Abstract
Aging leads to a progressive decline in immune function commonly referred to as immune senescence, which results in increased incidence and severity of infection. In addition, older males experience a significant disruption in their levels of circulating androgens, notably testosterone and dehydroepiandrosterone (DHEA), which has been linked to sarcopenia, osteoporosis, cardiovascular disease, and diabetes. Since sex steroid levels modulate immune function, it is possible that the age-related decline in androgen levels can also affect immune senescence. Therefore, in this study, we evaluated the pleiotropic effects of physiological androgen supplementation in aged male rhesus macaques (n = 7/group) on immune cell subset frequency and response to vaccination. As expected, frequency of naïve CD4 and CD8 T cells declined in aged non-treated macaques, while that of memory T cells increased. In contrast, frequency of naïve and memory T cells remained stable in androgen-supplemented males. In addition, levels of inflammatory cytokines increased less steeply in supplemented aged males compared to the aged controls. Despite these changes, androgen-supplemented animals only showed modest improvement in antibody responses following vaccination compared to age non-treated controls. These data indicate that short-term physiological androgen supplementation can improve some but not all aspects of immune senescence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-017-9979-5) contains supplementary material, which is available to authorized users.
Keywords: Andropause, Androgens, Immune senescence, Rhesus macaques, Inflammation, T cells
Introduction
Aging is accompanied by a decline in immune fitness referred to as immune senescence (Haberthur et al. 2010) that affects both innate and adaptive immunity. The most prominent changes include a severe loss of naïve T cells and accumulation of memory T cells, a decrease in CD4/CD8 T cell ratio and B cell numbers (Larbi et al. 2008), and upregulation of circulating pro-inflammatory cytokines, notably IL-6 and TNFα (De Martinis et al. 2005; Wikby et al. 2006). The shift from naïve to memory lymphocytes and the heightened systemic inflammation is due in part to reduced bone marrow and thymic output as well as the presence of chronic viral infections, especially cytomegalovirus (CMV) (Müller et al. 2017). Immune senescence exacerbates morbidity and mortality related to infections (Weinberger et al. 2008a, b), which remain one of the leading causes of death in the elderly (High 2004) and contributes to the development of age-related diseases such as Alzheimer’s, atherosclerosis and sarcopenia (Fulop et al. 2015). The increased susceptibility to infection is compounded by reduced vaccine efficacy. For example, seroconversion following influenza vaccine is 41–58% in persons 60–74 years of age compared to 90% in 18–45-year-old adults (Goodwin et al. 2006). Moreover, chronic CMV infection interferes with the generation of protective responses to seasonal influenza vaccination (Strindhall et al. 2016). Given that by 2030, 20% of the US population will be 65 years of age or older, it is imperative that new strategies are developed to delay immune senescence and improve immune responses to vaccination in the elderly.
In men, increasing age is associated with highly attenuated levels of bioactive androgens, especially testosterone and dehydroepiandrosterone (DHEA) (Harman et al. 2001). This phenomenon is termed andropause and is believed to contribute to perturbation in sleep-wake cycles (Bremner et al. 1983), sarcopenia (Vasto et al. 2007), osteoporosis (Tivesten et al. 2004; Vanderschueren et al. 2004), cardiovascular disease (Webb et al. 1999a, b), and diabetes (Malkin et al. 2004a, b). The prevalence of hypogonadism, which is defined as testosterone levels below the 2.5 percentile for young men, increases from 35% in 60-year-old men to 70% in 80-year-old men (Harman et al. 2001).
There is great controversy over diagnosing and treating hypogonadism in older men (Swerdloff and Wang 2011). Some studies have shown that testosterone supplementation exerts moderate benefit with respect to sexual function, some benefit with respect to mood and depressive symptoms, and no benefit with respect to vitality or walking distance (Cunningham et al. 2016; Snyder et al. 2016a, b). Other studies indicated that testosterone supplementation might increase the risk of polycythemia (Viallard et al. 2000), benign prostatic hyperplasia (Holmang et al. 1993), stroke, and heart attack (Basaria et al. 2010; Vigen et al. 2013; Finkle et al. 2014). In contrast to these findings, a third set of studies have demonstrated that testosterone supplementation in older men exerts several benefits ranging from improved spatial cognition (Janowsky et al. 1994; Cherrier et al. 2005a, b) and cognitive function (Tan and Pu 2003; Cherrier et al. 2005a, b) to increased muscle mass (Wang et al. 2004; Page et al. 2005) and bone density (Tivesten et al. 2004) and decreased severity of cardiovascular disease (Webb et al. 1999a, b).
