Abstract
The enteric nervous system (ENS) controls the function of the gastrointestinal tract and has been implicated in various diseases, including Parkinson’s disease (PD). PD is a neurodegenerative disease with Lewy bodies (LBs) and Lewy neurites (LNs) as the main pathological features. In addition to the typical motor symptoms in PD, attention has been drawn to non-motor symptoms, such as constipation, implying dysfunction of the ENS. In the present study, we characterized the age-dependent morphological alterations and aggregation of α-synuclein (α-syn), the primary protein component in LBs and LNs, in the ENS in an α-syn transgenic mouse model. We found that the expression and accumulation of α-syn increased gradually in neurons of Meissner’s and Auerbach’s plexuses of the gastrointestinal tract with age (from 1 week to 2 years). In addition, α-syn was increasingly phosphorylated at the serine 129 residue, reflecting pathological alterations of the protein over time. Furthermore, α-syn was present in different subtypes of neurons expressing vasoactive intestinal polypeptide, neuronal nitric oxide synthase, or calretinin. The results indicated that BAC-α-Syn-GFP transgenic mice provide a unique model in which to study the relationship between ENS and PD pathogenesis.
Keywords: Enteric system, Parkinson’s disease, α-synuclein, Phosphorylation, Protein aggregation
Introduction
Parkinson’s disease (PD), a common neurodegenerative disorder, is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta of the midbrain, and the presence of Lewy bodies (LBs) and Lewy neurites (LNs) in remaining neurons. Alpha-synuclein (α-syn) is the primary protein component in LBs and LNs; it is enriched in presynaptic components under physiological conditions and appears as aggregated forms in PD [1]. Clinically, patients with PD exhibit typical motor symptoms, such as resting tremor, bradykinesia, and rigidity. At early stages of the disease, PD patients often exhibit non-motor symptoms, such as olfactory malfunction, constipation, depression, and dementia, which severely affect their quality of life [2–5]. To date, the mechanisms underlying these non-motor symptoms have yet to be fully understood.
Constipation is a common early non-motor symptom of PD, implying gastrointestinal dysfunction. The majority (up to 80%) of PD patients suffer from gastrointestinal dysfunction [2–4]. Accumulating evidence has suggested that α-syn pathology is initiated in the periphery and spreads first to lower brain structures such as the dorsal motor nucleus of the vagus (DMNV) in the medulla oblongata and later to higher brain structures in a rostral-caudal fashion [6].
The most convincing evidence comes from the post-mortem histological studies conducted by Braak and colleagues, who demonstrated this topographic localization of Lewy pathology during disease progression. Braak also demonstrated that LBs appear in neurons of the gastric Meissner’s plexus of PD patients [7], further supporting the hypothesis of the periphery, specifically the enteric nervous system (ENS), as the initial structure affected during PD. The Lewy pathology spreads into the central nervous system and then to higher brain regions. Recently, we demonstrated that different forms of α-syn can indeed spread into the brain via the vagus nerve [8] and provided evidence of a link in the gut-brain axis, which has led to the proposition of prion-like mechanisms of propagation of α-syn in PD and related disorders [9, 10].
The ENS has been called the ‘second brain’, as enteric neurons independently control the secretion and motility of the gastrointestinal tract [11]. The ENS plays an important role in the pathogenic processes of PD [12]. Therefore, it is of current urgency to study the relationship between the ENS and the pathological development of PD. Here, we report the age-dependent presence, accumulation, and phosphorylation of α-syn in the enteric neurons of a BAC-α-Syn-GFP mouse model, which provides a unique tool in which to further study PD pathology in the ENS and beyond.
Materials and Methods
Mouse Strains
The BAC-α-Syn-GFP transgenic mice have been described elsewhere [13]. Briefly, the transgenic mice were produced by pronuclear injection of recombinant BAC (bacterial artificial chromosome) DNA containing the human α-syn gene fused with green fluorescent protein (GFP) into fertilized eggs from C57 black6 mice. BAC DNA is obtained by inserting human α-syn cDNA into the pAcGFP1-C1 vector at the initiation codon of mouse α-syn gene [13]. The mice were housed five to six per cage under a 12 h light-dark cycle with ad libitum access to food and water. All work involving animals was approved by the Ethical Committees for use of laboratory animals at Lund University, Sweden, and at Northeastern University, China.
Tissue Preparation
BAC-α-Syn-GFP transgenic mice aged 1 week to 24 months (n = 3 at each age) were sacrificed by transcardial perfusion and fixation with 4% paraformaldehyde (PFA) in 0.1 mol/L PBS. The small intestine and colon were dissected, post-fixed in 4% PFA overnight, and then immersed consecutively in 10%, 20%, and 30% sucrose in 0.1 mol/L PBS. The intestine was cut transversely into 14-μm sections on a cryostat (Leica, Germany), and the sections were mounted on gelatin-coated glass slides for immunohistochemical processing.
