Abstract
Physical activity has been proposed as a promising intervention to improve cognition and decrease the risk of dementia in older adults. Brain-derived neurotrophic factor (BDNF) appears to mediate, at least partially, these effects of exercise. However, intervention studies of the effects of multimodal exercises on cognition and BDNF levels are scarce and composed by small samples. Thus, the generalization of the conclusions of these studies depends on the reproducibility of the results. In order to contribute to the knowledge on the field, the present study evaluated the effects of a physical activity intervention composed by muscle strengthening and aerobic conditioning on BDNF levels and cognition in older women. Independent and non-demented subjects (≥75 years) were assigned to a 3-month physical activity intervention (n = 22, 60 min exercise sessions three times a week) or to a control condition (n = 10, no exercise). Clinical (anxiety and depression symptoms), neuropsychological (Digit Span, Stroop, Trail Making, and Contextual Memory tests), physical (upper and lower limb strength, aerobic conditioning), and physiological (serum BDNF) parameters were evaluated immediately before, 1 month, and 3 months after starting intervention. Results indicated that controls had stable levels for all measured variables, whereas the intervention group improved on physical fitness, depressive symptoms, cognitive performance, and BDNF levels. Moreover, a linear regression identified an association between aerobic conditioning and BDNF levels. In conclusion, combined muscle strengthening and aerobic conditioning was able to improve cognitive performance and increase BDNF levels. Aerobic conditioning seems to be an important mediator of these outcomes.
Keywords: BDNF, Physical activity, Depression symptom, Cognitive performance
Introduction
The global population is aging and the increase in longevity is associated with an increase of older individuals with cognitive impairment (Prince et al. 2013). Considering the devastating effects of cognitive decline on life quality and general health, the establishment of appropriate interventions to prevent, rehabilitate, and/or manage these age-related dysfunctions are important goals for public health.
Previous studies suggest that physical activity interventions can improve cognition (e.g., enhancing memory, attention, executive functions) (Desjardins-Crepeau et al. 2016; Tarazona-Santabalbina et al. 2016) and decrease the risk of dementia (Lista and Sorrentino 2010). These effects of physical activity could be mediated by its capacity to address dementia risk factors (e.g., diabetes mellitus, hypertension, obesity, depression) and modulate the levels of neurotransmitters and neurotrophins (Floel et al. 2010; Neeper et al. 1995), such as the brain-derived neurotrophic factor (BDNF) (Vaynman et al. 2004). BDNF can modulate several mechanisms that improve and/or protect cognitive functioning, such as neurogenesis, neuronal plasticity, and survival (Novkovic et al. 2015).
It is generally assumed that peripheral (plasma/serum) and central (brain) BDNF levels are correlated (O’Bryant et al. 2011; Rasmussen et al. 2009) and that BDNF decreases with aging (Gunstad et al. 2008; Lommatzsch et al. 2005) and increases with physical activity (Lustosa et al. 2011; Nascimento et al. 2014; Pereira et al. 2013). However, there are relatively few studies that evaluate the effects of multimodal exercises (combined muscle strength and aerobic conditioning protocols) on BDNF levels. From a global health perspective, these are the more advantageous exercises for the elderly. Resistance training counteracts the loss of muscle mass, strength, and potency, all of which impair functionality of older adults (Volpi et al. 2004; Wolfson et al. 1995). On the other hand, aerobic conditioning can protect from cardiovascular dysfunctions (Fleg 2002; Jaureguizar et al. 2016; Tadjibaev et al. 2014) and metabolic syndrome (Chodzko-Zajko et al. 2009), which are among the most common chronic conditions associated with aging (Vaes et al. 2012).
The aim of this study was to expand the knowledge about the effects of physical activity on cognition in older adults. Intervention studies that showed increased BDNF levels and improved cognitive performance after a period of multimodal exercises were composed by small samples of community-dwelling older adults (Nascimento et al. 2014; Vaughan et al. 2014), probably because of the difficulties inherent to this type of research. Thus, the generalization of the conclusions of these studies depends on the reproducibility of the results in different samples and conditions. In this context, we evaluated the effects of a 3-month physical activity intervention, composed by a combination of resistance and aerobic conditioning exercises, on BDNF levels and on cognitive functions in a sample of elderly women, all of them residing in the same retirement home.
Methods
Sample
Participants included 31 women (80 to 97 years), recruited from a residential home for elderly located in Porto Alegre, Brazil. The participants were assigned to a 3-month physical activity intervention (60 min exercise sessions three times a week, n = 22) or to a control condition (no exercise, n = 10). All volunteers were autonomous, being able to perform their daily activities without assistance and leave the residential home whenever they wanted. Even so, they usually stayed at the retirement home, following its routine, which was mainly determined by mealtimes.
