Abstract
Purpose
To evaluate the outcomes of 5-year ranibizumab treatment in neovascular age-related macular degeneration (nAMD) in a single center and real life clinical setting.
Methods
The records of nAMD patients who were treated with ranibizumab between January 2010 and June 2011 were retrospectively reviewed. Patients who completed 5 years of follow-up were included. Main outcome measures were change in best-corrected visual acuity, central retinal thickness, and visit and injection numbers.
Results
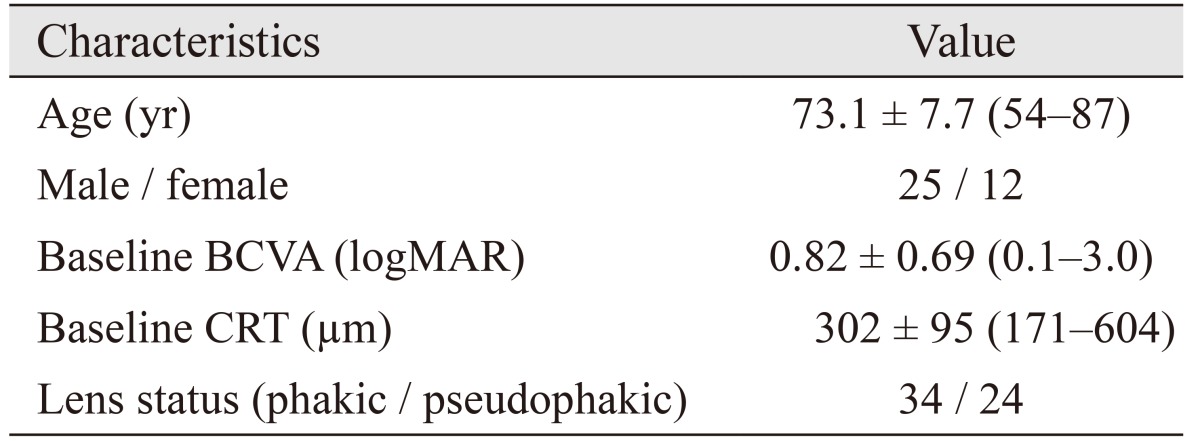
Forty-four eyes of 37 patients were included. Mean best-corrected visual acuity decreased from 0.82 ± 0.69 to 1.11 ± 0.65 logarithm of minimal angle of resolution after 5 years. Twenty-four eyes (54.5%) had visual acuity loss ≥3 lines, and 20 eyes (45.5%) had stable or improved vision (loss <3 lines, remained stable, or gained ≥1 line) at month 60. The mean total number of visits was 25.3 ± 5.8 (range, 14 to 42), and the mean total number of injections was 12.6 ± 6.4 (range, 3 to 26) at month 60.
Conclusions
Half of the ranibizumab-treated nAMD patients maintained their vision during the 5 years of follow-up. Visit and injection numbers were found to be lower than in prospective studies, reflecting a real world clinical practice.
Keywords: Age-related macular degeneration, Ranibizumab, Visual acuity
Neovascular age-related macular degeneration (nAMD) is a major cause of vision loss among the elderly population in Western countries [1,2]. Before the era of anti-vascular endothelial growth factor treatment, visual loss prevention was only achieved in a limited number of patients despite the use of different treatment modalities [3,4,5,6,7]. Bevacizumab, ranibizumab, and finally aflibercept treatments have led to conservation of baseline visual acuity (VA) in the vast majority of patients and significantly increased VA in approximately one-third of patients [8,9,10,11,12]. Multicenter studies have shown that ranibizumab treatment can prevent significant visual loss in up to 95% of patients, and VA improvement can be achieved in up to 40% of the patients [13,14]. However, most of these studies were performed for 2 or 3 years [10,11,12,13]. The efficacy of ranibizumab treatment over 3 years in nAMD has been investigated in only a few studies [15,16,17]. Therefore, in this study, we aimed to evaluate the treatment outcomes of ranibizumab in nAMD for 5 years in a single center and a real life clinical setting.