Fluorescent Immunohistochemistry and Analyses
After antigen retrieval with citrate buffer (pH 6.0) for 10 min, the sections were pre-incubated with blocking solution containing 5% normal donkey serum and 0.3% Triton X-100 in 0.1 mol/L PBS for 1 h. Double immunostaining was performed with primary antibodies (α-syn antibody (211) with respective double-labeling antibodies) (Table 1) at room temperature overnight, followed by incubation with donkey anti-mouse, rabbit, or goat secondary antibodies tagged with Alexa-488, Cy2, or Cy3. The sections were mounted with an anti-fading medium for confocal microscopy (Leica TCS SP8). Semi-quantification of the α-syn-transgenic-GFP and phospho-α-syn intensity was performed by measuring the fluorescence intensity of selected areas (at 4, 8, and 12 o’clock locations) of the intestinal sections. Two-way ANOVA and one-way ANOVA were used to analyze fluorescence intensity of α-syn and phospho-α-syn immunoreactivity, respectively, and all were followed by a Least Significant Difference (LSD) post hoc test in the software IBM SPSS Statistics 2.0 (IBM Svenska AB, Stockholm, Sweden). Logarithmic transformation was used when the variances of the statistics were not equal.
Table 1.
Primary and secondary antibodies.
| Antigen detected by primary antibodies | Working dilution | Source | Cat. # |
|---|---|---|---|
| Anti-α-synuclein (211) (mouse) | 1:200 | Santa Cruz Biotechnology | sc-12767 |
| Anti-α-synuclein (rabbit) | 1:200 | Santa Cruz Biotechnology | sc-7011-r |
| Anti-calretinin (N-18) (goat) | 1:400 | Santa Cruz Biotechnology | sc-11644 |
| Anti-HuC/HuD (mouse) | 1:400 | Life Technology | A21271 |
| Anti-neuronal nitric oxide synthase (nNOS) (rabbit) | 1:800 | Abcam | ab76067 |
| Anti-vasoactive intestinal polypeptide (VIP) (rabbit) | 1:800 | Abcam | ab22736 |
| Anti-α-synuclein (phosphoS129) | 1:1000 | Abcam | ab51253 |