Similarly, the effects of androgens on immune function in older men in the immune system remain controversial. Previous studies have reported that androgen ablation in mice and patients with prostate cancer results in increased naive T and B cell output (Ellis et al. 2001; Olsen and Kovacs 2001; Sutherland et al. 2005). Moreover, medical castration in young men results in a reduced frequency of regulatory T cells (Page et al. 2006). These data suggest that androgens suppress lymphopoiesis and promote generation of regulatory T cells. On the other hand, androgen supplementation in hypogonadal older men reduced plasma levels of pro-inflammatory cytokines TNFα and IL-1β while increasing those of regulatory cytokine IL-10 (Malkin et al. 2004a, b). In addition, medical castration of healthy young men reduced IFNγ production by CD8 T cells (Page et al. 2006). These data suggest that hypogonadism leads to increased inflammation and reduced T cell cytokine responses, both hallmarks of immune senescence.
This study examines the impact of short-term testosterone/DHEA supplementation on immune senescence and the immune response to vaccination in aged male rhesus macaques. Like humans, aged rhesus macaques experience a profound loss of naïve T cells and accumulation of terminally differentiated memory T cells (Asquith et al. 2012), a reduced ability to respond to vaccination and infection (Goodwin et al. 2006; Cicin-Sain et al. 2010; Engelmann et al. 2011; Josset et al. 2012), and increased plasma levels of inflammatory cytokines (Haberthur et al. 2010). Importantly, like men but unlike most rodents, male rhesus macaques also experience a significant age-related decline in circulating testosterone and DHEA sulfate (DHEAS) levels as well as a marked attenuation of their circadian plasma profiles (Urbanski and Sorwell 2012), thereby providing a robust animal model in which to investigate the interplay between androgen supplementation and immune senescence. We used a novel administration paradigm that can restore both the magnitude as well as circadian rhythm profiles of circulating androgens in aged male macaques (Urbanski et al. 2014) to determine the impact of androgen supplementation on circulating inflammatory cytokine levels, lymphocyte homeostasis, and the immune response to vaccination.
Our data shows that frequency of naïve CD4 and CD8 T cells remained stable in supplemented aged male macaques, while they declined in aged non-treated macaques. Similarly, levels of inflammatory cytokines increased at a much lower rate in supplemented aged males compared to the aged controls. Despite these improvements, androgen-supplemented animals exhibited only modest improvements in the antibody response to H1N1 influenza vaccination compared to age-matched controls, and no improvement in T cell responses was detected. Collectively, these data indicate that a 6-month physiological androgen supplementation can improve some but not all aspects of age-related decline in immune function.
Materials and methods
Animals and sample collection
Three groups of male rhesus macaques were studied (n = 7/group): (1) young adult receiving placebo, (2) aged receiving placebo, and (3) aged receiving testosterone/DHEA. Mean (±SEM) age of young animals was 11.3 ± 0.9 years, aged controls was 24.1 ± 1.0 years, and aged supplemented was 23.7 ± 0.5 years. No significant differences in dietary intake or body weight were observed between groups or with testosterone treatment: mean (±SEM) body weights at the end of the study were 10.0 ± 0.8 kg (young), 10.6 ± 0.6 kg (aged controls), and 11.9 ± 0.9 kg (aged supplemented). Each aged animal in the treatment group acted as its own control. All aged animals were in generally good health and free of major chronic diseases (diabetes, cardiovascular disease, frailty, or significant cognitive decline). All animals were CMV positive. Animals were euthanized after 8 months of treatment, 35 days after seasonal influenza vaccination.
