Abstract
Rapid eye movement sleep behavior disorder (RBD) is one of the most common non-motor symptoms of parkinsonism, and it may serve as a prodromal marker of neurodegenerative disease. The mechanism underlying RBD is unclear. Several prospective studies have reported that specific non-motor symptoms predict a conversion risk of developing a neurodegenerative disease, including olfactory dysfunction, abnormal color vision, autonomic dysfunction, excessive daytime sleepiness, depression, and cognitive impairment. Parkinson’s disease (PD) with RBD exhibits clinical heterogeneity with respect to motor and non-motor symptoms compared with PD without RBD. In this review, we describe the main clinical and pathogenic features of RBD, focusing on its association with other non-motor symptoms of parkinsonism.
Keywords: Parkinson’s disease, Rapid eye movement sleep behavior disorder, Risk factors, Parkinsonism
Introduction
Rapid eye movement sleep behavior disorder (RBD) is a parasomnia characterized by the loss of normal muscle atonia, which contributes to violent motor manifestations of undesirable dreams. RBD can be either idiopathic or secondary to drugs or other diseases. Idiopathic RBD (iRBD) has attracted increasing attention because patients may eventually be diagnosed with parkinsonism, such as Parkinson’s disease (PD), multiple system atrophy (MSA), or dementia with Lewy bodies (DLB) [1]. The primary goals of this review are to summarize the mechanism and predictive factors underlying the conversation from RBD to parkinsonism.
The prevalence of iRBD in otherwise asymptomatic individuals has not yet been investigated, partly due to the general ignorance of the public who incorrectly believe that dream-related motor manifestations are normal. The prevalence of probable RBD is 4.6%–7.7% in the elderly Caucasian population (aged 60–97 years) as assessed by the RBD Screening Questionnaire and the Innsbruck RBD Inventory [2]. Another study reported that the prevalence of polysomnographically-confirmed iRBD is 1.15% in the elderly Korean population [3]. Approximately 50%–90% of patients with iRBD eventually develop parkinsonism [1, 4], and RBD confirmed by polysomnography (PSG) has greater predictive strength and specificity than other prodromal markers (e.g., olfactory loss and constipation) [5]. Therefore, RBD has been used as a characteristic marker of premotor neurodegenerative disorders.
RBD is a common comorbidity with α-synuclein-related diseases. It is associated with PD in 33%–46% of patients [6, 7], with DLB in 75% [8], and with MSA in essentially 100% [9]. Many investigators have studied the clinical characteristics of patients with PD and RBD; these patients tend to have the akinetic/rigid-dominant subtype of PD [10] and exhibit severe non-motor symptoms [11]. RBD could be a potential marker of more severe disease manifestations in PD. It may precede PD motor manifestations or develop after PD onset, and the clinical manifestations of PD could vary depending on the timing of RBD onset. Our previous work has suggested that a shorter interval between RBD and PD onset may be associated with worse cognition [12]. We conducted a literature search in PubMed for all the relevant articles and selected authoritative research and objective case-control studies to summarize the conclusions. Future research should pay more attention to the timing of RBD onset and patient outcomes.
Diagnosis of RBD
The definitive diagnosis of RBD requires video-PSG to confirm the loss of REM atonia, termed REM sleep without atonia (RSWA). According to the International Classification of Sleep Disorders, Third Edition [13], four diagnostic criteria must be met to diagnose RBD. (1) The patient displays repeated episodes of sleep-related vocalization and/or complex motor behaviors (i.e., dream-enactment behavior). (2) These behaviors are documented by PSG as occurring during REM sleep [generally via electromyography (EMG) of chin muscles], or are presumed to occur during REM sleep based on the clinical history of dream enactment. (3) PSG recordings demonstrate RSWA. (4) The disturbance cannot be explained by another sleep or mental disorder, or by drug or substance use. Currently, there is no consensus on quantitative scoring rules and EMG activity cut-off values for a diagnosis of RBD. The diagnostic accuracy of RSWA obtained with both visual and automatic methods was high [14]. The majority of Chinese chin EMG activity results, including ours, showed slightly lower EMG activity than those of European patients [15].
Considering the time, expense, and disruption to patients’ lives required for PSG, several questionnaires have been developed for the screening of RBD, such as the REM Sleep Behavior Disorder Screening Questionnaire [16], the RBD Questionnaire-Hong Kong (RBDQ-HK) [17, 18], the Mayo Sleep Questionnaire [16], the REM Sleep Behavior Disorder Single-Question Screen [19], and the RBD Inventory Questionnaire (RBD-I) [20]. The sensitivity and specificity of these questionnaires are 60%–90%. In East China, we found the overall scale of RBDQ-HK was scored at 17 of 100 for a high-accuracy screen of RBD [18].
