Abstract
Interventions that target biological mechanisms of aging have great potential to enhance quality of life by delaying morbidity and mortality. The FDA-approved drug rapamycin is a compelling candidate for such an intervention. In a previous study, it was reported that 3 months of rapamycin treatment is sufficient to increase life expectancy and remodel the gut microbiome in aged mice. Transient treatment with rapamycin or a rapamycin derivative has also been shown to delay immune stem cell senescence and rejuvenate immune function in aged mice and elderly people. Periodontal disease is an important age-related disease involving altered immune function, pathological changes to the oral microbiome, and systemic inflammation. Periodontal disease is defined clinically by loss of alveolar bone and by connective tissue degeneration. Here, we describe significant alveolar bone loss during aging in two different mouse strain backgrounds and report that rapamycin treatment is sufficient to reverse age-associated periodontal disease in mice. Partial restoration of youthful levels of alveolar bone is observed in 22-month-old rapamycin-treated mice as rapidly as 8 weeks after initiation of treatment. To the best of our knowledge, this represents the first intervention shown to substantially prevent or reverse age-associated alveolar bone loss. These findings suggest the possibility that inhibition of mTOR with rapamycin or other pharmacological agents may be useful to treat a clinically relevant condition for which there is currently no effective treatment.
Electronic supplementary material
The online version of this article (10.1007/s11357-017-9994-6) contains supplementary material, which is available to authorized users.
Keywords: mTOR, Rapamycin, Aging, Healthspan, Oral health, Gum disease, Teeth, Dental health, Microbiome, Inflammation, Immune function, Mice
Introduction
Age is the single greatest risk factor for many diseases including Alzheimer’s disease, heart disease, diabetes, and periodontal disease (Kaeberlein 2013a; Eke et al. 2012; Sierra and Kohanski 2017). Aging is associated with failure to maintain homeostasis resulting from degradation of cellular maintenance and repair processes (Lopez-Otin et al. 2013; De Martinis et al. 2005). Studies in both mice and humans have shown that activation of the aged immune system results in dysregulated inflammation (Shaw et al. 2013). By delaying the biological aging process, it may be possible to reduce the impact of age-related diseases, which could have profound benefits for quality of life and reduced healthcare costs (Goldman et al. 2013; Kaeberlein et al. 2015).
The FDA-approved drug rapamycin, a specific inhibitor of mTOR, is a promising candidate to increase healthspan in people (Johnson et al. 2013a). Treatment with rapamycin has been shown to enhance longevity in numerous species (Johnson et al. 2015) and to delay or reverse multiple age-associated phenotypes including cancers (Anisimov et al. 2011), immune senescence (Chen et al. 2009), declining muscle function (Bitto et al. 2016; Fischer et al. 2015), and cognitive decline (Halloran et al. 2012; Majumder et al. 2012) in mice. Transient treatment with rapamycin beginning during middle age is sufficient to increase remaining life expectancy in mice by up to 60% (Bitto et al. 2016), enhance immune response to influenza vaccine (Chen et al. 2009), and improve cardiac dysfunction in both middle-aged mice (Dai et al. 2014; Flynn et al. 2013; Neff et al. 2013) and companion dogs (Urfer et al. 2017a; Urfer et al. 2017b). Recently, a 6-week treatment with rapamycin derivative RAD001 was reported to rejuvenate and improve immune function in healthy elderly people as measured by response to influenza vaccine (Mannick et al. 2014).
Periodontitis is an age-associated, bacterial-induced inflammatory disease of the oral cavity, which results in alveolar bone loss and tissue degradation (Darveau 2010; Socransky and Haffajee 1994). The damage to the periodontium is caused indirectly by the host response to the underlying, predominately anaerobic microbiome. Recent epidemiologic data in the US population assessed through the National Health and Nutrition Examination Survey (NHANES) suggest that more than 60% of adults aged 65 years and older have periodontitis (Eke et al. 2012), with about 35% of adults developing periodontitis between the ages of 35 and 49 (Eke et al. 2015). Further, periodontal disease is strongly associated with other age-related conditions such as heart disease, diabetes, and Alzheimer’s disease (Gil-Montoya et al. 2015; Kim and Amar 2006; Razak et al. 2014). Thus, periodontal disease represents an important health concern among elderly people, for which there is not currently any effective treatment to reverse the condition.