Testosterone was administered orally in sesame oil at the start of the night (12 mg/kg at 7 p.m.), while DHEA was administered in the morning (0.04 mg/kg at 7 a.m. and again at 10 a.m.). This hormone supplementation regimen faithfully recapitulated the 24-h androgen profiles of young animals in aged male macaques. As recently reported (Urbanski et al. 2014), the young animals used in this study over the same study period had a total (free + bound) nocturnal plasma testosterone peak of ∼25 ng/ml whereas the old animals used in this study over the same study period had a peak of ∼10 ng/ml; supplementation with androgens raised that peak level in the latter group to ∼25 ng/ml. Similarly, that study showed that in young animals, DHEAS had a plasma of ∼150 ng/ml in the morning, whereas the peak was <50 ng/ml in the old animals; supplementation with androgens raised that peak level in the latter group to ∼200 ng/ml (Urbanski et al. 2014). Blood samples were collected from each animal on a monthly basis including a pre-treatment/placebo time point. Analysis of immune subsets and cytokines focused on the pre-treatment and the 6- and 8-month time point respectively.
Immunization
After 6 months of androgen/placebo treatment, the animals first received Modified Vaccinia Virus (MVA) vaccination (108 pfu intramuscularly), and blood samples were obtained at days 0, 7, 14, and 28 post-vaccination. The animals then received the seasonal influenza (Flu) vaccine (on day 28 post-MVA vaccination), and blood samples were again collected on days 7, 14, and 35 post-vaccination. Animals continued to receive androgen supplementation during the vaccination periods.
Measurement of T and B cell frequency
PBMCs were surface stained with antibodies against CD8β (Beckman Coulter, Brea, CA), CD4 (eBioscience, San Diego, CA), CD28 (BioLegend, San Diego, CA), CD95 (BioLegend), and CCR7 (BD Pharmingen, San Diego, CA), which allowed for the delineation of naive (CD28+ CD95- CCR7+), central memory (CM; CD28+ CD95+ CCR7+), transitional effector memory (TEM; CD28+ CD95+ CCR7−), and effector memory (EM; CD28− CD95+ CCR7−) CD4 and CD8 T cells (Supplemental Fig. 1a). PBMCs were also surface stained with antibodies against CD20 (BioLegend), IgD (Southern Biotech, Birmingham, AL), and CD27 (BioLegend) to delineate naive (IgD+ CD27-), marginal zone-like (MZ-like; IgD+ CD27+), memory (IgD− CD27+), and double-negative (DN; IgD− CD27−) B cell subsets (Supplemental Fig. 1a). The samples were analyzed using the LSRII instrument (Becton Dickenson, San Jose, CA) and FlowJo software (TreeStar, Ashland, OR).
Identification of innate immune cell populations
A second tube of PBMC was stained with antibodies against CD3 (BD Pharmingen), CD20 (Beckman Coulter), CD14 (BioLegend), HLA-DR (BioLegend), CD11c (BioLegend), CD123 (BioLegend), and CD8α (Parmingen) to delineate (1) monocytes (CD3− CD20− CD14+ HLA-DR+), dendritic cells (DCs; CD3− CD20− CD14− HLA-DR+), and NK cells (CD3− CD20− CD8a+). DCs were further defined into myeloid (mDCs; CD123− CD11c+) and plasmacytoid (pDCs; CD123+ CD11c−) DCs (Supplemental Fig. 1b). Cells were analyzed using the LSRII instrument (Becton Dickenson) and FlowJo software (TreeStar).
Intracellular cytokine staining
To measure the frequency of responding T cells, PBMCs were stimulated ex vivo with MVA (multiplicity of infection = 1) or with Influenza A (H1N1) 2009 Monovalent Vaccine (0.15 μg HA antigen/test) for 1 h followed by the addition of brefeldin A (Sigma, St. Louis, MO) for an additional 14 h. After incubation, cells were stained with antibodies directed against CD4, CD8β, CD95, and CD28. Samples were fixed, permeabilized (BioLegend), and stained using antibodies against IFN-γ (eBioscience), TNF-α (eBioscience), and IL-2 (BioLegend) (Supplemental Fig. 1c). Samples were analyzed using the LSRII instrument and FlowJo software. T cell responses following vaccination were measured on the day of vaccination and then on days 7, 14, and 28 or 35 (for MVA and H1N1 respectively). Responding T cells were identified as those producing TNFα, TNFα/IFNγ, and IFNγ following stimulation (quadrants 2–4, supplemental Fig. 1c). Background responses detected on the day of vaccination were subtracted from responses detected on days 7, 14, and 28 or 35 post-vaccination.