| Anti-neurofilament 200 (rabbit) | 1:400 | Sigma-Aldrich | N4142 |
| Secondary antibodies | Dilution | Source | Cat. # |
|---|---|---|---|
| Cy3-donkey anti-mouse | 1:200 | Jackson ImmunoResearch | 715-165-150 |
| Cy2-donkey anti-rabbit | 1:800 | Jackson ImmunoResearch | 711-225-152 |
| Alexa 488-donkey anti-mouse | 1:200 | Jackson ImmunoResearch | 715-546-151 |
| Cy3-donkey anti-rabbit | 1:800 | Jackson ImmunoResearch | 711-165-152 |
| Alexa 488-donkey anti-goat | 1:400 | Jackson ImmunoResearch | 705-545-147 |
Results
Age-Dependent Presence and Accumulation of α-Syn in Enteric Neurons of the Small Intestine
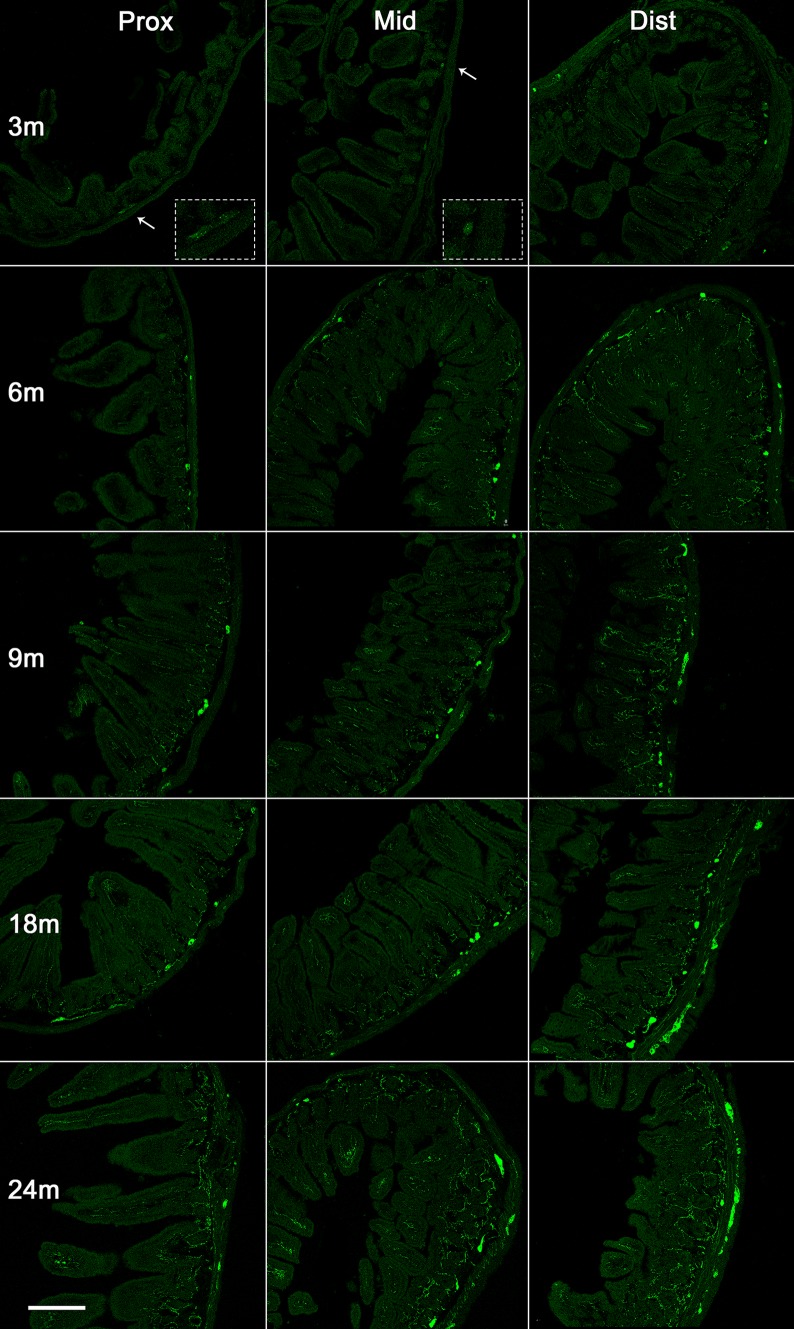
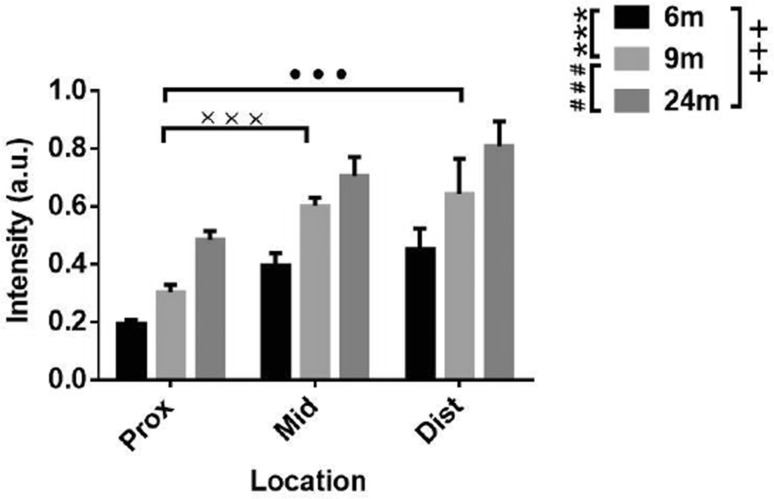
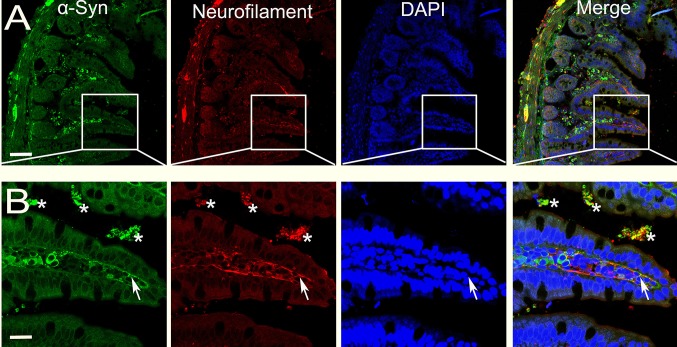
We first analyzed α-syn expression in the small intestine in the proximal (close to the stomach, i.e. the duodenum), middle, and distal (close to the colon) regions at different ages. α-Syn was barely present in the enteric neurons at 1 week and very scattered at one month. From three months onwards, an α-syn signal was detected and substantially increased with age. The high α-syn expression level was sustained in the oldest mice (Figs. 1, 2). The expression of α-syn-GFP increased gradually. This is consistent with the protein expression in the brain, in which initial low expression can start at the late embryonic stage, but is more robust at 1 week after birth [8, 14]. The α-syn expression was significantly more robust in the distal than in the proximal small intestine. By 9 months of age, similar levels of α-syn were observed throughout the intestinal segments examined. α-Syn was first mainly located in the somata of enteric neurons in both Meissner’s (submucosal) and Auerbach’s (myenteric) plexuses at the younger age, and then became evident in the nerve processes extending to the tips of microvilli of the mucosa (Figs. 1, 3).
