.Linher K., Cheung Q., Baker P., Bedecarrats G., Shiota K., Li J. (2009) An epigenetic mechanism regulates germ cell-specific expression of the porcine deleted in azoospermia-like (DAZL) gene. Differentiation 77, 335–349 [DOI] [PubMed] [Google Scholar]
- 37.Weber M., Hellmann I., Stadler M. B., Ramos L., Pääbo S., Rebhan M., Schübeler D. (2007) Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat. Genet. 39, 457–466 [DOI] [PubMed] [Google Scholar]
- 38.Hammoud S. S., Low D. H., Yi C., Carrell D. T., Guccione E., Cairns B. R. (2014) Chromatin and transcription transitions of mammalian adult germline stem cells and spermatogenesis. Cell Stem Cell 15, 239–253 [DOI] [PubMed] [Google Scholar]
- 39.Teng Y. N., Chang Y. P., Tseng J. T., Kuo P. H., Lee I. W., Lee M. S., Kuo P. L. (2012) A single-nucleotide polymorphism of the DAZL gene promoter confers susceptibility to spermatogenic failure in the Taiwanese Han. Hum. Reprod. 27, 2857–2865 [DOI] [PubMed] [Google Scholar]
- 40.Larsson N. G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G. S., Clayton D. A. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231–236 [DOI] [PubMed] [Google Scholar]
- 41.Ramalho-Santos J., Varum S., Amaral S., Mota P. C., Sousa A. P., Amaral A. (2009) Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 15, 553–572 [DOI] [PubMed] [Google Scholar]