ELISA
Plasma IgG antibody titers were measured on the day of vaccination and then on days 7, 14, and 28 or 35 (for MVA and H1N1 respectively) by enzyme-linked immunosorbent assay (ELISA) using plates coated with modified vaccinia virus (MVA) lysate (1 μg/mL) or with Influenza A (H1N1) 2009 Monovalent Vaccine overnight at 4 °C (0.15 μg HA antigen/mL Sino Biological, Inc., Beijing, China). Plates were then incubated with heat-inactivated (56 °C, 30 min) plasma samples in threefold dilution in triplicates. Plates were developed using horseradish peroxidase (HRP)-conjugated anti-rhesus IgG (Open Biosystems, Rockford, IL) and o-phenylenediamine dihydrochloride (OPD) substrate (Sigma). The reaction was stopped with the addition of 1 M HCl. IgG endpoint titers were calculated using the log–log transformation of the linear portion of the curve and 0.1 optical density (Goodwin, Viboud et al.) units as the cutoff. IgG titers were standardized using a positive control sample that was included in every ELISA plate.
Cytokine, chemokine, growth factor analysis
Plasma samples (stored at −80 °C) collected at baseline and at necropsy (after 8 months of supplementation) were thawed, and the circulating cytokines were measured using non-human primate Cytokine/Chemokine/GF 37-plex panel as per the manufacturer’s instructions (eBioscience). Concentrations for IFNγ, IFNα, TNFα, IL1RA, IL1b, IL2, IL4, IL5, IL6, IL7, IL8, IL10, IL12p70, IL13, IL15, IL17A, IL18, IP10, IL23, sCD40L, SCF, MCP1, MIP1α, MIP1β, MIG, Eotaxin, ITAC, BLC, SDF1α, VEGFA, VEGFD, GCSF, GMCSF, BDNF, FGF2, NGFß, and PDGFBB were determined for all samples. Values below the limit of detection of the assay were assigned a value half that of the lowest standard. The levels of IL-4, IL-5, IL-17, GM-CSF, and IL-8 were below limit of detection for all animals at all time points analyzed. For the rest of the analytes, the number of samples below the limit of detection did not exceed 5 out of 42 samples.
Statistical analysis
Graphing was performed with GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Longitudinal analysis of the frequency of responding immune cells was carried out using a one-way repeated-measures ANOVA model with a compound symmetric variance–covariance structure, followed by Dunnett’s multiple comparison post-test to explore differences between the three groups post-vaccination. Cytokine, chemokine, and growth factor levels between the three groups were compared using a two-way ANOVA, followed by Bonferroni’s multiple comparison post-test. ELISA titers were log transformed with base 10 to hold the normal distribution assumption. Total antibody responses were determined using area under curve (AUC), which was calculated by trapezoidal integration. Mann–Whitney–Wilcoxon test was used for assessing differences in IgG AUCs between aged controls, supplemented, and young animals. Two-way ANOVA and Bonferroni’s multiple comparison post-test were used to determine differences in intracellular cytokine levels post-vaccination between the three groups. Statistical significance for all comparisons was determined at the alpha level of 0.05.
Results
Impact of short-term androgen supplementation on complete blood counts
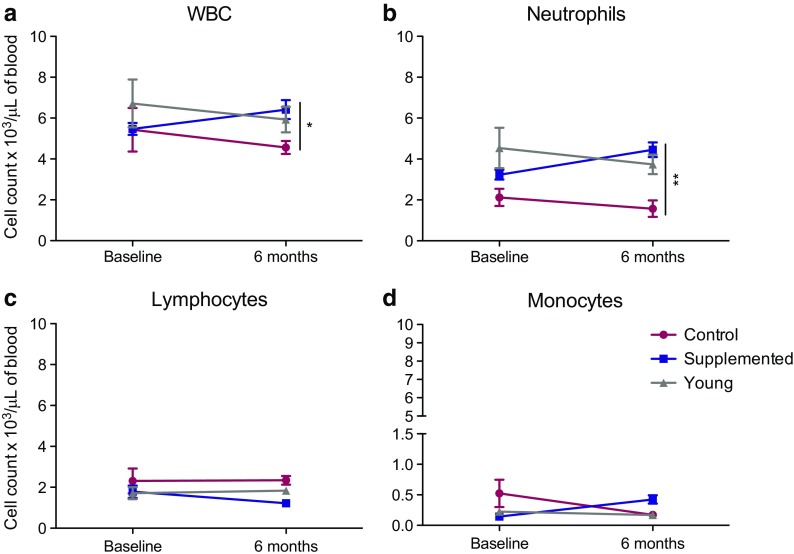
We first investigated the impact of androgen supplementation on total white blood cells (WBCs), neutrophils, lymphocytes, and monocytes (Fig. 1). At baseline, the animals in the androgen supplemented group had similar WBC numbers as the control group. However, after 6 months of androgen supplementation, the supplemented macaques had a higher WBC count than did the aged control macaques (Fig. 1a). This increase in WBC numbers was mediated by an increase in neutrophil counts after 6 months of supplementation (Fig. 1b). On the other hand, no significant changes were observed in lymphocyte and monocyte counts (Fig. 1c, d).