Inclusion criteria for either experimental group were age (≥75 years) and not being physically active in the last 6 months. Thus, they should not be practicing physical activity (recreational or oriented) for at least 6 months. Exclusion criteria were lower limb amputation or bone fractures in the past 6 months, neurological injures (e.g., traumatic brain injury, stroke), disorders (e.g., neurodegenerative diseases), or report of neurosurgical procedures, major unstable medical illnesses (e.g., metastatic cancer, acute inflammatory conditions), medications that interfere with cognitive functions and BDNF levels, and scores on Mini-Mental State Examination (MMSE) indicative of dementia (Folstein et al. 1975). Cutoff values for the Brazilian version of MMSE were <18 for middle educational level (4 to 8 years of formal education) and <26 for high educational level (more than 8 years of formal education) (Bertolucci et al. 1994).
We also controlled the experimental groups for depressive and anxiety symptoms, since we know that they can be modulated by physical activity and influence the results of cognitive tests. Symptoms of depression and anxiety were assessed with the Brazilian adapted and validated version of Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI) (Cunha 2011).
Participants selected for the intervention group were also required to obtain consent from their personal physician to participate in the 3-month physical activity program, which was composed of muscle strengthening and aerobic conditioning activities. The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and approved by the Research and Ethics Committee of Pontifical Catholic University, Porto Alegre, Brazil. All participants gave their written informed consent.
Experimental procedures
All subjects and experimental variables (depression and anxiety symptoms, muscle strength, aerobic conditioning, BDNF levels, and neuropsychological measures) were analyzed at three sampling times: immediately before (baseline), 1 month, and 3 months after starting the physical intervention program.
Measurement of muscle strength and aerobic conditioning
Aerobic conditioning of participants was estimated with the 6-min walk test (6MWT) (Bean et al. 2002; Solway et al. 2001), lower limb strength (LLS) was evaluated with the 30-s chair-stand test (Jones et al. 1999), and handgrip strength was used as a measure of upper limb strength (ULS) (Baltzopoulos and Brodie 1989) and evaluated with an Hydraulic Dynamometer SH5001 (Saehan).
Serum BDNF measurement
For BDNF analysis, 5 ml of blood was collected from each subject by venipuncture into a free-anticoagulant vacuum tube. The blood was immediately centrifuged at 4000×g for 10 min, and serum was frozen at −80 °C until further analysis. BDNF levels were measured by Sandwich ELISA (Merck Millipore, Darmstadt, Germany) following manufacturer’s instructions.
Neuropsychological measures
The effects of the physical activity intervention on attention and working memory were evaluated with the Forward and Backward Digit Span Tests of the Wechsler Adult Intelligence Scale (Wechsler 1997). The Trail Making A and B Tests (Strauss et al. 2006) were administered for processing speed and executive function assessment. The Logical Memory Tests I and II (Wechsler 1987) were used to analyze immediate and delayed recall. Response inhibition capacity was analyzed with the Stroop Color–Word test (Strauss et al. 2006).
All procedures related to the neuropsychological assessments followed the recommended guidelines and were briefly described elsewhere (Correa et al. 2015).
Physical activity intervention: muscle strengthening and aerobic conditioning
Physical activity protocols were performed only with the intervention group (n = 20) in the courtyard of the residential home, oriented and supervised by a physiotherapist and his assistants. The physical activity intervention was done three times a week and lasted 3 months. During this period, all volunteers were instructed to maintain their usual activities and to refrain from initiating other physical exercise programs.
All sessions lasted 60 min and started and ended with warm-up and cooldown stretching exercises accompanied by relaxing music. During each session, participants engaged in toning exercises (using resistance bands) for the following muscles: rectus femoris, hamstrings, triceps surae, and psoas. The lower limb strengthening intervention was then performed for hip flexion, abduction, adduction, extension, knee flexion, and mini-squat. Upper limb strengthening was also done with the aid of resistance bands for the biceps, triceps, and deltoid muscles.
The appropriate training load for each subject was obtained based on its maximal resistance (MR). Initially, exercises were done at 50% of the estimated 1MR load and then gradually increased until reaching at least 75% of 1MR from the third week of training (Lustosa et al. 2011; Watanabe et al. 2014). Each exercise was done in three sets with an interval of 30 s between them. Each set consisted of ten repetitions with a 10-s isometric hold (Kern et al. 2002). After completion of the strengthening exercises, which lasted about 30 min, participants were instructed to walk a specified distance in the residential courtyard. The walking distance was increased every week until reaching a 30-min walk. Heart and breath rates, as well as arterial pressure and oxygen saturation (O2 sat), were monitored during and at the end of the physical activity. All exercises were done in the 75–85% range of the maximum heart rate value (Blair et al. 1991; Strath et al. 2000).
Volunteers of the control group (n = 10) were instructed to maintain their usual activities and to refrain from initiating any physical exercise programs during data collection for this study.