Materials and Methods
In this retrospective and interventional study, we reviewed the records of nAMD patients who were treated with intravitreal ranibizumab (IVR) in Beyoglu Eye Training and Research Hospital on an as-needed treatment regimen basis between January 2010 and June 2011. Written informed consent was obtained from all patients before treatment, and the study adhered to the tenets of the Declaration of Helsinki.
To be included in the study, each patient had to meet the following criteria: age ≥50 years, newly diagnosed as treatment-naïve nAMD, and minimum follow-up time of 60 months. Patients were not included in the study if they had retinal disease other than nAMD (e.g., diabetic retinopathy, retinal vein occlusion), if they had received previous intravitreal injection or photodynamic therapy, or if they had been diagnosed with polypoidal choroidal vasculopathy or retinal angiomatous proliferation.
Data collected from patient records were age, gender, lens status, best-corrected visual acuity (BCVA), and central retinal thickness (CRT) at baseline and months 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60. The total number of injections for each year was recorded. All patients underwent a standardized examination including measurement of BCVA via the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 4 m, slit-lamp biomicroscopy, fundus examination, and measurement of intraocular pressure via applanation tonometry. Fundus photography, fluorescein angiography (HRA-2; Heidelberg Engineering, Heidelberg, Germany), and optical coherence tomography (OCT) imaging (Stratus OCT; Carl Zeiss Meditec, Dublin, CA, USA or Spectralis, Heidelberg Engineering) were performed before treatment. All examinations were repeated monthly during the first 2 years, except fluorescein angiography. Fluorescein angiography was repeated only when the cause of VA deterioration could not be clarified with the clinical examination and other imaging methods. OCT was used for detecting subretinal fluid and measurement of CRT. CRT, defined as the mean thickness of the neurosensory retina in the central 1-mm diameter area, was computed using OCT mapping software generated by the device. In the case of suspicion of polypoidal choroidal vasculopathy or retinal angiomatous proliferation in patients with occult/minimally classic CNV, indocyanine green angiography was performed. Patients diagnosed with polypoidal choroidal vasculopathy or retinal angiomatous proliferation were excluded from the study.
All injections were performed under sterile conditions after topical anesthesia and 10% povidone-iodine (Betadine; Purdue Pharma, Stamford, CT, USA) scrub were applied to lids and lashes, and 5% povidone-iodine was then administered on the conjunctival sac. IVR (Lucentis; Novartis, Basel, Switzerland) was injected through the pars plana at 3.5 mm to 4 mm posterior to the limbus with a 27-gauge needle. After injection, an ophthalmic solution of 0.5% moxifloxacin (Vigamox; Alcon Laboratories, Fort Worth, TX, USA) was administered five times a day for 1 week. Patients were instructed to consult the hospital if they experienced decreased vision, eye pain, or any new symptoms.
All patients received three loading doses of monthly IVR injections (0.5 mg/0.05 mL) initially. Patients were called for monthly visits especially for the first 2 years. There was not a strict follow-up regimen after the third year. A single injection of IVR was repeated when VA decreased by one or more ETDRS lines from the last visit, with newly developed macular hemorrhage, or evidence of subretinal fluid on OCT. Primary outcome measures in this study included BCVA, OCT defined CRT measurements, change in BCVA, and CRT between baseline and months 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60.
Statistical analysis
Statistical analyses were performed using the SPSS software ver. 20.0 (IBM Corp., Armonk, NY, USA). Data were first analyzed for normality using the Shapiro-Wilk test. Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as number and percentage. To compare two dependent variables, repeated measures t-test was used. A p-value <0.05 was considered statistically significant.