Because rapamycin is the most effective pharmacological agent for improving healthspan in mice (Johnson et al. 2013b; Kaeberlein 2013b) and has known anti-inflammatory (Hurez et al. 2015) and microbiome-modulatory effects (Bitto et al. 2016), we assessed the impact of rapamycin on periodontal bone levels in aged mice. Mice not only have increased susceptibility to infection-induced periodontal bone loss (Baker et al. 2000) but also like humans, mice exhibit age-associated periodontal bone loss beginning in young adulthood and continuing throughout life (Liang et al. 2010). Here, we report that aged mice of two different strain backgrounds have increased alveolar bone levels after receiving rapamycin either throughout life or transiently for 8 weeks.
Results
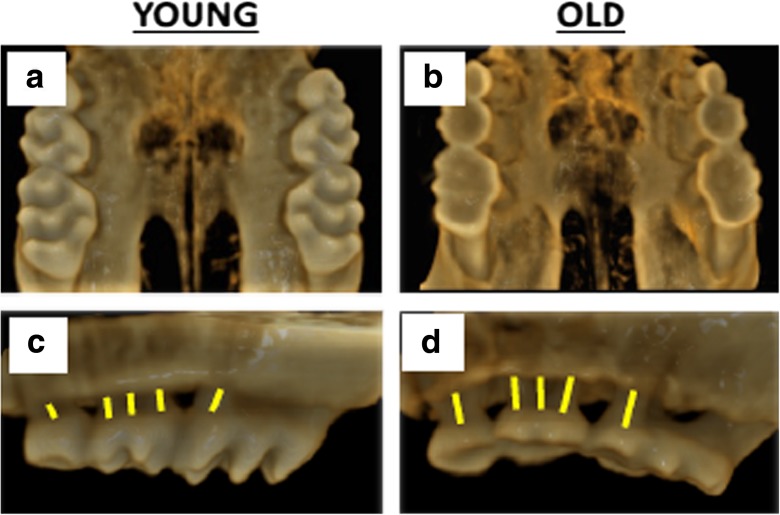
In order to quantitate periodontal disease in mice, we evaluated alveolar bone levels by high-resolution microCT imaging of the maxilla (Fig. 1). We compared young (2–3 month) and old (24 month) female C57BL/6JNia mice and observed a loss of alveolar bone with age (Fig. 1). This was quantified by the distance between the cementoenamel junction (CEJ) and the alveolar bone crest (ABC) at 14 predetermined maxillary sites, bilaterally (Fig. S1).
Fig. 1.
Aging is associated with alveolar bone loss in female C57BL6/JNia mice. Representative a, b palatal and c, d buccal aspect microCT scans of alveolar bone in young (2–3 month) and old (24 month) mice. Yellow lines represent distance between the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) as landmarked by an observer who was blinded to the identity of each animal. The larger distance in panel d compared to panel c is indicative of alveolar bone loss in the aged animal compared to the young animal
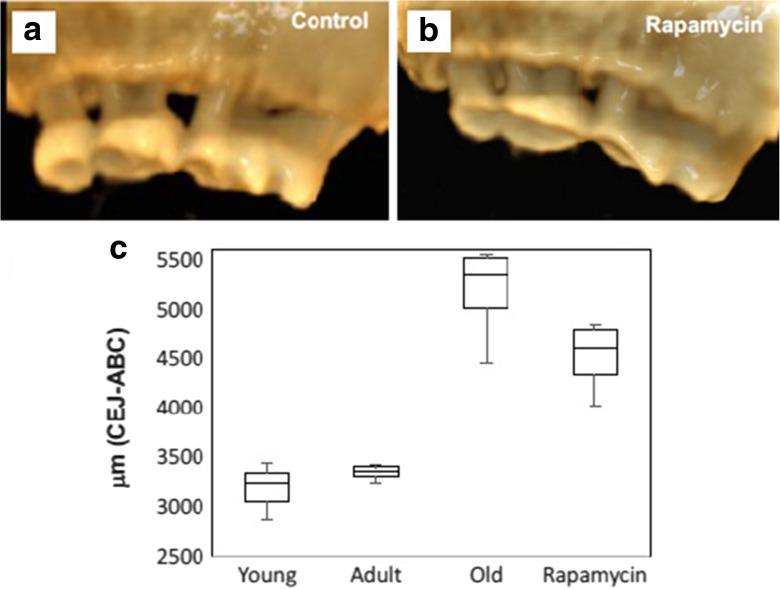
We next quantified alveolar bone loss in young, adult (9–10 month), and old female C57BL/6JNia mice and in aged mice treated with encapsulated rapamycin (eRAPA) at 14 ppm in the diet for 8 weeks beginning at 22 months of age. A significant reduction in alveolar bone, corresponding to a 55% increase in total distance between CEJ-ABC, was observed in old control mice compared to 9–10-month-old adult mice. Rapamycin-treated old mice showed significantly greater alveolar bone levels relative to age-matched controls after 8 weeks of treatment, corresponding to only 35% increase in distance between CEJ-ABC compared to young control animals. Thus, we estimate that 8 weeks of treatment with rapamycin at 14 ppm in the diet from 22 months of age is sufficient to prevent at least one third of the alveolar bone loss experienced by C57BL/6JNia mice between 3 and 22 months of age (Fig. 2, p < 0.005).