Fig. 1.
Expression of α-syn in the small intestine of transgenic mice with age. Representative images of α-syn-GFP expression in the small intestine proximal to the stomach (left), the middle of the small intestine (middle), and the distal intestine (right) at 3, 6, 9, 18, and 24 months (m). Arrows, high-power images in insets; scale bar 250 μm.
Fig. 2.
Semi-quantitative analysis of α-syn fluorescence (GFP) intensity in the small intestine of transgenic mice at different ages. Two-way ANOVA (F = 32.041, P < 0.001, in age; F = 24.110, P < 0.001, in location), followed by LSD post hoc test (***P < 0.005, 6 vs 9 months; ### P < 0.005, 9 vs 24 months; +++ P < 0.005, 6 vs 24 months; ••• P < 0.005, Prox vs Dist; ××× P < 0.005, Prox vs Mid.); error bars, standard error of the mean.
Fig. 3.
Overlap of α-syn and neurofilament in the distal segment of the small intestine. α-Syn- and neurofilament-positive profiles largely overlap in the cell bodies of Auerbach’s and Meissner’s plexuses, and also in nerve terminals extending into the microvilli (arrows in B). *Non-specific labels of residual contents in the lumen; scale bars, 75 μm in A and 25 μm in B.
Age-Dependent Phosphorylation of α-Syn in Enteric Neurons
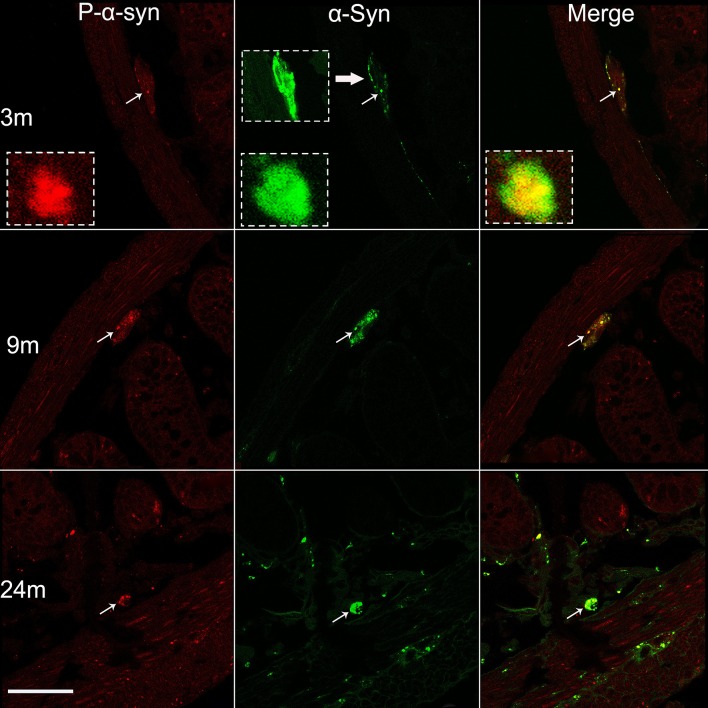
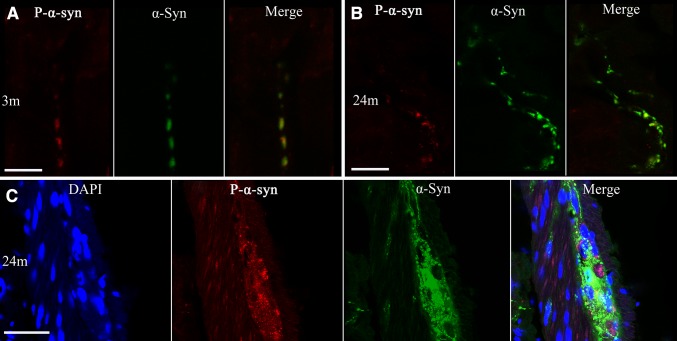
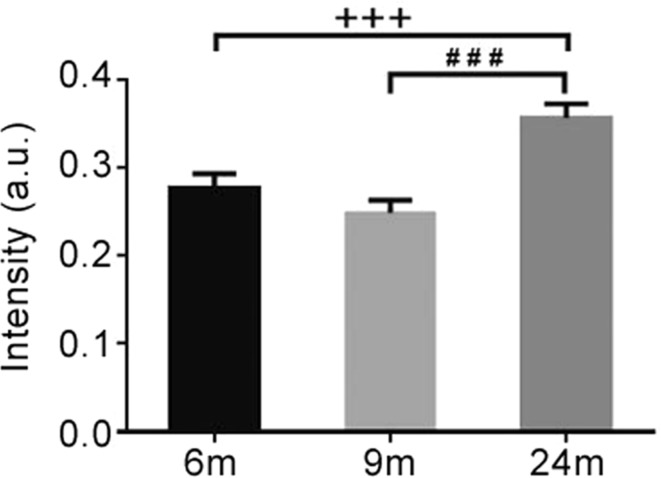
Phosphorylation is one of the most common post-translational modifications to α-syn [15], playing an important role in PD pathology. Therefore, we then focused on whether and how α-syn was phosphorylated in the enteric neurons of BAC α-syn-GFP mice. Using a phospho-α-syn antibody specific for the serine 129 residue we detected small amounts of phosphorylated α-syn in the enteric neurons of Meissner’s and Auerbach’s plexuses (Figs. 4, 5) at 3 months of age. Compared to the pan-α-syn for the same age group, the signal of phospho-α-syn was much weaker. Semi-quantitative analyses showed that α-syn phosphorylation (pS129) became more evident with age (Fig. 6). It was not only present in the cell bodies of enteric neurons, but also appeared in processes in the mucosal and muscular layers as well as in the nerve terminals around enteric neurons (Fig. 5).