Results
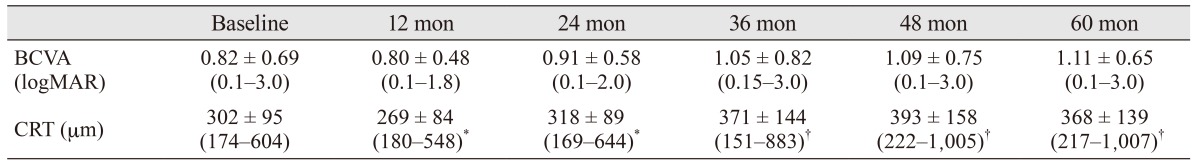
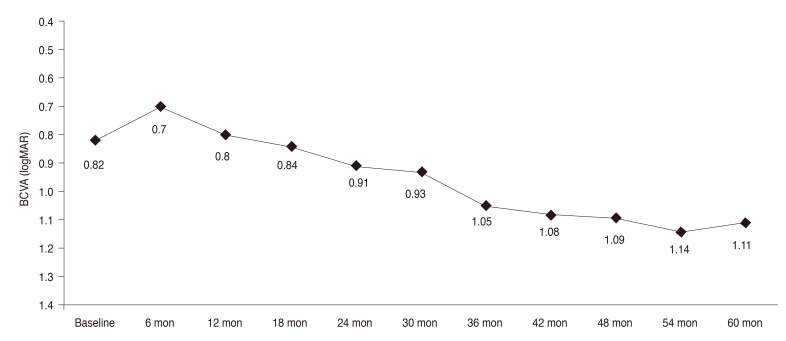
Forty-four eyes of 37 patients were included. Choroidal neovascularization was located subfoveally in all patients. The general characteristics of the patients are summarized in Table 1. The mean BCVA values of the patients are summarized in Table 2 and Fig. 1. Mean BCVA did not show a statistically significant difference until month 36 (p = 0.2 for month 6, p = 0.8 for month 12, p = 0.8 for month 18, p = 0.3 for month 24, p = 0.3 for month 30, and p = 0.07 for month 36). However, there was a significant decrease in BCVA from month 42 to month 60 (p = 0.04 for month 42, p = 0.03 for month 48, p = 0.01 for month 54, and p = 0.01 for month 60).
Table 1. General characteristics of patients.
Values are presented as mean ± standard deviation (range) or number.
BCVA = best-corrected visual acuity; logMAR = logarithm of minimal angle of resolution; CRT = central retinal thickness.
Table 2. Mean BCVA and CRT values over time.
Values are presented as mean ± standard deviation (range).
BCVA = best-corrected visual acuity; CRT = central retinal thickness; logMAR = logarithm of the minimum angle of resolution.
*Measurements obtained via time domain optical coherence tomography; †Measurements obtained via spectral domain optical coherence tomography.
Fig. 1. Change in logarithm of minimal angle of resolution (logMAR) visual acuity from baseline to year 5. BCVA = best-corrected visual acuity.
The Stratus OCT was used during the first 2 years, then OCT imaging was performed via Spectralis OCT. As CRT measurements of the two devices were not obtained with the same technique [18], statistical calculations for CRT were performed only for the first 2 years. CRT values are presented in Table 2. Mean CRT was found to be statistically different from baseline to month 12. However, there was not a significant difference in the other time points (p = 0.06 for month 6, p = 0.01 for month 12, p = 0.1 for month 18, and p = 0.4 for month 24).
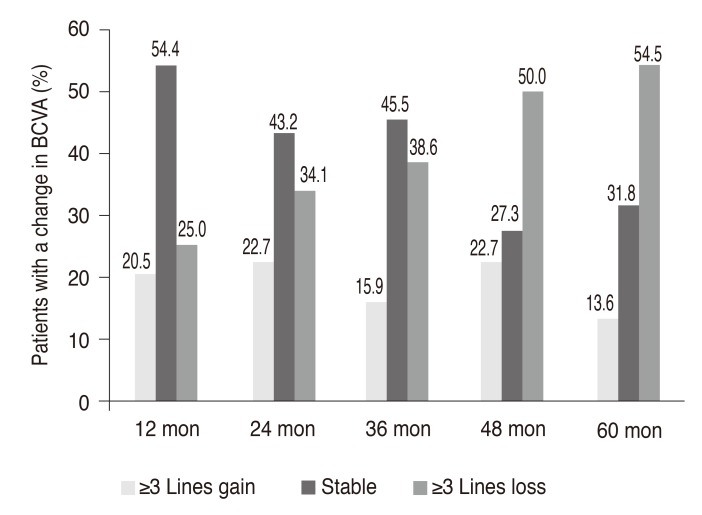
Twenty-four eyes (54.5%) had VA loss ≥3 lines, and 20 eyes (45.5%) had stable or improved vision (loss of <3 lines, remained stable, or gained ≥1 line) at month 60. At the 5 year visit, five eyes (11.4%) had VA ≥0.3 logarithm of minimal angle of resolution (logMAR) (≥20 / 40), and 25 eyes (56.8%) had a VA ≤1.0 logMAR (≤20 / 200). The VA changes over 5 years are summarized in Fig. 2.