Fig. 2.
A single 8 week treatment with rapamycin attenuates alveolar bone loss in aged C57BL/6JNia mice. Female 22-month-old C57BL/6JNia mice were treated with either a control diet or 14 ppm encapsulated rapamycin diet for 8 weeks. MicroCT image analysis indicated less alveolar bone loss in the rapamycin-treated mice at the end of the treatment period compared to control animals. Representative images of a control and b rapamycin-treated mice after 8 weeks. c Measured periodontal bone levels in 2–3 month young (n = 3), 9–10 month adult (n = 4), 24-month-old control (n = 7) animals, and 24-month-old animals that had received rapamycin for 8 weeks (n = 6). Boxplot shows median, 25th, and 75th percentile, with the whiskers at the 10th and 90th percentile. Total distance was measured from CEJ-ABC buccal and palatal aspect for all mice. Rapamycin-treated animals had significantly greater alveolar bone levels compared to age-matched controls (p < 0.005)
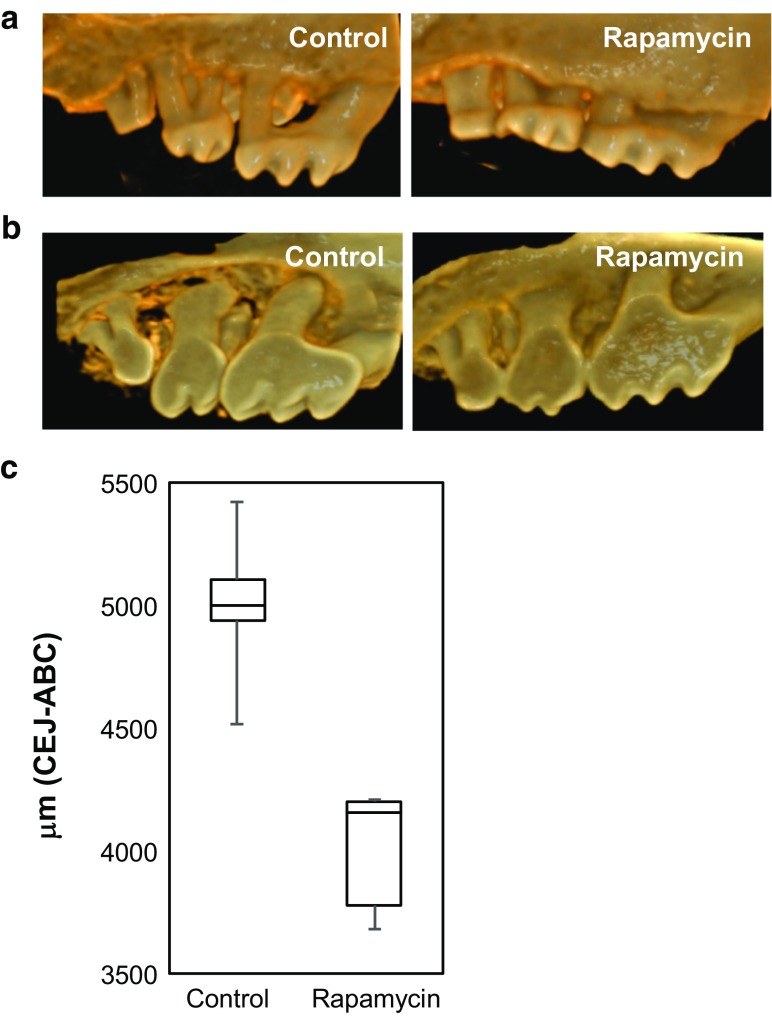
Because the influence of rapamycin on age-related periodontal disease has never before been described, and there is no other report of alveolar bone restoration during aging in any animal, we wished to assess the generality of this observation in a second mouse strain. The National Institute on Aging Interventions Testing Program (ITP) has published several studies showing that dietary eRAPA can increase lifespan in the genetically heterogeneous UMHET3 mouse strain at doses ranging from 4.6 to 42 ppm (Harrison et al. 2009; Miller et al. 2011; Wilkinson et al. 2012; Miller et al. 2014). We obtained skulls from female UMHET3 mice treated with control chow or with chow including 42 ppm eRAPA from 9 months of age until 35–37 months of age and examined their alveolar bone levels by high-resolution microCT imaging of the maxilla.
The aged UMHET3 mice had severe tooth wear on the palatal aspect that was not apparent in 24-month-old C57BL/6JNia mice (Fig. 3a, b), which caused the palatal landmarks to be difficult to identify. This greater palatal degeneration in the UMHET3 may reflect strain background differences or may be due to the later age range at which the UMHET3 mice were analyzed. As a result, only the buccal aspect was evaluated at the eight predetermined sites, bilaterally, for quantitation of alveolar bone levels. As in transiently treated C57BL/6JNia mice, UMHET3 mice treated with rapamycin throughout adult life showed significantly greater alveolar bone levels, corresponding to 25% less total distance between CEJ-ABC, compared to age-matched controls (Fig. 3c, p < 0.005).