Fig. 1.
Absolute numbers of cell populations in whole blood. a Total white blood cell (WBC), b neutrophil, c lymphocyte, and d monocyte counts were taken at 0 and 6 months post-androgen supplementation. *P < 0.05; **P < 0.01; supplemented aged macaques compared to aged non-treated controls
Impact of short-term androgen supplementation on T and B cell populations
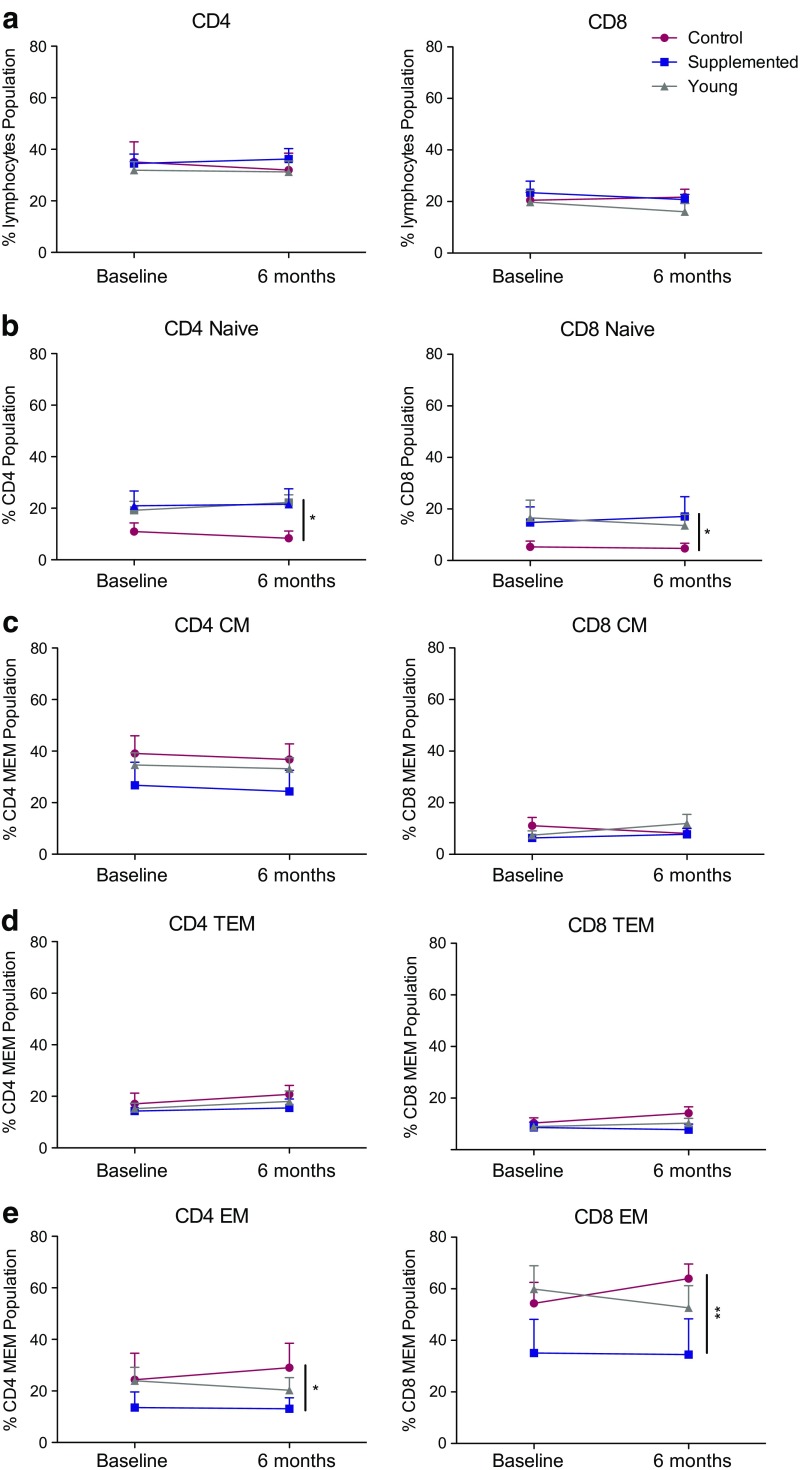
Loss of naïve T cells and accumulation of highly differentiated memory T cells is a hallmark of immune senescence. Therefore, we next investigated the impact of 6-month androgen supplementation on circulating T (CD4, CD8) and B cell (CD20) subset frequencies. In line with the lack of differences in lymphocyte numbers reported above, flow cytometry analysis showed no significant changes in total circulating T and B cell numbers (Fig. 2a and Supplement Fig. 2a). We next investigated changes in the naïve and memory subsets after 6 months of androgen supplementation. As expected, the frequency of naïve CD4 and CD8 T cells slightly declined in aged non-treated macaques, while those of effector memory (EM) CD4 and CD8 T cells increased (Fig. 2b, e). In contrast, the frequency of naïve and effector memory CD4 and CD8 T cells remained stable in androgen-supplemented macaques (Fig. 2b, e). Although the longitudinal changes within each group were not statistically significant, together, they resulted in a higher frequency of naïve T cells (Fig. 2b) and a lower frequency of EM T cells (Fig. 2e) in supplemented animals compared to non-treated controls. No significant changes were seen in CD4 and CD8 central memory (CM) (Fig. 2c) and transitional effector memory (TEM) populations (Fig. 2d). Similarly, no significant changes in naïve and memory B cell subsets were observed over the 6-month period (Supplement Fig. 2b–e).