Fig. 2. The percentage of patients who gained ≥3 lines of visual acuity, remained stable (<3 lines of visual acuity loss, experienced no change, or improved), and lost ≥3 lines of visual acuity at different time points. BCVA = best-corrected visual acuity.
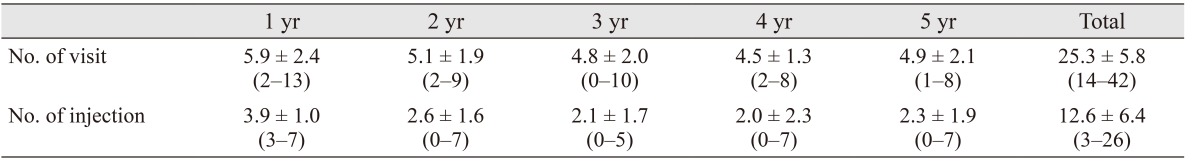
Mean visit and injection numbers throughout 5 years are summarized in Table 3. The mean total number of visits was 25.3 ± 5.8 (range, 14 to 42) at month 60. The percent of patients who had ≥5 visits during years 1, 2, 3, 4, and 5 was 72.7%, 65.9%, 54.5%, 43.2%, and 55.8%, respectively. The mean total number of injections was 12.6 ± 6.4 (range, 3 to 26) at month 60. The percent of patients who had ≥3 injections during years 1, 2, 3, 4, and 5 was 100%, 52.3%, 47.7%, 36.4%, and 40.9%, respectively. The percent of patients who did not receive any injections during years 2, 3, 4, and 5 was 13.6%, 20.5%, 45.5%, and 22.7%, respectively. No injection-related endophthalmitis was noted after a total of 555 injections.
Table 3. Mean visit and injection numbers over time.
Values are presented as mean ± standard deviation (range).
Discussion
In this study, we evaluated the 5-year outcomes of ranibizumab treatment in nAMD in a real life clinical setting. We obtained similar results to other real world clinical studies. Visit and injection numbers were very low compared with those in pivotal prospective multicenter studies [13,14]. Although follow-up examinations were not properly performed, visual and anatomical outcomes were acceptable for a real life practice. In 45.5% of the patients, VA was stable at the last follow-up visit at month 60, which was comparable with the study by Rofagha et al. [15] in which the nAMD patients were evaluated after 7 years of follow-up. Their study reported that 43% of patients had improved or stable VA [15]. We also detected some interesting findings with regard to visit and injection numbers over the 5 years of follow-up. The mean visit number during the first year was 5.9, which gradually decreased to 4.5 at the fourth year. However, it increased to 4.9 at the fifth year. In parallel to the mean visit number, the mean injection number decreased from 3.9 to 2.0 from the first year to the fourth year and then increased to 2.3 in the last year. The last year follow-up examinations were usually performed between 2013 and 2015. During these years, some additional retreatment criteria were added to the nAMD treatment and follow-up protocols [12,13,14]. Similarly, the real life outcomes of nAMD treatment with anti-vascular endothelial growth factor agents were increasingly published [12,14]. These new data might have affected our clinical practice and might have caused an increase in our clinic's visit and injection numbers. Indeed, the increased percentage of patients who had ≥5 visits and ≥3 injections and the decrease in the percentage of the patients who did not receive any injections throughout the follow-up period supports that supposition. The percent of patients who had ≥5 visits during the first year was 72.7% and decreased to 43.2% during the fourth year. Again, this percent interestingly increased to 55.8% during the fifth year. The percent of patients who had ≥3 injections during the first year was 100% and decreased to 36.4% during the fourth year, then increased to 40.9% during the fifth year. The percent of patients who did not receive any injections was 45.5% during year 4, which like the other parameters, decreased during year 5, to 22.7%.