Pathophysiology of RBD and Conversion to PD
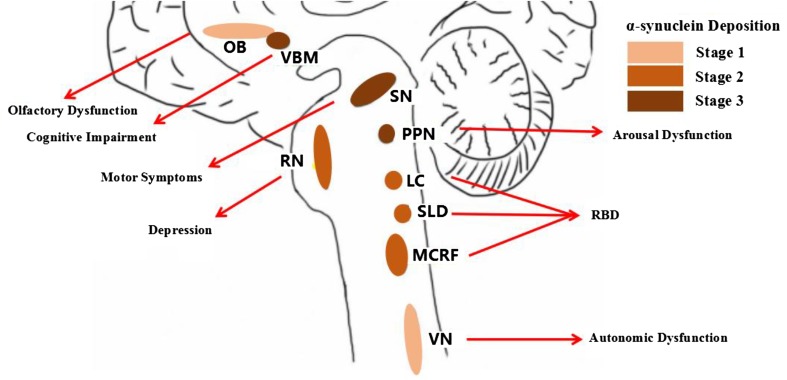
The underlying pathogenesis of RBD is still not known. Some animal and human studies have suggested that dysfunction in brainstem structures can cause RBD [21, 22]. Lesions in the pre-coeruleus and sub-laterodorsal nucleus, magnocellular reticular nucleus, and/or peri-locus coeruleus structures may reduce the inhibition of motor neurons, leading to cranial and skeletal muscle activity. There is a close relationship between RBD and α-synuclein deposition (Fig. 1). Vilas et al. detected α-synuclein aggregates in eight of nine iRBD patients [23]. Another study showed that α-synuclein deposition is present in the brain in 94% of participants who also have RBD with or without neurodegenerative disease [24]. In PD patients, Postuma et al. reported that possible RBD symptoms are associated with a greater range and density of α-synuclein deposition in the brain, while no significant differences in α-synuclein deposition were observed in Alzheimer’s disease pathology (amyloid or tau) or cerebral white-matter rarefaction [25].
Fig. 1.
The mechanism underlying non-motor symptoms may be reflected in the progression of α-synuclein deposition (Braak stage). LC locus coeruleus, MCRF magnocellular reticular nucleus, NBM nucleus basalis of Meynert, OB olfactory bulb, PPN pedunculopontine nucleus, RN raphe nucleus, SLD sub-laterodorsal nucleus, SN substantia nigra, VN vagus nerve.
Risk of Neurodegenerative Disease
A longitudinal, prospective study of iRBD has determined that the 5-year conversion risk of neurodegenerative disease (we call this process conversion, and the converted patients convertors) is 17.7%, the 10-year risk 40.6%, and the 12-year risk 52.4% [1]. A rapidly developing area of research has been the delineation in longitudinal studies of sensory, motor, autonomic, imaging, gene, and biochemical biomarkers that may help predict whether iRBD will progress to a diagnosable neurodegenerative disorder before disease onset [4]. Risk factor profiles between convertors and non-convertors have important commonalities and differences. However, it is difficult to predict whether a patient with iRBD will develop PD, DLB, or MSA. Some of the studies of RBD and/or comorbid signs and symptoms are not conclusive and further work is required. The study of RBD provides a window into neurodegenerative synucleinopathies in their prodromal stages, before parkinsonism or dementia becomes fully manifest.
A mean duration of 10–12 years between RBD diagnosis and PD motor syndromes has been reported [26, 27]. One study claimed that up to 81% of elderly males with newly-developed iRBD eventually develop a parkinsonian syndrome [28]. Other studies have reported that 30%–80% of PD patients have RBD symptoms, and RBD may precede the development of PD motor syndromes by 3–13 years [6, 29, 30]. Many non-motor symptoms can help to determine whether iRBD has a higher risk of conversion to parkinsonism in individual patients; these include advanced age (hazard ratio [HR] = 1.07), olfactory loss (HR = 2.8), abnormal color vision (HR = 3.1), subtle motor dysfunction (HR = 3.9), and non-use of antidepressants (HR = 3.5). Some marker combinations can identify >80% of patients with 5-year conversion rates [31, 32].
Timeline of Non-motor Symptoms During the Development and Conversion to Parkinsonism
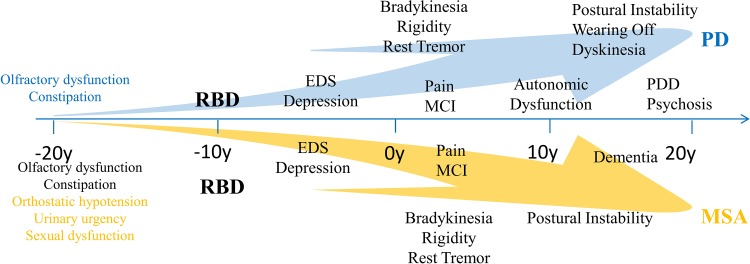
RBD plays an important role in the conversion to parkinsonism. The probability of conversion increases with the development time. RBD combined with different non-motor symptoms can be used to predict parkinsonism. The most prominent and common symptoms for the conversion of RBD to PD are olfactory dysfunction and constipation. Sleep disorders and depression may appear before the onset of motor symptoms, and the cognitive impairment often appears last. Orthostatic hypotension, urinary urgency, and sexual dysfunction are the most common characteristic symptoms for conversion of RBD to MSA (Fig. 2). We can rely on these prodromal symptoms to identify parkinsonism.
Fig. 2.
Timeline of non-motor symptoms during the development of Parkinsonism. RBD has an important role in this conversion when combined with other non-motor symptoms. Prominent manifestations for Parkinsonism and neurodegenerative diseases are shown in different colors according to the onset of non-motor symptoms. EDS excessive daytime sleepiness, MCI mild cognitive impairment, MSA multiple system atrophy, PD Parkinson’s disease, PDD PD with dementia, RBD rapid eye movement sleep behavior disorder.