Statistical analysis
All variables were tested for homogeneity of variances and normality of distribution by means of the Levene and Shapiro-Wilk tests, respectively, and met parametric assumptions. Continuous demographic and clinical data were compared with independent sample t tests, whereas categorical variables were compared with chi-square statistics. Fitness indicators (muscle strength and aerobic conditioning), psychiatric symptoms, BDNF levels, and performance on neuropsychological tests were analyzed with dependent measures ANOVAs to evaluate alterations of these variables along the sampling times in each experimental group. One-way ANOVAs were used to compare intervention and control groups in each sampling time. Bonferroni’s post hoc test was used whenever necessary. All statistical procedures employed for the analysis of BDNF levels and performance on neuropsychological tests were repeated to evaluate the effect of age, education, and/or scores on BDI as covariates (see rationale for doing so below). Finally, relations between physical conditioning (muscle strength and aerobic conditioning) and BDNF levels in the intervention group were evaluated with linear regression analysis. Statistical power of all analysis was ≥0.80, significance level was set at α = 0.05 (two-tailed), and continuous variables were expressed as mean ± standard deviation. Statistical analyses were performed using the Statistical Package for Social Sciences, SPSS Statistics 17.0 software (SPSS Inc., Chicago, IL, USA), and figure preparation was performed with Prism 5.0 (GraphPad Software Inc. San Diego, CA).
Results
Demographic and clinical characteristics
Two of the volunteers initially admitted in the intervention group were excluded from statistical analysis because they began antidepressant medication use (which could interfere in the evaluation of psychiatric symptoms and BDNF levels) during the physical activity program. In the control group, one volunteer was excluded because she began to attend to an oriented physical activity (swimming).
Table 1 shows the demographic and psychiatric characteristics of the intervention (n = 20) and control (n = 9) groups. As can be seen, no significant group differences were seen for age, BMI, and baseline scores on MMSE, BDI, and BAI [all p > 0.05]. However, education levels were somewhat higher in the control group [t = 2.176, df = 29, p = 0.038].
Table 1.
Demographic and clinical data in the control and intervention groups (mean ± SD)
| Control n = 9 | Intervention n = 20 | p | |
|---|---|---|---|
| Age (years) | 77.33 ± 9.89 | 83.00 ± 6.53 | 0.068 |
| Education (years) | 10.11 ± 3.82 | 7.40 ± 3.39 | 0.038 |
| BMI | 24.88 ± 3.95 | 24.83 ± 3.76 | 0.916 |
| MMSE | 24.77 ± 3.30 | 24.10 ± 3.30 | 0.725 |
| Baseline BDI | 9.77 ± 6.18 | 10.55 ± 6.00 | 0.622 |
| Baseline BAI | 4.77 ± 4.89 | 5.05 ± 3.29 | 0.423 |
BMI body mass index, MMSE Mini-Mental State Examination, BDI Beck Depression Inventory, BAI Beck Anxiety Inventory
Effects of physical activity on muscle strength, aerobic conditioning, and psychiatric symptoms
As shown in Table 2, physical fitness parameters and psychiatric symptoms remained constant along sampling times in the control group [all p > 0.05]. However, improvements in muscle strength of lower limbs [F(2,38) = 43.387, η2ρ = 0.695, p < 0.001], aerobic conditioning [F(2,38) = 17.975, η2ρ = 0.486, p < 0.001], and depressive symptoms [F(2,38) = 9.09, ƞ2ƿ = 0.324, p = 0.001] were seen in the intervention group. Bonferroni’s post hoc test indicated that lower limb strength and aerobic conditioning had a stepwise improvement during the physical activity protocol [all p < 0.05 between the baseline and first month, and between the first and third month]. In turn, improvements in depressive symptoms were observed only at the third month of exercise [p = 0.001 in comparison to baseline]. Even so, all these improvements were modest, since they did not lead to significant group differences, as shown by the one-way ANOVAs for each sampling time [all p > 0.05].
Table 2.