Fig. 3.
Lifelong treatment with rapamycin preserves alveolar bone levels in UM-HET3 mice. a Representative buccal aspect scans from 35 to 37-month female UM-HET3 mice treated either drug-free control chow (n = 5) or 42 ppm encapsulated rapamycin (n = 6) beginning at 9 months of age. Visual inspection indicates preservation of alveolar bone levels in rapamycin-treated mice compared to vehicle controls. b Representative palatal aspect scans. c Boxplot showing total distance measured from CEJ-ABC for the buccal aspect only. Rapamycin-treated animals had significantly greater alveolar bone levels compared to controls (p < 0.005)
Discussion
Periodontal disease is a major health concern among the elderly and may contribute to comorbidities including diabetes, cancers, dementia, and cardiovascular disease. Currently, there is no effective treatment for age-related periodontal disease beyond preventative dental care, periodontal surgeries, and tooth removal, and approaches to prevention are imperfect. In this report, we demonstrate that the geroprotective compound rapamycin improves alveolar bone levels in aging mice. Indeed, mice treated with rapamycin for 8 weeks beginning around 22–24 months of age (roughly the mouse equivalent of 70 years of age in people) show only about half the age-related alveolar bone loss as age-matched controls. Although we cannot definitively state that this short-term treatment with rapamycin induces rejuvenation of alveolar bone levels in the aged oral cavity, due to the lack of longitudinal data for each animal, it is unlikely that this magnitude of effect could be achieved simply be preventing bone loss over an 8-week period. It is noteworthy that rapamycin can extend longevity, improve vaccine response, and enhance cardiac function in mice even when started as late as 20–24 months of age (Chen et al. 2009; Dai et al. 2014; Flynn et al. 2013; Harrison et al. 2009), consistent with the idea that it may be able to reverse some of the age-dependent pathology already seen in middle-aged and older mice.