Relationships Between RBD and Other Symptoms
Olfactory Dysfunction
Olfactory dysfunction is frequently reported in iRBD patients tested by reliable methods such as the University of Pennsylvania Smell Identification Test, the Brief Smell Identification Test, or Sniffin’ Sticks, and positive results indicate a possible premotor phase of PD. Olfactory dysfunction in iRBD patients manifests as impairment in odor threshold, including discrimination, and impaired identification [33]. Approximately 97% of iRBD patients have a pathologically increased olfactory threshold, 63% have an impaired odor discrimination score, and 63% have a decreased identification score [34]. Assessment of olfactory function, particularly odor identification, may help predict an early transition to parkinsonism in patients with iRBD over a relatively short time period, with a high diagnostic accuracy of 82.4% and a 7.3-fold risk [35].
Although olfactory testing can predict neurodegenerative disease in many patients, it is not perfect because some patients with parkinsonism have normal olfaction at diagnosis. The olfactory system has relatively direct connections to the hippocampus, thalamus, and frontal cortex, which are implicated in memory and emotion. Brain imaging studies using single-photon emission computed tomography have reported that patients with RBD have reduced perfusion in the frontal, temporal, and parietal cortical regions compared with controls [36]. Therefore, the mesolimbic pathway is important in the pathogenesis of RBD, and is thought to be key to the pathogenesis of olfactory dysfunction and RBD.
Numerous studies have reported that olfactory dysfunction occurs in 80%–96% of PD patients, and it usually develops several years before the onset of motor syndromes. The degree of impairment appears to be independent of disease duration or severity [37], and is accompanied by olfactory bulb atrophy [38]. Olfactory dysfunction should be considered a significant marker for both iRBD and PD, suggesting the early involvement of olfactory structures in neurodegenerative diseases [39].
Color Vision
Abnormal color vision discrimination has been described in iRBD. Five-year parkinsonism-free survival with normal color vision is 70.3%, while it is 26.0% with impaired vision [39]. Similar to olfaction, color vision changes with aging [39]. Combined abnormalities of olfaction and color vision are present up to 5 years before the diagnosis of neurodegenerative disease, with no substantial worsening closer to onset [40]. In a 10-year prospective study, Postuma et al. showed that iRBD patients with abnormal color vision have a 3.1-times higher risk of developing synucleinopathy than those without [31]. Color vision impairment may provide better prediction of dementia. The mechanisms underlying color vision loss in the pathogenesis of neurodegenerative diseases may be the degeneration of dopaminergic retinal neurons [41] or abnormalities in the visual/association cortex [42]. Currently, these mechanisms are not clearly understood.
We found that patients with RBD (idiopathic or secondary to PD) display a significant reduction in retinal nerve fiber layer (RNFL) thickness compared with groups without RBD. The RNFL thickness may be associated with an increased probability of comorbid RBD in PD [43].
Autonomic Dysfunction
Autonomic dysfunction is another potential preclinical marker that commonly arises in early neurodegenerative disease and iRBD [44, 45]. The pattern of autonomic dysfunction in iRBD is similar to that reported in PD and MSA, such as abnormal cardiac activation [46], constipation, erectile dysfunction, and orthostatic blood-pressure changes.
Cardiac Activation
Heart rate variability (HRV) during wakefulness is significantly reduced in patients with iRBD, suggesting functional abnormalities in both the sympathetic and parasympathetic systems [47]. HRV also decreases in PD, suggesting that both iRBD and PD have the same pathological mechanisms [48, 49].
Constipation
Many studies have shown that iRBD patients have constipation more frequently than controls. The prevalence of constipation increases slowly over time during prodromal PD, and may start 15 years before the diagnosis of PD [44, 50]. Several studies have reported that constipation occurs early during the development of PD in half of patients [51, 52]. These combined results indicate that constipation in both iRBD and PD have a common early pathophysiological origin, which is probably located in the enteric nervous system and the dorsal motor nucleus of the vagus in the medulla [53, 54].
Orthostatic Hypotension
RBD patients have greater systolic blood pressure changes during orthostasis than controls [55]. One logistic regression analysis indicated that at least 5 years before conversion, there are differences in the change of orthostatic blood pressure between controls and iRBD patients, detected as decreased systolic pressure [50]. Patients with early or mild PD also have an increased prevalence of systolic pressure decline, suggesting that orthostatic hypotension may be closely associated with the occurrence of RBD [44, 56]. Other studies have reported that orthostatic hypotension is a significant risk factor for the development of progressive disability and mortality in PD and MSA [57, 58].
Autonomic dysfunction may be the earliest prodromal marker in patients with parkinsonism, and essentially all patients with onset of iRBD have autonomic dysfunction [31]. The mechanism may be mediated by lesions of autonomic centers in the brainstem before characteristic changes in the substantia nigra pars compacta (SNc) occur [59]. Notably, a different study reported no differences in autonomic dysfunction between iRBD patients who did not develop parkinsonism and dementia- and age-matched controls [50]. Further studies with larger cohorts are required to determine whether autonomic dysfunction is associated with RBD, and whether autonomic dysfunction combined with RBD is a good way to predict prodromal PD.
Excessive Daytime Sleepiness (EDS)
EDS is defined as the inability to remain awake and alert during the major waking episodes of the day, with sleep occurring unintentionally or at inappropriate times almost daily for at least 3 months [13]. In a retrospective study, EDS was closely associated with iRBD in patients <50 years old [60]. A different study reported that patients with iRBD score ~3 points higher on the Epworth Sleepiness Scale (ESS) than controls. An ESS score >8 should be considered a red flag for earlier conversion to parkinsonism in patients with iRBD, especially if the patients are old and the RBD symptoms have persisted for a long time. ESS is associated with a shorter time from RBD onset or RBD diagnosis to parkinsonism. A longitudinal study reported that sleepy adults were 3.3 times more likely than non-sleepy adults to develop PD later in life; therefore, sleepiness may be an independent preclinical marker for PD [61, 62]. These results support the proposal that EDS can be used as a marker to predict PD in iRBD [63].