Psychiatric and physical conditioning parameters of the control and intervention groups along the experimental period [mean (±SD)]
| Control n = 9 | Intervention n = 20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6MWT | LLS | ULS | BDI | BAI | 6MWT | LLS | ULS | BDI | BAI | |
| Baseline | 227.25 (±123.77) | 7.75 (±4.49) | 16.86 (±6.08) | 9.77 (±6.18) | 4.77 (±4.89) | 185.05 (±65.85) | 6.85 (±3.37) | 14.90 (±4.45) | 10.30 (±4.11) | 6.51 (±5.75) |
| 1 month | 232.87 (±125.06) | 7.75 (±4.09) | 16.21 (±6.32) | 8.55 (±5.65) | 3.77 (±3.30) | 204.45* (±61.69) | 9.25* (±2.80) | 14.76 (±4.07) | 7.40 (±5.20) | 4.75 (±5.75) |
| 3 months | 245.37 (±125.74) | 8.62 (±4.30) | 16.20 (±4.92) | 7.66 (±8.37) | 5.66 (±7.61) | 240.20** (±8.66) | 12.45** (±3.99) | 16.42 (±4.45) | 4.35* (±3.43) | 3.150 (±3.42) |
| p | 0.063 | 0.202 | 0.836 | 0.723 | 0.743 | p < 0.001 | p < 0.001 | 0.126 | p < 0.001 | p = 0.056 |
BAI Beck Anxiety Inventory, BDI Beck Depression Inventory, ULS upper limb strength, LLS lower limb strength, 6MWT 6-min walk test (6MWT), p repeated measures ANOVA
*p < 0.05 in comparison to baseline; **p < 0.05 in comparison to the baseline and first month of physical activity intervention
Effects of physical activity on serum BDNF levels
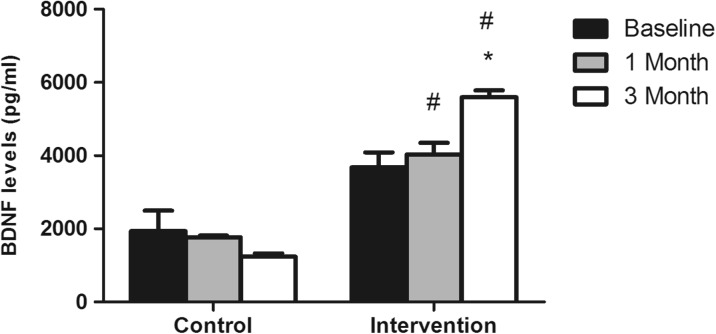
The BDNF levels remained without significant alterations in the control group [F(2,16) = 1.193, η2ρ = 0.329, p = 0.32] during the 3-month period of the study, but increased in the intervention group [F(2,38) = 6.301, η2ρ = 0.249, p = 0.004]. As indicated by Bonferroni’s post hoc test, the highest BDNF values were achieved at the third month of the physical activity intervention [p = 0.006 in comparison to baseline and p = 0.007 in comparison to first month values] (Fig. 1). Moreover, the comparison of the intervention and control groups at each sampling time indicated that BDNF levels were significantly higher in the intervention group at the first [F(1,27) = 23.84, η2ρ = 0.451, p < 0.01] and third [F(1,27) = 243.166, η2ρ = 0.893, p < 0.01] months of the physical activity program.
Fig. 1.
Serum BDNF concentration (pg/ml) before (baseline), 1 month, and 3 months after the beginning of the physical activity intervention. *p < 0.01 in comparison to baseline and first month levels of the intervention group; # p < 0.05 in comparison to the control group in the same sampling times
To strengthen the internal validity and generalizability of our results, we also evaluated if age, education, and scores on BDI could be confounding variables in the statistical analysis of BDNF levels. The rationale in doing so is related to the composition of our sample (which showed a relatively broad age distribution, 73 to 97 years, and significant between group differences in years of education and to the significant effects of the exercise intervention on BDI scores. Literature suggests that age and depressive symptoms can have negative relations with BDNF levels (Archer et al. 2014; Bus et al. 2012; Erickson et al. 2010; Martinowich et al. 2007). On the other hand, years of education is often used as a proxy variable to estimate cognitive reserve (Barulli and Stern 2013) and different studies suggest that the extent of cognitive reserve could be associated with increased BDNF availability (Beeri and Sonnen 2016; Ward et al. 2015). Thus, education could have a positive association with BDNF levels.
The results of the covariance analysis indicated that age, years of education, and scores on BDI had no significant effects as covariates (all p > 0.05) and, consequently, the results previously described for the BDNF analysis remained unchanged.
Finally, regression analysis indicated a significant association between the improvement of aerobic conditioning and the increase in circulating BDNF levels in the intervention group [R = 0.52; B = 9.07; p = 0.02].
Effects of physical activity on neuropsychological measurements
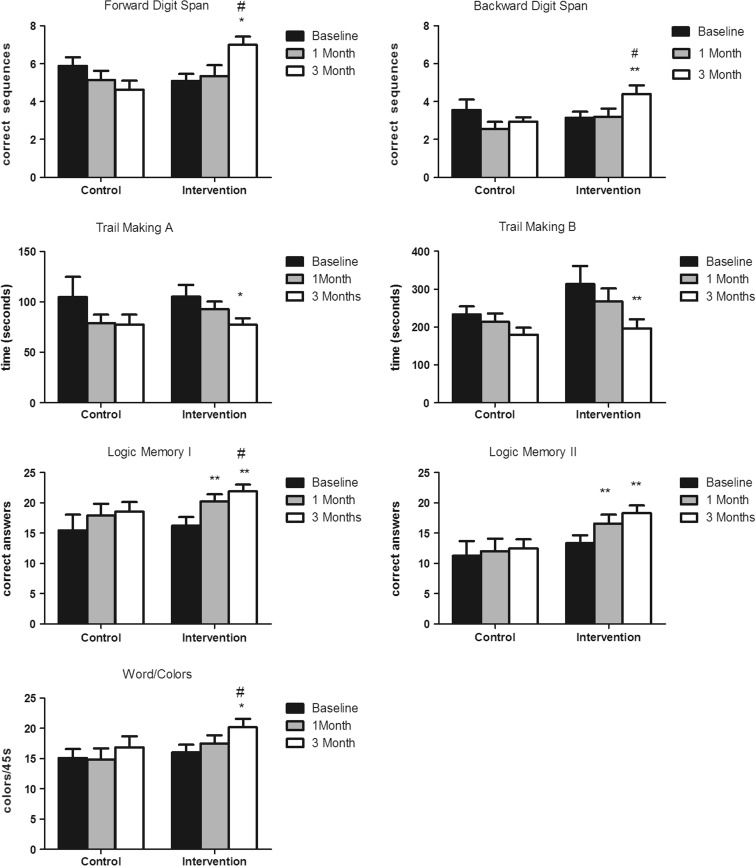