In a study by Rofagha et al. [15], treatment outcomes of nAMD patients were evaluated after a mean follow-up period of 7.3 years. All of the included patients were participants in one of three major multicenter pivotal studies [11,13,19], and after completion of these studies, the patients who had continued follow-up were evaluated. It was reported that 23% of the patients had VA ≥20 / 40 at the last follow-up, 37% had a VA ≤20 / 200, and 34% had lost ≥15 letters. The mean injection number was found to decrease dramatically after the termination of prospective studies. For example, patients who exited the HORIZON study were reported to receive a mean of 6.8 injections during the mean follow-up period of 3.4 years [19]. Interestingly, the HORIZON subgroup patients who received ≥11 or more injections during follow-up had better visual outcomes. In another study by Cvetkova et al. [16] the 5-year treatment outcomes of nAMD patients who were treated with ranibizumab were evaluated. The study included 66 patients and was mostly focused on anatomical and functional outcomes. The mean number of injections after 5 years was reported to be 8.8, which is lower than in our study. Interestingly, with regard to injection numbers, there was a trend of decrease in the first 2 years, leading to poor outcomes, and an opposite trend in the following 3 years, resulting in better outcomes. Over 5 years, 20% of the patients were reported to have stable VA, 12.5% had increased VA, and 67.5% had decreased VA. The visual results were similar to our study, but the percentage of patients who lost ≥3 lines of VA was slightly higher than our findings (67.5% vs. 54.5%), which may be secondary to the low mean injection numbers (8.8 vs. 12.6).
Several studies have shown that patients received a smaller number of injections in real life practice than in prospective studies, which usually resulted in worse outcomes [15,16,17]. In another 5-year follow-up study in which CATT study patients continued follow-up visits for 3 years after study completion, the mean number of injections was reported to be 25.3 during the first 2 years and 15.4 during the next 3 years. There were relatively high numbers compared with our study and the other long follow-up studies [14,15,16,17]. Likely parallel to the high injection numbers, the visual results were better than other studies as only 20% of the patients had VA ≤20 / 200, and 50% of the patients had VA ≥20 / 50. As shown in the AURA study, ≥5.1 injections were needed to maintain VA during the first year, and a total of ≥8.3 injections were needed during the first 2 years of treatment in nAMD patients [20]. Also, it was reported that ≥7.9 injections during the first year and 16.1 injections during the first 2 years were required to significantly increase VA. The mean injection numbers were very low in our study compared to the calculated injection numbers in the AURA study, and we achieved VA of ≥20 / 50 in only 11.4% eyes.
The visual results of our study and other long-term studies showed that VA usually decreased after 2 or 3 years despite treatment [15,16,17]. In our study, VA decreased from 0.82 logMAR to 1.11 logMAR after 5 years, which was equal to 2.9 logMAR lines. During the 5-year follow-up period, the most significant VA decreases occurred in the second and third years. It is worth recalling here that this is still better than the natural history of the disease, which was evaluated prospectively in the MARINA study [13] in which patients who received only sham injections lost around 15 letters (3 logMAR lines) in 24 months. Therefore, regarding the natural history of the disease, we propose that, although patients are undertreated in the real world clinical setting, ranibizumab treatment may delay significant visual loss until 5 years.
The main limitation of the study was its retrospective design. However, real world clinical studies are conducted with this method, and the study may prove useful for nAMD treatment. Another limitation was that we did not use any VA cut-off levels in the inclusion criteria. Also, patients were not evaluated in regard to scatrization or geographic atrophy during follow-up, which might alter VA outcomes. The strengths of our study are the satisfactory patient number and long follow-up period.
Although ranibizumab treatment was proven to be very effective in a large series of prospective multicenter studies, long-term follow-up data is limited [11,13,14]. Therefore, we believe that this study will make a useful contribution to the literature. In this study, we showed that VA decreased gradually over a 5-year period in ranibizumab-treated nAMD patients. In contrast to the decrease in VA, patients improved anatomically and showed a gradual decrease in CRT. Half of the patients maintained their vision for 5 years. However, the visual gains reported in prospective studies could not be obtained in this real world clinical setting in either the medium or long-term. The main reasons for this failure were the dramatic decreases in visit and injection numbers after the first year, reflecting undertreatment. With a proper follow-up schedule, strict injection criteria might be obeyed over the entire period, and better treatment outcomes might be expected.