EDS is a frequent complaint in patients with PD, DLB, and MSA, and affects up to one-third of these patients [64]. The incidence of EDS increases by 6% per year in patients with PD [8, 64–66]. The mechanism mediating EDS in neurodegenerative synucleinopathy is multifactorial, and includes non-restorative sleep, sedative effects of treatment, and lesions in arousal systems [61].
Depression
Depression is common in iRBD, and the use of antidepressants leads to the development of secondary RBD in some patients [67, 68]. If antidepressants independently result in RBD, this cannot be used to predict the onset of neurodegenerative disorders. If depression is associated with iRBD, it can be used as a marker of neurodegeneration in iRBD. A recent study performed transcranial sonography and used hyperechogenicity of the brainstem raphe and SN to predict depression in iRBD, with a sensitivity of 23.1% and a specificity of 97.1%. This result suggested that dysfunction of the serotonergic dorsal raphe may be involved in the pathophysiology of depression in iRBD [69].
Several studies have indicated that a depressive episode occurs on average 10.1 years before PD diagnosis, and ~35% of PD patients have clinically significant symptoms of depression [70, 71]. The underlying mechanism may be that depression in PD patients arises from multiple neurotransmitter dysfunctions, including dopamine (SNc), serotonin (raphe nuclei), and noradrenaline (locus coeruleus). This implies the involvement of both the raphe nuclei and locus coeruleus at Braak stage 2. These combined results support the hypothesis that depression can be used in a similar way to iRBD as a prodromal symptom of PD [72].
Cognitive Impairment
Approximately 50% of patients with iRBD present mild cognitive impairment. Investigators have explored this in iRBD patients using a range of complicated neuropsychological tests; however, there is no consensus on the domains impaired. Predominant deficits have been reported in visuospatial function [73, 74], memory [75], attention [74, 75], and executive functions [76], while language is less affected. A longitudinal study identified cognitive deterioration in patients with iRBD, primarily involving visuospatial abilities, nonverbal logic, attention, and executive functions, suggesting the development of an underlying degenerative process [77]. Patients with iRBD who perform poorly in cognitive assessment tests are more likely to develop DLB and PD dementia [31].
Many studies have reported that the prevalence of cognitive impairment is significantly higher in PD patients with RBD than in those without it [75]. The pedunculopontine nucleus plays an important role in the mechanism mediating RBD, and it provides the majority of the cholinergic input to the thalamus and nucleus basalis of Meynert [78]. In most studies, PD patients with RBD perform poorly in attention/executive and visuospatial tasks [79–81]. We found that poor cognition is associated with shorter intervals between the onset of RBD and the subsequent PD onset, but the mechanism underlying this process remains to be elucidated [11].
Imaging Results
Radiotracer Imaging
The most commonly used imaging method is the dopamine transporter (DAT) scan, which measures the density of presynaptic DATs. RBD patients with normal presynaptic uptake might not convert. Rupprecht et al. performed transcranial sonography (TCS) and 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)-nortropane (123I-FP-CIT) single-photon emission computed tomography in patients with PD, and reported that severe motor dysfunction and RBD are associated with neuroimaging abnormalities, whereas olfactory dysfunction is not [82]. In a longitudinal study, Iranzo et al. reported that 3 of 20 patients who developed PD had the lowest 123I-FP-CIT uptake at baseline, and mean reductions at 3 years of 32.81% in the left putamen, 30.40% in the right putamen, 26.51% in the left caudate nucleus, and 23.75% in the right caudate nucleus [83].
Magnetic Resonance Imaging (MRI)
The results from conventional structural MRI analysis of RBD have been highly inconclusive. Voxel-based morphometry (VBM) has detected some distinguishing characteristics in iRBD patients. Scherfler et al. reported increased grey-matter density in the hippocampi and parahippocampal gyri of patients with iRBD [84]. In contrast, another VBM study reported that grey matter is reduced in the left parahippocampal gyrus of iRBD patients [85]. Recent studies have performed resting-state functional MRI (rs-fMRI) analysis in patients with iRBD. Rolinski et al. performed rs-fMRI and DAT to compare the basal ganglia dysfunction in patients with iRBD and PD; rs-fMRI identified minor differences, while DAT identified major differences in the basal ganglia [86].
Transcranial Sonography (TCS)
Hyperechogenicity of the SN is detected in most patients with PD. Therefore, several studies have investigated whether TCS can identify convertors with iRBD. Iranzo et al. reported that the sensitivity of baseline SN hyperechogenicity for the development of a synucleinopathy is 42.1%, with specificity of 67.7%, positive predictive value of 44.4%, and negative predictive value of 65.6%. The low positive predictive value and the high negative predictive value indicate that TCS alone may not be a useful method of identifying the risk of synucleinopathy in iRBD [32]. Iranzo et al. also reported that a combination of DAT and TCS provides 100% sensitivity and 55% specificity to predict iRBD conversion to synucleinopathy after 2.5 years [87]. In conclusion, multiple approaches and longitudinal studies are indispensable to explore conversion.