Fig. 4.
Age-dependent phosphorylation of α-syn in Meissner’s plexus of the small intestine. Profiles of phospho-α-syn (P-α-syn, left), pan-α-syn (middle), and their co-localization (right), in 3, 9, and 24 month-old mice. The photomicrographs acquired under a confocal microscope show increased phosphorylation of α-syn with age (left). Subsets of pan-α-syn-positive profiles (middle) are overlapped with phospho-α-syn-positive profiles (right) (arrows and the insets). Scale bar 50 μm.
Fig. 5.
Co-localization of α-syn and phosphorylated α-Syn (P-α-syn) in transgenic mice at indicated ages. A–B Photomicrographs acquired under a confocal microscope show increased phosphorylation of α-syn with age (A, 3 months; B 24 months) in the mucous layer and in Auerbach’s plexus of the distal segment of the small intestine. Subsets of pan-α-syn-positive are overlapped with phospho-α-syn-positive profiles (A–C). Scale bars 2.5 μm (A), 10 μm (B), and 50 μm (C).
Fig. 6.
Semi-quantitative analysis of fluorescence intensity of phospho-α-syn immunoreactivity in the small intestine (distal segment) of transgenic mice at different ages. One-way ANOVA (F = 12.029, P = 0.001) followed by LSD post hoc test (### P < 0.005, 9 vs 24 months; +++ P < 0.005, 6 vs 24 months); error bars SEM.
Localization of α-Syn in Subtypes of Enteric Neurons
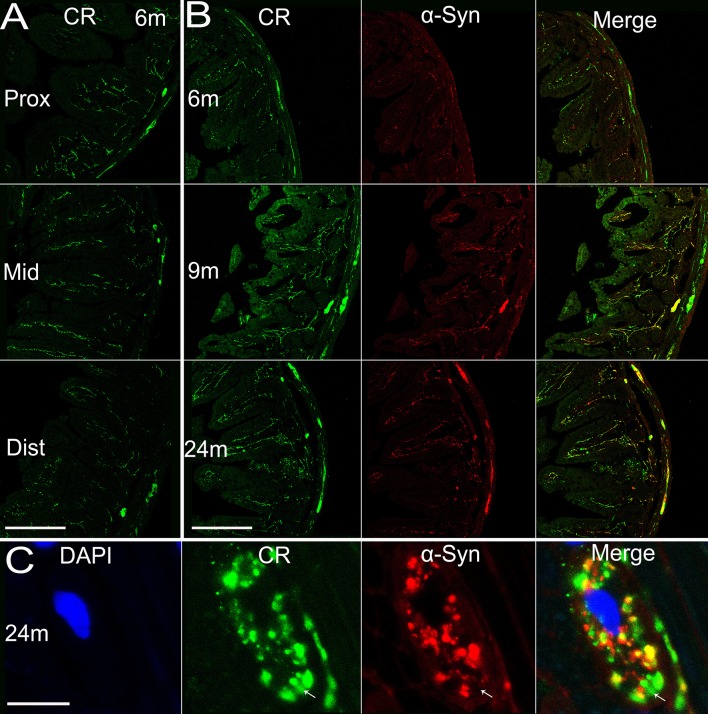
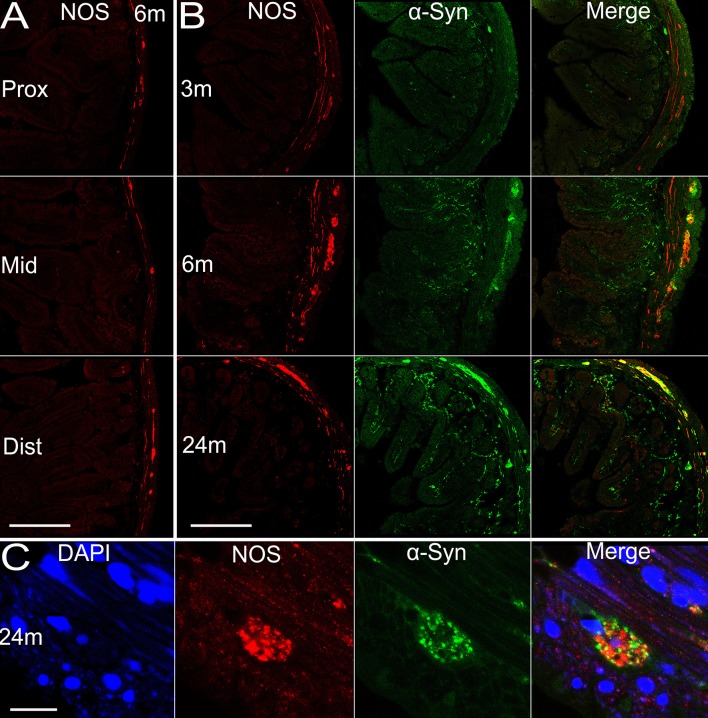
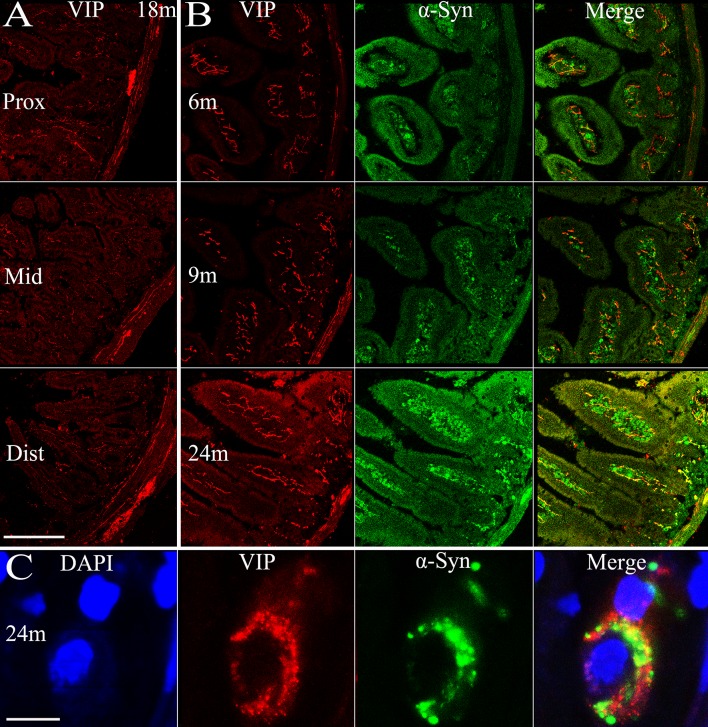
There are multiple subtypes of neurons in the enteric nervous system that exert different functions, use different neurotransmitters, and behave differently in electrophysiological tests [16]. Considering this, we examined the localization of α-syn in different neuronal subtypes. Most calretinin-positive neurons in Auerbach’s plexus of the mouse small intestine are excitatory motor neurons to the circular and longitudinal