Because rapamycin is provided in the food, we are unable at this time to determine whether its effects on periodontal disease result from local or systemic actions of the drug. It is possible, for example, that periodontal tissues are exposed to rapamycin as food passes through the oral cavity. Given that periodontal disease in the elderly is associated with proliferation of unfavorable bacteria in the oral microbiome, perhaps resulting from chronic inflammation and immune senescence (Preshaw et al. 2017), we speculate that the beneficial effects of rapamycin treatment seen here result at least in part from restoration of youthful immune function, as has been previously reported from transient treatment with mTOR inhibitors in aged mice (Chen et al. 2009) and people (Mannick et al. 2014). An alternative, but not mutually exclusive possibility, is that aging induces mTOR hyperactivation in the gingival tissue, perhaps due to changes in the oral microbiome and increased oral inflammation, which is prevented by rapamycin treatment. Future studies examining mTOR signaling in the gingival tissue and local administration of mTOR inhibitors may be able to resolve these questions.
Although this is the first demonstration of a geroprotective agent that can enhance oral health during aging, we predict that other geroprotective interventions may have similar effects on periodontal disease. Consistent with this idea, there is evidence that metformin may help preserve alveolar bone in diabetics (Agarwal 2013). Additionally, we speculate that senolytic compounds which target and kill senescence cells (Kirkland and Tchkonia 2015) may also be able to improve oral health during aging by reducing systemic inflammatory signals. In this regard, it is worth noting that rapamycin attenuates the pro-inflammatory senescence-associated secretory profile (Laberge et al. 2015; Wang et al. 2017), which could underlie some of the effects seen here. It will likewise be of interest to determine whether other interventions associated with enhanced lifespan or healthspan in mice, such as acarbose (Harrison et al. 2014), 17-alpha-estradiol (Harrison et al. 2014), fasting and fasting mimicking diets (Mattson et al. 2016), and caloric restriction, can have similar effects.
It is our belief that this work has particularly high translational potential. Periodontal disease, a major health concern in the aging population, and alveolar bone levels are easily evaluated through standard dental examination. Because rapamycin and rapamycin derivatives have been used clinically for many years, safety and dosing are well established and are compatible with short-term treatment in otherwise healthy people (Mannick et al. 2014). We propose that rapamycin could easily be tested in a clinical population to assess whether it can attenuate periodontal disease and restore youthful alveolar bone levels in older people. If successful, such a demonstration may represent a paradigm shift in treatment of this significant health problem.
Materials and methods
Animal care
Skulls utilized in this study came from previously completed studies, and no additional animals were used for this work. All animal procedures were previously approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Washington and the University of Michigan, in compliance with established Federal and State policies. The carcasses were stored in 10% neutral buffered formalin and then transferred in 1× PBS or 70% ethanol. The heads were then retrieved for alveolar bone analysis. IACUC approval was not needed for scanning, as samples were considered incidental tissue. Detailed information on the studies where the animals were collected is stated below.
C57BL/6JNia mice
C57BL/6JNia mice in this study were obtained from the National Institute on Aging Aged Rodent Colony and housed as previously described (Dai et al. 2014). Female mice arrived at 17 months old and were aged in house to 22 months, at which point they were given either rapamycin-containing diet (14 ppm rapamycin encapsulated in Eudragit, “eRapa,” in LabDiet 5053 chow) or control diet containing the Eudragit encapsulation alone. All animals were monitored daily. After 8 weeks, the animals were sacrificed by cervical dislocation.
UM-HET3 mice
UM-HET3 mice were produced at the University of Michigan using the protocols of the National Institute on Aging Interventions Testing Program (ITP). In brief, genetically heterogeneous UM-HET3 mice were produced by a cross between CByB6F1/J mothers (JAX No. 100009) and C3D2F1/J fathers (JAX No. 100004). They were housed at four female mice per cage from weaning, and at 9 months of age were given a diet containing encapsulated rapamycin (LC Labs) at 42 ppm (mg of drug per kg of food). Control mice received Purina 5LG6 food without added drug. Mice that died were not replaced, so that cage density declined at older ages. Cages were inspected daily. Date of death was noted for mice found dead, and mice found to be so ill that they were expected to die within the next 24–48 h were euthanized, with the date of euthanasia taken as the date of death for life table calculations. Mice chosen for this study were all females and were selected because they had died at ages between 1054 and 1132 days. Mean age at death for the selected control mice (1070 days) did not differ from that of the selected rapamycin-treated mice (1096 days), to minimize age at death as a possible confounding variable.