Genes Involved in PD and RBD
Patients with RBD share genetic profiles similar to those with PD. The combination of genetic analysis with other diagnostic methods may facilitate the identification of individuals susceptible to PD. The associations between genes and RBD are not clear. Recently, two studies have reported strong associations between glucocerebrosidase (GBA) mutations and RBD; patients with GBA mutations have a higher incidence of RBD, although this association has not been tested in patients with iRBD [88, 89]. Single-nucleotide polymorphisms in the SCARB2 and MAPT regions, which were previously associated with a reduced risk for PD, have also been associated with a reduced risk for RBD [90]. Most cross-sectional studies indicate that carriers of leucine-rich repeat kinase 2 (LRRK2) mutations exhibit subtle non-motor symptoms, including RBD [91, 92]. A few studies have shown that the incidence of RBD in patients with LRRK2 mutations is higher, especially with the mutations G2019S and G2385R [93–95]. Patients with synuclein (SNCA) mutations also have a higher incidence of RBD [96, 97].
These studies suggest that RBD is associated with several genetic markers for PD. A future longitudinal study is needed to determine whether RBD diagnosis combined with the analysis of selected genes can be used as a preclinical marker for phenoconversion to PD.
Conclusions
Increasing evidence indicates that iRBD may be associated with some non-motor symptoms of patients with parkinsonism, including olfactory dysfunction, color vision impairment, cognitive abnormalities, depression, and EDS. RBD provides a therapeutic window for the development of disease-modifying therapies. However, it is difficult to predict disease progression because conversion of RBD to PD often takes decades and has low sensitivity. The conversion risk increases when RBD is combined with non-motor symptoms, including those from aberrant imaging results and gene analyses. On the contrary, if RBD patients suffer from fewer non-motor symptoms or have normal presynaptic uptake on DAT, they might not convert. In conclusion, clinicians should pay more attention to longitudinal follow-up and meta-analysis studies on RBD and other non-motor symptoms to preclude the development of a neurodegenerative disease.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (91649114), the Jiangsu Provincial Special Program of Medical Science, China (BL2014042), a Jiangsu Provincial Medical Key Discipline Project, the Suzhou Clinical Research Center of Neurological Disease (Szzx201503), Jiangsu Province Ordinary University Professional Degree Graduate Practice Innovation, China (SJZZ16-0242), and the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Footnotes
Hong Jin and Jin-Ru Zhang contributed equally to this review.
References
- 1.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahlknecht P, Seppi K, Frauscher B, Kiechl S, Willeit J, Stockner H, et al. Probable RBD and association with neurodegenerative disease markers: A population-based study. Mov Disord. 2015;30:1417–1421. doi: 10.1002/mds.26350. [DOI] [PubMed] [Google Scholar]
- 3.Kang SH, Yoon IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36:1147–1152. doi: 10.5665/sleep.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iranzo A, Fernández-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9:e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30:1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon JF, Bédard MA, Fantini ML, Petit D, Panisset M, Rompré S, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59:585–589. doi: 10.1212/WNL.59.4.585. [DOI] [PubMed] [Google Scholar]
- 7.Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011;77:1048–1054. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- 8.Ferman TJ, Boeve BF, Smith GE, Lin SC, Silber MH, Pedraza O, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77:875–882. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetrugno R, Provini F, Cortelli P, Plazzi G, Lotti EM, Pierangeli G, et al. Sleep disorders in multiple system atrophy: a correlative video-polysomnographic study. Sleep Med. 2004;5:21–30. doi: 10.1016/j.sleep.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79:1117–1121. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- 11.Nomura T, Inoue Y, Kagimura T, Nakashima K. Clinical significance of REM sleep behavior disorder in Parkinson’s disease. Sleep Med. 2013;14:131–135. doi: 10.1016/j.sleep.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, Xiong KP, Mao CJ, Shen Y, Hu WD, Huang JY, et al. Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Med. 2014;15:647–653. doi: 10.1016/j.sleep.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Ito E, Inoue Y. The International Classification of Sleep Disorders, third edition. American Academy of Sleep Medicine. Includes bibliographies and index. Nihon Rinsho. 2015;73:916–923. [PubMed] [Google Scholar]
- 14.Figorilli M, Ferri R, Zibetti M, Beudin P, Puligheddu M, Lopiano L, et al. Comparison between automatic and visual scorings of REM Sleep without atonia for the diagnosis of REM sleep behavior disorder in Parkinson disease. Sleep 2017, 4. doi: 10.1093/sleep/zsw060. [DOI] [PubMed]