Repeated measures ANOVAs indicated that the performance of the control group on the neuropsychological tests remained without significant alterations during the 3 months of the study [all p > 0.05]. On the other hand, performances of the intervention group improved in all neuropsychological tasks, as indicated by the repeated measures ANOVAS for Forward Digit Span [F(2,38) = 8.665, η2ρ = 0.95, p < 0.001], Backward Digit Span [F(2,38) = 3.994, η2ρ = 0.174, p = 0.027], Logical Memory I [F(2,38) = 10.797, η2ρ = 0.238, p < 0.001], Logical Memory II [F(2,38) = 7.507, η2ρ = 0.320, p = 0.003], Trail Making A [F(2,38) = 6.627, η2ρ = 0.241, p = 0.003], Trail Making B [F(2,38) = 6.627, η2ρ = 0.241, p = 0.037], and Stroop [F(2,38) = 8.332, η2ρ = 0.149, p = 0.002] tasks. Bonferroni’s post hoc test indicated that the scores of the intervention group on Logical Memory I and II already increased in the first month of the intervention [all p < 0.05 in comparison to baseline scores], remaining stable until the last sampling time [all p > 0.05 in comparison to the first month]. All other neuropsychological tasks showed significant improvements only at 3 months of physical activity intervention [all p < 0.05 in comparison to baseline and p > 0.05 in comparison to the first month].
Further comparisons between the control group and intervention group at each experimental sampling time indicated no significant differences between groups at baseline and 1 month. However, at 3 months, the scores of the intervention group were significantly better than the control group on Forward Digit Span [F(1,27) = 10.775, η2ρ = 0.093, p = 0.003], Backward Digit Span [F(1,27) = 16.334, η2ρ = 0.451, p = 0.032], Logical Memory I [F(1,27) = 435.271, η2ρ = 0.185, p < 0.001], and Stroop [F(1,27) = 5.058, η2ρ = 0.175, p = 0.033] tasks.
The results of the covariance analysis indicated that age, education, and BDI scores had no significant effects as covariates (all p > 0.05) and, consequently, the results previously described for the analysis of neuropsychological tests remained unchanged.
To summarize, performance on all neuropsychological tasks improved in the intervention group. However, the improvements were modest and mainly seen in the third month of the physical activity protocol (Fig. 2).
Fig. 2.
Performance of the control and intervention groups on neuropsychological tests evaluated at baseline, 1 month, and 3 months after the beginning of the physical activity intervention. *p < 0.05 in comparison to the other sampling times of this group; **p < 0.05 in comparison to baseline of this group; # p < 0.05 in comparison to the third month of the control group
Discussion
The main findings of this study indicate that the 3-month intervention protocol, composed of muscle strengthening and aerobic conditioning, was capable to increase BDNF levels and induce improvements on attention, working memory, declarative memory, and executive function (inhibitory response capacity) in older women residing in an assisted living home. Moreover, the BDNF increase was significantly and positively associated with the improvement in aerobic conditioning.
The exercise program used in this study was able to increase muscle strength and aerobic conditioning in the intervention group along the 3-month intervention. However, since the duration of the exercise protocol was relatively short, increases in physical fitness were modest and resulted in the lack of significant differences between the intervention and control groups at the end of the experimental period. Similar results were observed in multimodal exercise interventions that lasted between 10 and 16 weeks (Nascimento et al. 2014; Suzuki et al. 2013), and suggest that longer exercise protocols can lead to greater physical fitness improvements (Pahor et al. 2014; Van der Bij et al. 2002). Depressive symptoms also showed a modest improvement with the combined physical activity intervention, clearly indicating that it has potential for mental, as well as physical, health modulation. It is worth noting that our study subjects were mainly at the minimum symptoms range for depression (Cunha 2011), in which physical interventions usually are more effective, but not less important, since these psychiatric symptoms are related to impairments in activities of daily living (Strait and Lakatta 2012).