Fig. 2.
Frequencies of circulating naïve and effector memory T cell populations. a–e The frequencies (means ± SEM) of CD4 T, and CD8 T and central memory (CM; CD95+ CD28+ CCR7+), transitional effector memory (TEM; CD95+ CD28+ CCR7−), effector memory (EM; CD95+ CD28− CCR7−), and naïve (CD28+ CD95-) subsets in CD4 and CD8 T cells in PBMC were measured by flow cytometry. *P < 0.05; **P < 0.01; supplemented aged macaques to aged controls
Impact of androgen supplementation on innate immune cells
We next investigated the impact of androgen supplementation on the frequency of monocytes, dendritic cells (DCs), and natural killer (NK) cell subsets after 6 months of supplementation (Supplement Fig. 3). No significant differences in frequency of monocytes, DCs, and NK cells or their subsets were observed after treatment or between the three groups (Supplement Fig. 3). Monocyte frequency decreased over the 6-month period, but the decline was comparable between the three groups and not significant (Supplement Fig. 3a). No changes or differences in DC numbers were noted over the 6-month placebo or androgen supplementation (Supplement Fig. 3b). NK cell numbers increased over the 6-month period, but once again, the change was comparable and not significant among all three groups (Supplement Fig. 3c). Although initially, the number of myeloid dendritic cell (mDCs) was lower in the androgen-treated macaques, this difference was no longer significant between the two groups after 6 months of supplementation due to a slight increase in the supplemented group (Supplement Fig. 3e).