Microcomputed tomography
Fixed heads were scanned in a SkyScan 1076 microcomputed tomography (microCT) system at the Small Animal Tomographic Analysis Facility (SANTA) at Seattle Children’s Research Institute. Resolutions were at 18 or 35 μm with following settings: 55 kV, 179μA, 360-ms exposure, 0.5 AI filter, 0.7° rotation step, and 3-frame averaging. Raw scan data were reconstructed with NRecon 1.6.9, and three-dimensional (3D) renderings were generated with Drishti 2.4 (Limaye 2012).
Alveolar bone loss assay and landmark
3D rendered images (see Figs. S2–S5 for representative images) were randomized and landmarked by five-independent observers. Periodontal bone loss was measured as distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) on eight predetermined landmarks on the buccal surfaces of the maxillary molars and six predetermined landmarks on the palatal surfaces of the maxillary molars. The CEJ-ABC distances were totaled for each mouse through the Drishti software. Landmarks were completed by five-independent observers and means were calculated. A two-tailed t test was performed. p < 0.05 was taken as the criterion for significance.
Electronic supplementary material
(PDF 1.64 MB)
Acknowledgements
JA was supported by NIH training grants T90DE021984 and ARCS Foundation. EQ was supported by NIH training grants T32AG000057. This work was supported by grants from the Glenn Foundation to MK and RAM and by NIH grant AG022303 to RAM.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s11357-017-9994-6) contains supplementary material, which is available to authorized users.
References
- Agarwal A. Osteogenic action of anti-diabetic drug metformin in periodontal disease. J Pharm Bioallied Sci. 2013;5:327. doi: 10.4103/0975-7406.120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun. 2000;68:5864–5868. doi: 10.1128/IAI.68.10.5864-5868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. elife. 2016;5:e16351. doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13:529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Eke PI, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eke PI, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KE, et al. Health effects of long-term rapamycin treatment: the impact on mouse health of enteric rapamycin treatment from four months of age throughout life. PLoS One. 2015;10:e0126644. doi: 10.1371/journal.pone.0126644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Montoya JA, de Mello AL, Barrios R, Gonzalez-Moles MA, Bravo M. Oral health in the elderly patient and its impact on general well-being: a nonsystematic review. Clin Interv Aging. 2015;10:461–467. doi: 10.2147/CIA.S54630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DP, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff. 2013;32:1698–1705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran J, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurez V, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14:945–956. doi: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M. Preserving youth: does rapamycin deliver? Sci Transl Med. 2013;5:211fs240. doi: 10.1126/scitranslmed.3005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdiscip Top Gerontol. 2015;40:107–127. doi: 10.1159/000364974. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. doi: 10.12703/P5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. mTOR inhibition: from aging to autism and beyond. Scientifica (Cairo) 2013;2013:849186. doi: 10.1155/2013/849186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350:1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 2015;68:19–25. doi: 10.1016/j.exger.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J Periodontal Res. 2010;45:574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11:326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- M. P. Mattson, V. D. Longo, M. Harvie (2016) Impact of intermittent fasting on health and disease processes. Ageing Res Rev [DOI] [PMC free article] [PubMed]
- Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff F, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preshaw PM, Henne K, Taylor JJ, Valentine RA, Conrads G. Age-related changes in immune function (immune senescence) in caries and periodontal diseases: a systematic review. J Clin Periodontol. 2017;44(Suppl 18):S153–S177. doi: 10.1111/jcpe.12675. [DOI] [PubMed] [Google Scholar]
- Razak PA, et al. Geriatric oral health: a review article. J Int Oral Health. 2014;6:110–116. [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Kohanski R. Geroscience and the trans-NIH geroscience interest group. GSIG Gerosci. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol. 1994;2000(5):7–25. doi: 10.1111/j.1600-0757.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Urfer SR, et al. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39:117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, et al. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience. 2017;39:43–50. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Yu Z, Sunchu B, Shoaf J, Dang I, Zhao S, Caples K, Bradley L, Beaver LM, Ho E, Lohr CV, Perez VI (2017) Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 16:564-574 [DOI] [PMC free article] [PubMed]
- Wilkinson JE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1.64 MB)