muscle layers [17], innervating smooth muscles of the intestine. We observed an age-dependent pattern of calretinin expression in the enteric neurons of both Meissner’s and Auerbach’s plexuses (Fig. 7B), similar to α-syn. Calretinin expression was similar in amount among the proximal, middle, and distal segments of the small intestine (Fig. 7A), with calretinin-positive nerve terminals extending to the tips of the microvilli. It appeared that most, if not all, α-syn-positive profiles overlapped with calretinin, though a small fraction of calretinin-positive fibers and terminals did not express α-syn (Fig. 7B, C). In contrast to calretinin, the majority of nNOS-positive neurons are inhibitory motor neurons to circular muscle [17]. We found that nNOS expression reached a plateau by 6 months of age and maintained a high level. Very interestingly, the protein expression was restricted to Meissner’s and Auerbach’s plexuses in the submucosal and muscular layers, and the nNOS-positive profiles barely extended into the mucosal layer and microvilli. The co-localization of α-syn and nNOS was evident mainly in Auerbach’s plexus of the myenteric layers, while they were separate from each other in other regions (Fig. 8). Vasoactive intestinal polypeptide (VIP)-positive profiles were widely distributed in the small intestine of transgenic mice. They were present in Meissner’s and Auerbach’s plexuses and the nerve fibers in different intestinal layers, without clear age-related alterations or differences among the proximal, middle, and distal segments of the intestine (Fig. 9). We only observed partial co-localization between VIP and α-syn, much less than that between calretinin/nNOS and α-syn. In high-power images, VIP and α-syn immunoreactivity was largely separate (Fig. 9C), indicating that they are localized in different subcellular compartments.