The BDNF levels of the intervention group increased from baseline to the third month of physical activity, already showing higher BDNF levels than the control group at the first month of the exercise intervention. Moreover, the increase in the mean BDNF level was accompanied by a considerable decrease in the standard deviation (from 3746.61 ± 1891.01 pg/ml at baseline to 5559.69 ± 807.87 pg/ml at 3 months), suggesting that our exercise protocol had a greater effect on subjects with the lowest BDNF levels. Former studies using multimodal exercise protocols also observed increases in BDNF levels in healthy elderly and subjects with mild cognitive impairment (MCI), but only when the intervention lasted more than 10 weeks (Nascimento et al. 2014; Suzuki et al. 2013; Vaughan et al. 2014). The relative contribution of resistance versus aerobic conditioning exercises in promoting the BDNF increases are still a matter of debate (Baker et al. 2010a, b; Coelho et al. 2013; Erickson et al. 2011; Pereira et al. 2013). Interestingly, the elevation of BDNF seen in this study correlated with aerobic conditioning, but not with the resistance gain. However, we find it premature to rule out an effect of resistance training on the increase in BDNF levels, since our study has some limitations that must be emphasized, as discussed below. Thus, the result that must be highlighted here is that a combined muscle strength and aerobic conditioning intervention can increase BDNF levels, and that aerobic conditioning is at least partially responsible for that improvement.
The literature suggests that BDNF could be a key component of the beneficial effects of physical activity on cognitive functioning, since this neurotrophin can modulate neurogenesis (Wei et al. 2015), neuroplasticity (Leal et al. 2017), and neuronal survival (Park and Poo 2013), all of which could improve cognitive processing (Bekinschtein et al. 2008; Novkovic et al. 2015) and protect against age-related cognitive declines (Carlino et al. 2013; Erickson et al. 2012). In accordance with this notion, our results showed that the BDNF increase seen in the intervention group was accompanied by improved performances on all neuropsychological tests evaluated. Therefore, at the end of the 3-month exercise protocol, scores on attention, working memory, declarative memory, and executive function (inhibitory response capacity) were higher in the intervention group in comparison to the sedentary control group. These results are in accordance with previous studies that reported positive effects of multimodal exercise interventions on cognitive functioning in healthy as well as in MCI older adults (Lee et al. 2015; Nascimento et al. 2014; Suzuki et al. 2013; Vaughan et al. 2014).
Although tempting, the experimental design of the present study does not allow the establishment of a causal relationship between the increases in BDNF levels and the improvement on cognitive performance. Besides BDNF modulation, physical activity can set in motion other mechanisms that can improve (directly or indirectly) cognitive functioning. In this context, we could cite different trophic factors (such as fibroblast, nerve, insulin-like and vascular endothelial growth factors), among other substances, that were also suggested to be associated with exercise-induced benefits on brain structures and cognitive performance (Colcombe and Kramer 2003). Even so, the results of this exploratory study give support to the hypothesis that BDNF could be a key intermediate of the effects of physical exercise on cognition.
Among the limitations regarding the current study are sample size and composition. Our sample was small, consisted only of women and all of them resided in the same retirement home, thus restricting the generalization of the results to the elderly population. However, the homogeneity of our sample, in terms of general life conditions (housing, diet type, daily routine, and activities) and participation in the physical activity protocol (all subjects participated in all exercise sessions), must be emphasized as a strength of this study. Literature suggests that BDNF levels are sensitive to life conditions (Bus et al. 2011; Rosas-Vargas et al. 2011) and style (Chan et al. 2008), and most studies with greater samples than ours suffer with the variability of these parameters among their volunteers, besides the difficulty to engage them continuously in their exercise programs (Carvalho et al. 2014). Moreover, former studies recruited volunteers from the community (Nascimento et al. 2014; Suzuki et al. 2013; Vaughan et al. 2014). We choose a sample of women residing in the same residential home because they could reflect the real importance of the introduction of a multimodal exercise program for the physical and mental health of older adults residing in care facilities, since they normally have different characteristics and needs in comparison to the more independent community volunteers.
In conclusion, our study shows that a multimodal exercise intervention can increase BDNF levels and enhance cognitive functioning (besides improving physical fitness and symptoms of mood disorders) in non-demented elderly women residing in an assisted living home. Thus, our results give support to former studies that showed the positive effects of multimodal exercises on BDNF levels and cognition. Moreover, our study reinforces the notion that multimodal exercises are promising, low-cost, and low-risk interventions able to simultaneously improve physical fitness and mental health. Future studies should analyze greater samples, include men, and extend the intervention period, in order to further the generalization of the results and evaluate the full potential of this kind of exercise protocol on the promotion of successful aging. Moreover, it would be very interesting to also evaluate other physiological parameters known to be sensible to physical activity interventions and capable to interact with BDNF levels and cognitive functions, such as other neurotrophic factors and inflammatory status markers.
Acknowledgments
E. Bromberg and I.I.L. Argimon are National Counsel of Technological and Scientific Development (CNPq) research fellows. K. Vedovelli has a Research Support Foundation of Rio Grande do Sul (FAPERGS)/Coordination for the Improvement of Higher Education Personnel (CAPES) fellowship, and B.L. Giacobbo, M.S. Corrêa, and A. Wieck have a CAPES fellowship.
Compliance with ethical standards
The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and approved by the Research and Ethics Committee of Pontifical Catholic University, Porto Alegre, Brazil. All participants gave their written informed consent.