- 15.Shen Y, Xiong KP, Li J, Mao CJ, Chen J, He PC, et al. Clinical correlates of rapid eye movement sleep without atonia in Parkinson’s disease. Clin Neurophysiol. 2015;126:1198–1203. doi: 10.1016/j.clinph.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Stiasny-Kolster K, Sixel-Döring F, Trenkwalder C, Heinzel-Gutenbrunner M, Seppi K, Poewe W, et al. Diagnostic value of the REM sleep behavior disorder screening questionnaire in Parkinson’s disease. Sleep Med. 2015;16:186–189. doi: 10.1016/j.sleep.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Li SX, Wing YK, Lam SP, Zhang J, Yu MW, Ho CK, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK) Sleep Med. 2010;11:43–48. doi: 10.1016/j.sleep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Shen SS, Shen Y, Xiong KP, Chen J, Mao CJ, Huang JY, et al. Validation study of REM sleep behavior disorder questionnaire-Hong Kong (RBDQ-HK) in east China. Sleep Med. 2014;15:952–958. doi: 10.1016/j.sleep.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27:913–916. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frauscher B, Ehrmann L, Zamarian L, Auer F, Mitterling T, Gabelia D, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012;27:1673–1678. doi: 10.1002/mds.25223. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 22.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 23.Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, et al. Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15:708–718. doi: 10.1016/S1474-4422(16)00080-6. [DOI] [PubMed] [Google Scholar]
- 24.Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14:754–762. doi: 10.1016/j.sleep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Postuma RB, Adler CH, Dugger BN, Hentz JG, Shill HA, Driver-Dunckley E, et al. REM sleep behavior disorder and neuropathology in Parkinson’s disease. Mov Disord. 2015;30:1413–1417. doi: 10.1002/mds.26347. [DOI] [PubMed] [Google Scholar]
- 26.Iranzo A, Molinuevo JL, Santamaría J, Serradell M, Martí MJ, Valldeoriola F, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 27.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–499. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 2015;14:57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 30.Iranzo A, Santamaría J, Rye DB, Valldeoriola F, Martí MJ, Muñoz E, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–252. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 31.Postuma RB, Gagnon JF, Bertrand JA, Génier MD, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015;84:1104–1113. doi: 10.1212/WNL.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iranzo A, Stockner H, Serradell M, Seppi K, Valldeoriola F, Frauscher B, et al. Five-year follow-up of substantia nigra echogenicity in idiopathic REM sleep behavior disorder. Mov Disord. 2014;29:1774–1780. doi: 10.1002/mds.26055. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto T, Miyamoto M, Iwanami M, Hirata K, Kobayashi M, Nakamura M, et al. Olfactory dysfunction in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11:458–461. doi: 10.1016/j.sleep.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Stiasny-Kolster K, Doerr Y, Möller JC, Höffken H, Behr TM, Oertel WH, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–137. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 35.Mahlknecht P, Iranzo A, Högl B, Frauscher B, Müller C, Santamaría J, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015;84:654–658. doi: 10.1212/WNL.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 36.Mazza S, Soucy JP, Gravel P, Michaud M, Postuma R, Massicotte-Marquez J, et al. Assessing whole brain perfusion changes in patients with REM sleep behavior disorder. Neurology. 2006;67:1618–1622. doi: 10.1212/01.wnl.0000242879.39415.49. [DOI] [PubMed] [Google Scholar]
- 37.Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63:167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, You H, Liu JF, Ni DF, Zhang ZX, Guan J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am J Neuroradiol. 2011;32:677–681. doi: 10.3174/ajnr.A2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyles K, Sharma JC. Olfactory loss as a supporting feature in the diagnosis of Parkinson’s disease: a pragmatic approach. J Neurol. 2013;260:2951–2958. doi: 10.1007/s00415-013-6848-8. [DOI] [PubMed] [Google Scholar]
- 40.Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol. 2011;69:811–818. doi: 10.1002/ana.22282. [DOI] [PubMed] [Google Scholar]
- 41.Price MJ, Feldman RG, Adelberg D, Kayne H. Abnormalities in color vision and contrast sensitivity in Parkinson’s disease. Neurology. 1992;42:887–890. doi: 10.1212/WNL.42.4.887. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso EF, Fregni F, Maia FM, Melo LM, Sato JR, Cruz AC, et al. Abnormal visual activation in Parkinson’s disease patients. Mov Disord. 2010;25:1590–1596. doi: 10.1002/mds.23101. [DOI] [PubMed] [Google Scholar]
- 43.Yang ZJ, Wei J, Mao CJ, Zhang JR, Chen J, Ji XY, et al. Retinal nerve fiber layer thinning: a window into rapid eye movement sleep behavior disorders in Parkinson’s disease. Sleep Breath. 2016;20:1285–1292. doi: 10.1007/s11325-016-1366-4. [DOI] [PubMed] [Google Scholar]
- 44.Postuma RB, Gagnon JF, Vendette M, Montplaisir JY. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain. 2009;132:3298–3307. doi: 10.1093/brain/awp244. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67:2236–2238. doi: 10.1212/01.wnl.0000249313.25627.2e. [DOI] [PubMed] [Google Scholar]