Fig. 7.
Expression of calretinin in the small intestine of transgenic mice with age and the co-localization of calretinin and α-syn. A Expression of calretinin in different sections (proximal, middle, and distal segments) of the small intestine in transgenic mice. B α-Syn and calretinin expressed in transgenic mice and their co-localization at 6, 9, and 24 months. C Co-localization of calretinin and α-syn, and the nucleus (DAPI) of an enteric neuron in Auerbach’s plexus at 24 months. Arrows, area where calretinin is expressed while α-syn not. Scale bars 200 μm in A, B; 10 μm in C. CR, calretinin.
Fig. 8.
Expression of nNOS in the small intestine of transgenic mice with age and the co-localization of nNOS and α-syn. A Expression of nNOS in different sections (proximal, middle, and distal segments) of the intestine in transgenic mice. B α-Syn and nNOS expressed in transgenic mice and the co-localization at 3, 6, and 24 months. C Co-localization of nNOS and α-syn, and the nucleus of an enteric neuron at 24 months. Scale bars 200 μm in A, B; 10 μm in C.
Fig. 9.
Expression of VIP in the small intestine of transgenic mice with age and the co-localization of VIP and α-syn. A Expression of VIP in different sections (proximal, middle, and distal segments) of the small intestine in transgenic mice. B α-Syn and VIP expressed in transgenic mice and their co-localization at 6, 9, and 24 months. C Co-localization of VIP and α-syn, and the nucleus (DAPI) of an enteric neuron at 24 months. Scale bars 200 μm in A, B; 10 μm in C.
Discussion
Gastrointestinal dysfunction is a common non-motor symptom in PD. It can manifest as gastric dis-motility, constipation, or anorectal dysfunction [18, 19]. Considering the presence of Lewy pathology in the enteric neurons of the gut and then the DMNV in the medulla oblongata at very early stages of PD, mostly preceding the onset of the motor symptoms, Braak et al proposed that PD pathology is initiated in the gastrointestinal tract [20], when the key protein component in LBs and LNs, α-syn, is aggregated in enteric neurons. Further studies using samples from biopsies and autopsies mostly favor the hypothesis of a link between the gut and the brain in PD pathogenesis [21–23], despite inconsistency with other reports [24, 25]. The current opinion of gut-brain communication is that the Lewy pathology initiated in the enteric neurons of the gut undergoes a retrograde transport from the intestine up to the DMNV in the medulla oblongata, and further up to higher brain regions. Our recent study demonstrated that, when applying different forms of exogenous α-syn to the intestinal wall, the monomeric, oligomeric or fibrillar forms of α-syn are taken up and actively transported to the DMNV via the vagus nerve [8]. Truncal vagotomy interrupts the transport of aggregated α-syn, blocks the protein propagation and aggregation in the brain [26], and decreases the risk of PD development [27]. Therefore, it is conceivable that, at least in subsets of PD cases, the origin of pathology is the enteric neurons of the gastrointestinal tract.
We here demonstrated an increase of α-syn presence and phosphorylation in enteric neurons with age. α-Syn appeared in both excitatory (positive for calretinin) and inhibitory enteric motor neurons (positive for nNOS); therefore, it is likely that accumulated and aggregated α-syn not only contributes to the spread of protein to the brain via the vagus, but also causes gastrointestinal dysfunction, leading to the gastrointestinal symptoms in PD. Available evidence has shown that increased intestinal permeability is associated with the presence of α-syn in the intestinal mucosa at the early stages of PD [28].
What causes α-syn accumulation and aggregation in the enteric neurons is still not clear. In the present study, we demonstrated that normal development and aging can be a major contributing factor. Recent studies have also suggested that multiple risk factors may individually or jointly contribute to this, for example, gut microbiota [29], intestinal inflammation [30], and enteric glial cell dysfunction [31]. The results here provide evidence that the BAC α-syn-GFP transgenic mouse model is a useful tool in which to study α-syn accumulation, aggregation, and spread in enteric neurons in relation to gastrointestinal function and PD pathogenesis. Understanding how the pathology is initiated in the gut will help treat the gastrointestinal dysfunction and perhaps aid in preventing or delaying the onset and progression of PD.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81430025) and Northeastern University, China, as well as the Swedish Research Council (K2015-61X-22297-03-4), the EU Joint Programme—Neurodegenerative Disease Research (aSynProtec and REfreAME), EU H2020-MSCA-ITN-2016 (Syndegen), Basal Ganglia Disorders Linnaeus Consortium-Excellence in Parkinson and Huntington Research, the Strong Research Environment MultiPark (Multidisciplinary Research on Parkinson’s disease), the Swedish Parkinson Foundation (Parkinsonfonden), the Torsten Söderbergs Foundation, and the Olle Engkvist Byggmästere Foundation. W. L. is supported by a scholarship from the China Scholarship Council.