References
- Archer T, Josefsson T, Lindwall M. Effects of physical exercise on depressive symptoms and biomarkers in depression. CNS Neurol Disord Drug Targets. 2014;13(10):1640–1653. doi: 10.2174/1871527313666141130203245. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22:569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzopoulos V, Brodie DA. Isokinetic dynamometry. Applications and limitations. Sports Med (Auckland, NZ) 1989;8(2):101–116. doi: 10.2165/00007256-198908020-00003. [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17(10):502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean JF, Kiely DK, Leveille SG, Herman S, Huynh C, Fielding R, Frontera W. The 6-minute walk test in mobility-limited elders: what is being measured? J Gerontol A Biol Sci Med Sci. 2002;57:M751–M756. doi: 10.1093/gerona/57.11.M751. [DOI] [PubMed] [Google Scholar]
- Beeri MS, Sonnen J (2016) Brain BDNF expression as a biomarker for cognitive reserve against Alzheimer disease progression. Neurology. United States. doi:10.1212/WNL.0000000000002389 [DOI] [PubMed]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neurosci Rev J Bringing Neurobiol Neurol Psychiatr. 2008;14(2):147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Bertolucci PH, Brucki SM, Campacci SR, Juliano Y. The Mini-Mental State Examination in a general population: impact of educational status. Arq Neuropsiquiatr. 1994;52(1):1–7. doi: 10.1590/S0004-282X1994000100001. [DOI] [PubMed] [Google Scholar]
- Blair SN, Dowda M, Pate RR, Kronenfeld J, Howe HGJ, Parker G, et al. Reliability of long-term recall of participation in physical activity by middle-aged men and women. Am J Epidemiol. 1991;133(3):266–275. doi: 10.1093/oxfordjournals.aje.a115871. [DOI] [PubMed] [Google Scholar]
- Bus BAA, Molendijk ML, Penninx BJWH, Buitelaar JK, Kenis G, Prickaerts J, et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36(2):228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Bus BAA, Tendolkar I, Franke B, de Graaf J, den Heijer M, Buitelaar JK, Oude Voshaar RC. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatr Off J World Fed Soc Biol Psychiatr. 2012;13(1):39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlino D, De Vanna M, Tongiorgi E. Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunctions? Neurosci Rev J Bringing Neurobiol Neurol Psychiatr. 2013;19(4):345–353. doi: 10.1177/1073858412469444. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Rea IM, Parimon T, Cusack BJ. Physical activity and cognitive function in individuals over 60 years of age: a systematic review. Clin Interv Aging. 2014;9:661–682. doi: 10.2147/CIA.S55520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, Tong KY, Yip SP. Relationship of serum brain-derived neurotrophic factor (BDNF) and health-related lifestyle in healthy human subjects. Neurosci Lett. 2008;447(2–3):124–128. doi: 10.1016/j.neulet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56:10–15. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Correa MS, Vedovelli K, Giacobbo BL, de Souza CEB, Ferrari P, de Lima Argimon II, et al. Psychophysiological correlates of cognitive deficits in family caregivers of patients with Alzheimer disease. Neuroscience. 2015;286:371–382. doi: 10.1016/j.neuroscience.2014.11.052. [DOI] [PubMed] [Google Scholar]
- Cunha JA. Escalas Beck—manual. 1. São Paulo: Casa do Psicólogo; 2011. [Google Scholar]
- Desjardins-Crepeau L, Berryman N, Fraser SA, Vu TTM, Kergoat M-J, Li KZ, et al. Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clin Interv Aging. 2016;11:1287–1299. doi: 10.2147/CIA.S115711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci Off J Soc Neurosci. 2010;30(15):5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neurosci Rev J Bringing Neurobiol Neurol Psychiatr. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg JL. Can exercise conditioning be effective in older heart failure patients? Heart Fail Rev. 2002;7(1):99–103. doi: 10.1023/A:1013758008044. [DOI] [PubMed] [Google Scholar]
- Floel A, Ruscheweyh R, Kruger K, Willemer C, Winter B, Volker K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? NeuroImage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Benitez A, Smith J, Glickman E, Spitznagel MB, Alexander T, et al. Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. J Geriatr Psychiatry Neurol. 2008;21:166–170. doi: 10.1177/0891988708316860. [DOI] [PubMed] [Google Scholar]
- Jaureguizar KV, Vicente-Campos D, Bautista LR, de la Pena CH, Gomez MJA, Rueda MJC, Fernandez Mahillo I. Effect of high-intensity interval versus continuous exercise training on functional capacity and quality of life in patients with coronary artery disease: a randomized clinical trial. J Cardiopulm Rehabil Prev. 2016;36(2):96–105. doi: 10.1097/HCR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- Kern H, Hofer C, Modlin M, Forstner C, Raschka-Hogler D, Mayr W, Stohr H. Denervated muscles in humans: limitations and problems of currently used functional electrical stimulation training protocols. Artif Organs. 2002;26(3):216–218. doi: 10.1046/j.1525-1594.2002.06933.x. [DOI] [PubMed] [Google Scholar]