- 46.Lanfranchi PA, Fradette L, Gagnon JF, Colombo R, Montplaisir J. Cardiac autonomic regulation during sleep in idiopathic REM sleep behavior disorder. Sleep. 2007;30:1019–1025. doi: 10.1093/sleep/30.8.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valappil RA, Black JE, Broderick MJ, Carrillo O, Frenette E, Sullivan SS, et al. Exploring the electrocardiogram as a potential tool to screen for premotor Parkinson’s disease. Mov Disord. 2010;25:2296–2303. doi: 10.1002/mds.23348. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt C, Herting B, Prieur S, Junghanns S, Schweitzer K, Globas C, et al. Valsalva manoeuvre in patients with different Parkinsonian disorders. J Neural Transm (Vienna) 2009;116:875–880. doi: 10.1007/s00702-009-0239-4. [DOI] [PubMed] [Google Scholar]
- 49.Friedrich C, Rüdiger H, Schmidt C, Herting B, Prieur S, Junghanns S, et al. Baroreflex sensitivity and power spectral analysis during autonomic testing in different extrapyramidal syndromes. Mov Disord. 2010;25:315–324. doi: 10.1002/mds.22844. [DOI] [PubMed] [Google Scholar]
- 50.Postuma RB, Gagnon JF, Pelletier A, Montplaisir J. Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov Disord. 2013;28:597–604. doi: 10.1002/mds.25445. [DOI] [PubMed] [Google Scholar]
- 51.Pont-Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study) Mov Disord. 2015;30:229–237. doi: 10.1002/mds.26077. [DOI] [PubMed] [Google Scholar]
- 52.Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 2013;80:276–281. doi: 10.1212/WNL.0b013e31827deb74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014;29:1010–1018. doi: 10.1002/mds.25776. [DOI] [PubMed] [Google Scholar]
- 54.Iranzo A, Gelpi E, Tolosa E, Molinuevo JL, Serradell M, Gaig C, et al. Neuropathology of prodromal Lewy body disease. Mov Disord. 2014;29:410–415. doi: 10.1002/mds.25825. [DOI] [PubMed] [Google Scholar]
- 55.Eschweiler J, Stromps JP, Fischer M, Schick F, Rath B, Pallua N, et al. A biomechanical model of the wrist joint for patient-specific model guided surgical therapy: Part 2. Proc Inst Mech Eng H. 2016;230:326–334. doi: 10.1177/0954411916635443. [DOI] [PubMed] [Google Scholar]
- 56.Kim JS, Park HE, Oh YS, Lee SH, Park JW, Son BC, et al. Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J Neurol Sci. 2016;362:59–63. doi: 10.1016/j.jns.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein DS, Holmes C, Sharabi Y, Wu T. Survival in synucleinopathies: A prospective cohort study. Neurology. 2015;85:1554–1561. doi: 10.1212/WNL.0000000000002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maetzler W, Liepelt I, Berg D. Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol. 2009;8:1158–1171. doi: 10.1016/S1474-4422(09)70291-1. [DOI] [PubMed] [Google Scholar]
- 59.Braak H, Del TK, Rüb U, de Vos RA, Jansen SEN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: 197–211. [DOI] [PubMed]
- 60.Bonakis A, Howard RS, Ebrahim IO, Merritt S, Williams A. REM sleep behaviour disorder (RBD) and its associations in young patients. Sleep Med. 2009;10:641–645. doi: 10.1016/j.sleep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Arnulf I, Konofal E, Merino-Andreu M, Houeto JL, Mesnage V, Welter ML, et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology. 2002;58:1019–1024. doi: 10.1212/WNL.58.7.1019. [DOI] [PubMed] [Google Scholar]
- 62.Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 63.Arnulf I, Neutel D, Herlin B, Golmard JL, Leu-Semenescu S, de Cock VC, et al. Sleepiness in idiopathic REM sleep behavior disorder and Parkinson disease. Sleep. 2015;38:1529–1535. doi: 10.5665/sleep.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA 287: 455–463. [DOI] [PubMed]
- 65.Moreno-López C, Santamaría J, Salamero M, Del SF, Albanese A, Pellecchia MT, et al. Excessive daytime sleepiness in multiple system atrophy (SLEEMSA study) Arch Neurol. 2011;68:223–230. doi: 10.1001/archneurol.2010.359. [DOI] [PubMed] [Google Scholar]
- 66.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58:480–486. doi: 10.1111/j.1532-5415.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 67.Teman PT, Tippmann-Peikert M, Silber MH, Slocumb NL, Auger RR. Idiopathic rapid-eye-movement sleep disorder: associations with antidepressants, psychiatric diagnoses, and other factors, in relation to age of onset. Sleep Med. 2009;10:60–65. doi: 10.1016/j.sleep.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 68.Ju YE, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12:278–283. doi: 10.1016/j.sleep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 69.Vilas D, Iranzo A, Pont-Sunyer C, Serradell M, Gaig C, Santamaria J, et al. Brainstem raphe and substantia nigra echogenicity in idiopathic REM sleep behavior disorder with comorbid depression. J Neurol. 2015;262:1665–1672. doi: 10.1007/s00415-015-7745-0. [DOI] [PubMed] [Google Scholar]
- 70.Alonso A, Rodríguez LA, Logroscino G, Hernán MA. Use of antidepressants and the risk of Parkinson’s disease: a prospective study. J Neurol Neurosurg Psychiatry. 2009;80:671–674. doi: 10.1136/jnnp.2008.152983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord 2008, 23: 183–189; quiz 313. [DOI] [PubMed]