References
- 1.Eschbach J, Danzer KM. Alpha-synuclein in Parkinson’s disease: pathogenic function and translation into animal models. Neurodegener Dis. 2014;14:1–17. doi: 10.1159/000354615. [DOI] [PubMed] [Google Scholar]
- 2.Cersosimo MG, Benarroch EE. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis. 2012;46:559–564. doi: 10.1016/j.nbd.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127:235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 4.Stirpe P, Hoffman M, Badiali D, Colosimo C. Constipation: an emerging risk factor for Parkinson’s disease? Eur J Neurol. 2016;23:1606–1613. doi: 10.1111/ene.13082. [DOI] [PubMed] [Google Scholar]
- 5.Cao M, Gu ZQ, Li Y, Zhang H, Dan XJ, Cen SS, et al. Olfactory dysfunction in Parkinson’s disease patients with the LRRK2 G2385R variant. Neurosci Bull. 2016;32:572–576. doi: 10.1007/s12264-016-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Del Tredici K, Rub U, De Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, De Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 9.Hansen C, Li JY. Beyond alpha-synuclein transfer: pathology propagation in Parkinson’s disease. Trends Mol Med. 2012;18:248–255. doi: 10.1016/j.molmed.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avetisyan M, Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest. 2015;125:899–907. doi: 10.1172/JCI76307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 13.Hansen C, Bjorklund T, Petit GH, Lundblad M, Murmu RP, Brundin P, et al. A novel alpha-synuclein-GFP mouse model displays progressive motor impairment, olfactory dysfunction and accumulation of alpha-synuclein-GFP. Neurobiol Dis. 2013;56:145–155. doi: 10.1016/j.nbd.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Hsu LJ, Mallory M, Xia Y, Veinbergs I, Hashimoto M, Yoshimoto M, et al. Expression pattern of synucleins (non-Abeta component of Alzheimer’s disease amyloid precursor protein/alpha-synuclein) during murine brain development. J Neurochem. 1998;71:338–344. doi: 10.1046/j.1471-4159.1998.71010338.x. [DOI] [PubMed] [Google Scholar]
- 15.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 17.Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini C, Antonioli L, Colucci R, Ballabeni V, Barocelli E, Bernardini N, et al. Gastric motor dysfunctions in Parkinson’s disease: Current pre-clinical evidence. Parkinsonism Relat Disord. 2015;21:1407–1414. doi: 10.1016/j.parkreldis.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrini C, Colucci R, Antonioli L, Barocelli E, Ballabeni V, Bernardini N, et al. Intestinal dysfunction in Parkinson’s disease: Lessons learned from translational studies and experimental models. Neurogastroenterol Motil. 2016;28:1781–1791. doi: 10.1111/nmo.12933. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 21.Aldecoa I, Navarro-Otano J, Stefanova N, Sprenger FS, Seppi K, Poewe W, et al. Alpha-synuclein immunoreactivity patterns in the enteric nervous system. Neurosci Lett. 2015;602:145–149. doi: 10.1016/j.neulet.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Gold A, Turkalp ZT, Munoz DG. Enteric alpha-synuclein expression is increased in Parkinson’s disease but not Alzheimer’s disease. Mov Disord. 2013;28:237–240. doi: 10.1002/mds.25298. [DOI] [PubMed] [Google Scholar]
- 23.Pouclet H, Lebouvier T, Coron E, Des Varannes SB, Neunlist M, Derkinderen P. A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson’s disease. Neurogastroenterol Motil. 2012;24:e202–e205. doi: 10.1111/j.1365-2982.2012.01887.x. [DOI] [PubMed] [Google Scholar]
- 24.Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124:665–680. doi: 10.1007/s00401-012-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbille AG, Letournel F, Kordower JH, Lee J, Shanes E, Neunlist M, et al. Evaluation of alpha-synuclein immunohistochemical methods for the detection of Lewy-type synucleinopathy in gastrointestinal biopsies. Acta Neuropathol Commun. 2016;4:35. doi: 10.1186/s40478-016-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’sullivan GA, Pal A, et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2012, 2: 898. [DOI] [PMC free article] [PubMed]
- 27.Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 28.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 30.Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Clairembault T, Leclair-Visonneau L, Neunlist M, Derkinderen P. Enteric glial cells: new players in Parkinson’s disease? Mov Disord. 2015;30:494–498. doi: 10.1002/mds.25979. [DOI] [PubMed] [Google Scholar]