Acknowledgements
The authors thank Dr. Hande Mefkure Ozkaya for English editing of the manuscript.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Soubrane G, Coscas G, Francais C, Koenig F. Occult subretinal new vessels in age-related macular degeneration: natural history and early laser treatment. Ophthalmology. 1990;97:649–657. doi: 10.1016/s0161-6420(90)32530-7. [DOI] [PubMed] [Google Scholar]
- 2.Roy M, Kaiser-Kupfer M. Second eye involvement in age-related macular degeneration: a four-year prospective study. Eye (Lond) 1990;4(Pt 6):813–818. doi: 10.1038/eye.1990.128. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Lee DE, Park YJ. ICG-enhanced digital angiography and photocoagulation of choroidal neovascularization in age-related macular degeneration. Korean J Ophthalmol. 1995;9:59–65. doi: 10.3341/kjo.1995.9.1.59. [DOI] [PubMed] [Google Scholar]
- 4.Kapran Z, Ozkaya A, Uyar OM. Hemorrhagic age-related macular degeneration managed with vitrectomy, subretinal injection of tissue plasminogen activator, gas tamponade, and upright positioning. Ophthalmic Surg Lasers Imaging Retina. 2013;44:471–476. doi: 10.3928/23258160-20130909-09. [DOI] [PubMed] [Google Scholar]
- 5.Kim HW, Kim JL, Lee MH, et al. Combined treatment of photodynamic therapy and bevacizumab for choroidal neovascularization secondary to age-related macular degeneration. Korean J Ophthalmol. 2011;25:231–237. doi: 10.3341/kjo.2011.25.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin GS, Bressler NM Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Effects of verteporfin therapy on contrast on sensitivity: results from the treatment of age-related macular degeneration with photodynamic therapy (TAP) investigation-TAP report No 4. Retina. 2002;22:536–544. doi: 10.1097/00006982-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Lee DK, Kim SH, You YS, Kwon OW. High dose intravitreal bevacizumab for refractory pigment epithelial detachment in age-related macular degeneration. Korean J Ophthalmol. 2016;30:265–271. doi: 10.3341/kjo.2016.30.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon DR, Lee DK, Kim SH, et al. Aflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 2015;29:226–232. doi: 10.3341/kjo.2015.29.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozkaya A, Alagoz C, Garip R, et al. The role of indocyanine green angiography imaging in further differential diagnosis of patients with nAMD who are morphologically poor responders to ranibizumab in a real-life setting. Eye (Lond) 2016;30:958–965. doi: 10.1038/eye.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozkaya A, Alkin Z, Yazici AT, Demirok A. Comparison of intravitreal ranibizumab in phakic and pseudophakic neovascular age-related macular degeneration patients with good baseline visual acuity. Retina. 2014;34:853–859. doi: 10.1097/IAE.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 12.Liu EM, Shah G, Blinder KJ, et al. Intravitreal aflibercept for neovascular AMD: short-term clinical effects of intravitreal aflibercept injection as a predictor of long-term results. Ophthalmic Surg Lasers Imaging Retina. 2015;46:1021–1027. doi: 10.3928/23258160-20151027-06. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 14.CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 16.Cvetkova NP, Holldobler K, Prahs P, et al. Ranibizumab in neovascular age-related macular degeneration: a 5-year follow-up. Clin Ophthalmol. 2016;10:1047–1051. doi: 10.2147/OPTH.S101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Maguire MG, Martin DF, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozkaya A, Alkin Z, Ozkaya HM, et al. Is spectral-domain optical coherence tomography essential for flexible treatment regimens with ranibizumab for neovascular age-related macular degeneration? J Ophthalmol. 2013;2013:786107. doi: 10.1155/2013/786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration? Ophthalmology. 2012;119:1175–1183. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Holz FG, Tadayoni R, Beatty S, et al. Determinants of visual acuity outcomes in eyes with neovascular AMD treated with anti-VEGF agents: an instrumental variable analysis of the AURA study. Eye (Lond) 2016;30:1063–1071. doi: 10.1038/eye.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]