- 72.Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27:617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 73.Terzaghi M, Sinforiani E, Zucchella C, Zambrelli E, Pasotti C, Rustioni V, et al. Cognitive performance in REM sleep behaviour disorder: a possible early marker of neurodegenerative disease. Sleep Med. 2008;9:343–351. doi: 10.1016/j.sleep.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 74.Ferini-Strambi L. Does idiopathic REM sleep behavior disorder (iRBD) really exist? What are the potential markers of neurodegeneration in iRBD. Sleep Med. 2011;12(Suppl 2):S43–49. doi: 10.1016/j.sleep.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Gagnon JF, Vendette M, Postuma RB, Desjardins C, Massicotte-Marquez J, Panisset M, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann Neurol. 2009;66:39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 76.Delazer M, Högl B, Zamarian L, Wenter J, Ehrmann L, Gschliesser V, et al. Decision making and executive functions in REM sleep behavior disorder. Sleep. 2012;35:667–673. doi: 10.5665/sleep.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manni R, Sinforiani E, Pacchetti C, Zucchella C, Cremascoli R, Terzaghi M. Cognitive dysfunction and REM sleep behavior disorder: key findings in the literature and preliminary longitudinal findings. Int J Psychophysiol. 2013;89:213–217. doi: 10.1016/j.ijpsycho.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 78.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26:2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 79.Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, Tognoni G, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:601–606. doi: 10.1136/jnnp-2011-301874. [DOI] [PubMed] [Google Scholar]
- 80.Levy G, Jacobs DM, Tang MX, Côté LJ, Louis ED, Alfaro B, et al. Memory and executive function impairment predict dementia in Parkinson’s disease. Mov Disord. 2002;17:1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 81.Zhang JR, Chen J, Yang ZJ, Zhang HJ, Fu YT, Shen Y, et al. Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in Parkinson’s disease. Chin Med J (Engl) 2016;129:379–385. doi: 10.4103/0366-6999.176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rupprecht S, Walther B, Gudziol H, Steenbeck J, Freesmeyer M, Witte OW, et al. Clinical markers of early nigrostriatal neurodegeneration in idiopathic rapid eye movement sleep behavior disorder. Sleep Med. 2013;14:1064–1070. doi: 10.1016/j.sleep.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Iranzo A, Valldeoriola F, Lomeña F, Molinuevo JL, Serradell M, Salamero M, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10:797–805. doi: 10.1016/S1474-4422(11)70152-1. [DOI] [PubMed] [Google Scholar]
- 84.Scherfler C, Frauscher B, Schocke M, Iranzo A, Gschliesser V, Seppi K, et al. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann Neurol. 2011;69:400–407. doi: 10.1002/ana.22245. [DOI] [PubMed] [Google Scholar]
- 85.Hanyu H, Inoue Y, Sakurai H, Kanetaka H, Nakamura M, Miyamoto T, et al. Voxel-based magnetic resonance imaging study of structural brain changes in patients with idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2012;18:136–139. doi: 10.1016/j.parkreldis.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 86.Rolinski M, Griffanti L, Piccini P, Roussakis AA, Szewczyk-Krolikowski K, Menke RA, et al. Basal ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson’s disease. Brain. 2016;139:2224–2234. doi: 10.1093/brain/aww124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iranzo A, Lomeña F, Stockner H, Valldeoriola F, Vilaseca I, Salamero M, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected] Lancet Neurol. 2010;9:1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- 88.Gan-Or Z, Mirelman A, Postuma RB, Arnulf I, Bar-Shira A, Dauvilliers Y, et al. GBA mutations are associated with Rapid Eye Movement Sleep Behavior Disorder. Ann Clin Transl Neurol. 2015;2:941–945. doi: 10.1002/acn3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72:201–208. doi: 10.1001/jamaneurol.2014.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gan-Or Z, Girard SL, Noreau A, Leblond CS, Gagnon JF, Arnulf I, et al. Parkinson’s disease genetic loci in rapid eye movement sleep behavior disorder. J Mol Neurosci. 2015;56:617–622. doi: 10.1007/s12031-015-0569-7. [DOI] [PubMed] [Google Scholar]
- 91.Saunders-Pullman R, Alcalay RN, Mirelman A, Wang C, Luciano MS, Ortega RA, et al. REM sleep behavior disorder, as assessed by questionnaire, in G2019S LRRK2 mutation PD and carriers. Mov Disord. 2015;30:1834–1839. doi: 10.1002/mds.26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernández-Santiago R, Iranzo A, Gaig C, Serradell M, Fernández M, Tolosa E, et al. Absence of LRRK2 mutations in a cohort of patients with idiopathic REM sleep behavior disorder. Neurology. 2016;86:1072–1073. doi: 10.1212/WNL.0000000000002304. [DOI] [PubMed] [Google Scholar]
- 93.Mirelman A, Alcalay RN, Saunders-Pullman R, Yasinovsky K, Thaler A, Gurevich T, et al. Nonmotor symptoms in healthy Ashkenazi Jewish carriers of the G2019S mutation in the LRRK2 gene. Mov Disord. 2015;30:981–986. doi: 10.1002/mds.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ehrminger M, Leu-Semenescu S, Cormier F, Corvol JC, Vidailhet M, Debellemaniere E, et al. Sleep aspects on video-polysomnography in LRRK2 mutation carriers. Mov Disord. 2015;30:1839–1843. doi: 10.1002/mds.26412. [DOI] [PubMed] [Google Scholar]
- 95.Bergareche A, Rodríguez-Oroz MC, Estanga A, Gorostidi A, de Munain AL, Castillo-Triviño T, et al. DAT imaging and clinical biomarkers in relatives at genetic risk for LRRK2 R1441G Parkinson’s disease. Mov Disord. 2016;31:335–343. doi: 10.1002/mds.26478. [DOI] [PubMed] [Google Scholar]
- 96.Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK. Autosomal dominant Parkinson’s disease caused by SNCA duplications. Parkinsonism Relat Disord. 2016;22(Suppl 1):S1–6. doi: 10.1016/j.parkreldis.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papadimitriou D, Antonelou R, Miligkos M, Maniati M, Papagiannakis N, Bostantjopoulou S, et al. Motor and nonmotor features of carriers of the p. A53T alpha-synuclein mutation: a longitudinal study. Mov Disord. 2016;31:1226–1230. doi: 10.1002/mds.26615. [DOI] [PubMed] [Google Scholar]