Androgen supplementation and plasma levels of circulating factors
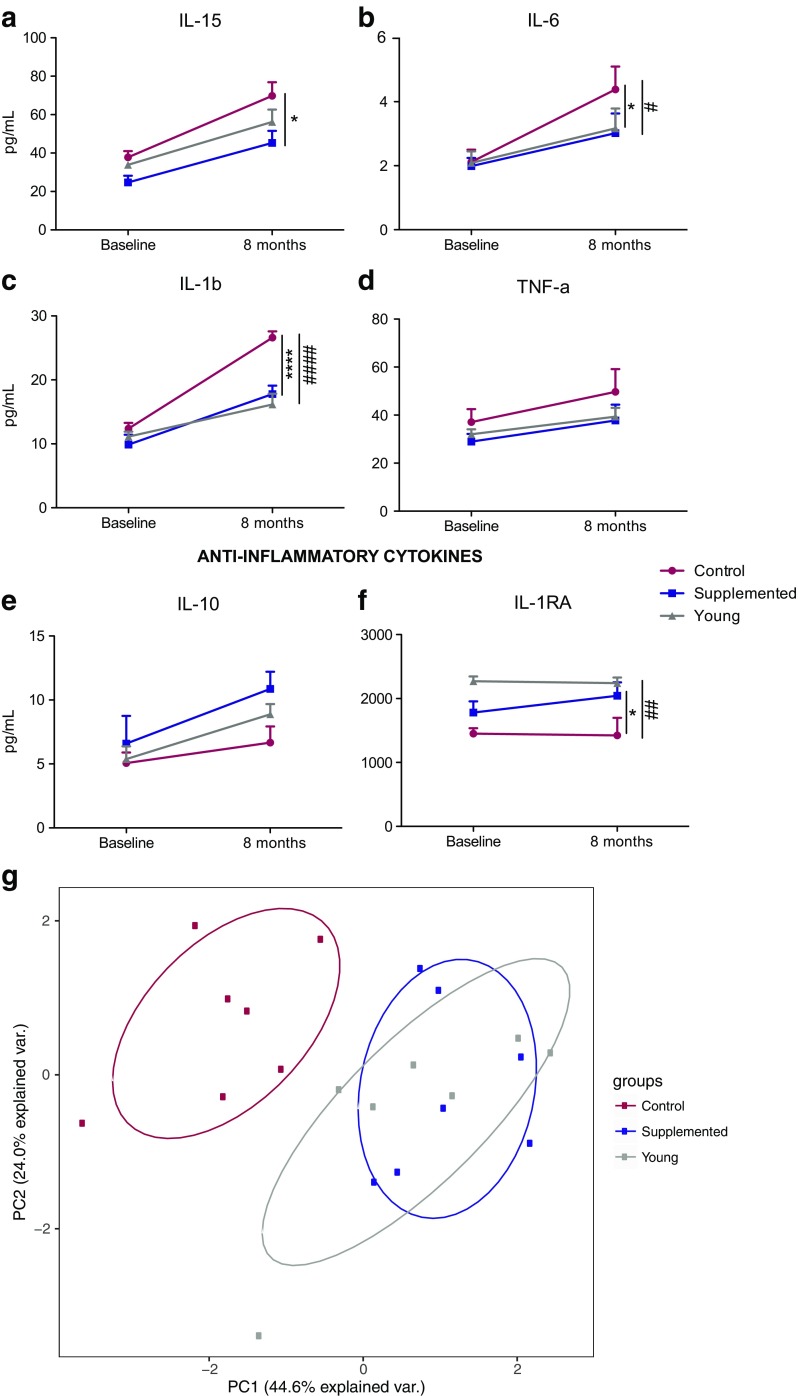
Aging is accompanied by a significant increase in pro-inflammatory and decrease in anti-inflammatory cytokine production. Therefore, we compared the circulating levels of cytokines, chemokines, and growth factors in the plasma between the three groups (Fig. 3). At baseline, there were no differences in cytokine levels between the two aged groups. However, after 6 months of supplementation, plasma levels of pro-inflammatory cytokines IL-15, IL-6, and IL-1β were lower in the androgen-supplemented males than in the non-treated animals (Fig. 3a–c), while the opposite was true for the anti-inflammatory cytokine, IL-1RA (Fig. 3e, f). A slower increase in TNFα and a higher increase in IL-10 levels were observed in supplemented animals compared to non-treated animals, but these changes did not result in significant differences after 8 months of supplementation (Fig. 3d, e). No other changes were detected in the other cytokines/chemokines or growth factors analyzed (data not shown). Principal component analysis (PCA) of these cytokines (IL-1β, TNFα, IL-15, IL-6, IL-10, IL-1RA) showed the supplemented and young animals clustering together, while the aged controls remained segregated after 8 months of androgen supplementation (Fig. 3g).
Fig. 3.