- Leal G, Bramham CRR, Duarte CBB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim J, Han ES, Chae S, Ryu M, Ahn KH, Park EJ. Changes in physical activity and cognitive decline in older adults living in the community. Age (Dordr) 2015;37(2):20. doi: 10.1007/s11357-015-9759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30:493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lustosa LP, Silva JP, Coelho FM, Pereira DS, Parentoni AN, Pereira LSM. Impact of resistance exercise program on functional capacity and muscular strength of knee extensor in pre-frail community-dwelling older women: a randomized crossover trial. Revista Brasileira de Fisioterapia (Sao Carlos (Sao Paulo, Brazil)) 2011;15(4):318–324. doi: 10.1590/S1413-35552011000400010. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Nascimento CMC, Pereira JR, de Andrade LP, Garuffi M, Talib LL, Forlenza OV, et al. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res. 2014;11(8):799–805. doi: 10.2174/156720501108140910122849. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C (1995) Exercise and brain neurotrophins. Nature. England. doi:10.1038/373109a0 [DOI] [PubMed]
- Novkovic T, Mittmann T, Manahan-Vaughan D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus. 2015;25(1):1–15. doi: 10.1002/hipo.22342. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Hobson VL, Hall JR, Barber RC, Zhang S, Johnson L et al, Texas Alzheimer’s Res C (2011) Serum brain-derived neurotrophic factor levels are specifically associated with memory performance among Alzheimer’s disease cases. Dement Geriatr Cogn Disord 31:31–36. doi:10.1159/000321980 [DOI] [PMC free article] [PubMed]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pereira DS, de Queiroz BZ, Miranda AS, Rocha NP, Felicio DC, Mateo EC, et al. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women—a randomized clinical trial. Arch Phys Med Rehabil. 2013;94:1443–1450. doi: 10.1016/j.apmr.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dement J Alzheimer’s Assoc. 2013;9(1):63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Rosas-Vargas H, Martinez-Ezquerro JD, Bienvenu T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch Med Res. 2011;42(6):482–494. doi: 10.1016/j.arcmed.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- Strait JBJ, Lakatta EEG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8(1):143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strath SJ, Swartz AM, Bassett DRJ, O’Brien WL, King GA, Ainsworth BE. Evaluation of heart rate as a method for assessing moderate intensity physical activity. Med Sci Sports Exerc. 2000;32(9 Suppl):S465–S470. doi: 10.1097/00005768-200009001-00005. [DOI] [PubMed] [Google Scholar]
- Strauss E, Spreen O, Sherman EMS (2006) A Compendium of Neuropsychological Tests: Administration, Norms, And Commentary. 3rd ed. Oxford University Press, editor, New York, p 1216
- Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Ito K, et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One. 2013;8(4):e61483. doi: 10.1371/journal.pone.0061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjibaev P, Frolova E, Gurina N, Degryse J, Vaes B. The relationship between physical performance and cardiac function in an elderly Russian cohort. Arch Gerontol Geriatr. 2014;59(3):554–561. doi: 10.1016/j.archger.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Tarazona-Santabalbina FJ, Gomez-Cabrera MC, Perez-Ros P, Martinez-Arnau FM, Cabo H, Tsaparas K, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17(5):426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Vaes B, Rezzoug N, Pasquet A, Wallemacq P, Van Pottelbergh G, Mathei C, et al. The prevalence of cardiac dysfunction and the correlation with poor functioning among the very elderly. Int J Cardiol. 2012;155(1):134–143. doi: 10.1016/j.ijcard.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Van der Bij AK, Laurant MGH, Wensing M. Effectiveness of physical activity interventions for older adults: a review. Am J Prev Med. 2002;22(2):120–133. doi: 10.1016/S0749-3797(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Wallis M, Polit D, Steele M, Shum D, Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing. 2014;43(5):623–629. doi: 10.1093/ageing/afu010. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7(4):405–410. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DD, Summers MJ, Saunders NL, Ritchie K, Summers JJ, Vickers JC. The BDNF Val66Met polymorphism moderates the relationship between cognitive reserve and executive function. Transl Psychiatry. 2015;5:e590. doi: 10.1038/tp.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Madarame H, Ogasawara R, Nakazato K, Ishii N. Effect of very low-intensity resistance training with slow movement on muscle size and strength in healthy older adults. Clin Physiol Funct Imaging. 2014;34(6):463–470. doi: 10.1111/cpf.12117. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1987) Manual for the Wechsler Memory Scale—revised. The psychological Corporation
- Wechsler D (1997) WAIS III: Administration and Scoring Manual. Harcourt, editor. San Antonio, TX
- Wei Z, Liao J, Qi F, Meng Z, Pan S. Evidence for the contribution of BDNF-TrkB signal strength in neurogenesis: an organotypic study. Neurosci Lett. 2015;606:48–52. doi: 10.1016/j.neulet.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50:64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]