Plasma cytokine profiles. a–f Pro-inflammatory (a–d) and anti-inflammatory e–f cytokine levels were determined using Luminex technology at the baseline and necropsy time points. Left y-axis, mean pg/ml ± SEM (*P < 0.05; ****P < 0.0001 supplemented macaques compared to aged non-treated controls; #P < 0.05; ####P < 0.0001 young macaques compared to non-treated aged controls). g Principal component analysis of the cytokines presented in a–f showing that androgen-supplemented animals are similar to young animals while control non-supplemented aged animals segregate away
Androgen supplementation and immune response to vaccination
We next investigated the impact of androgen supplementation on the immune response to MVA and seasonal H1N1 influenza vaccination (Fig. 4). First, we measured MVA and H1N1 influenza-specific IgG antibody end-point titers (Fig. 4a, b). Young animals generated a robust antibody response that peaked 14 days post-vaccination (Fig. 4a, b). Aged non-treated and supplemented animals generated lower IgG antibody responses than did the young group. Compared to the aged non-treated macaques, the aged supplemented macaques had slightly higher, but not significant, overall IgG response (area under the curve (AUC) = 3406 in supplemented versus 2203 in controls and 6046 in young animals). Following H1N1 influenza vaccination, both young animals and supplemented aged animals generated a more robust antibody response compared to the aged non-treated macaques. Similarly, overall IgG responses were higher in young and supplemented aged macaques compared to aged non-treated animals (AUC = 4272 aged supplemented compared to 4909 in young animals and 2381 in controls).
Fig. 4.
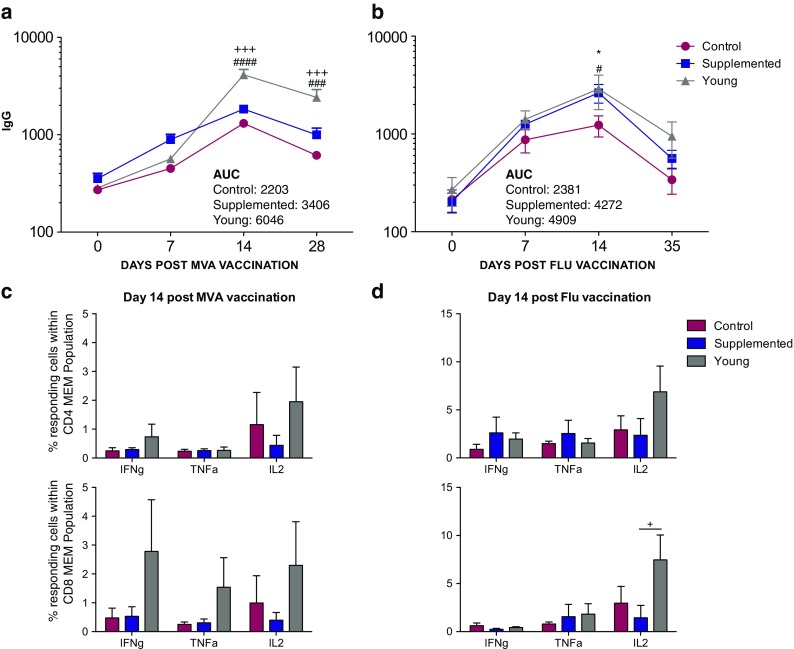
Humoral and cellular immune responses to MVA and Flu vaccinations. a, b MVA- and H1N1-specific IgG antibody endpoint titers (means ± SEM) were measured by standard ELISA in plasma on days 0, 7, 14, and 28/35 post-vaccinations (#P < 0.05; ###P < 0.001; ####P < 0.0001 young animals compared to aged non-treated controls; +++P < 0.001 young animals compared to aged supplemented animals; *P < 0.05 aged supplemented animals compared to aged non-treated controls). c, d The frequencies (means ± SEM) of MVA- and H1N1-specific CD4 and CD8 memory cells in PBMC samples producing IFN-γ, TNF-α, and IL-2 were measured on day 14 post-MVA (c) and Flu (d) vaccinations by intracellular cytokine staining following stimulation with VV lysate or Influenza A (H1N1) 2009 Monovalent Vaccine, respectively (+P < 0.05 supplemented macaques compared to young animals)
To further investigate the impact of androgen supplementation on T cell response to MVA and Flu vaccinations, we compared the frequency of responding T cells (assessed based on the production of antiviral cytokines TNFα and/or IFNγ; see “Methods and materials” section and Supplemental Fig. 1c) following the stimulation of PBMC with MVA lysate or Influenza A (H1N1) 2009 Monovalent Vaccine between the three groups (Fig. 4c, d). As described for the antibody response, T cell responses also peaked 14 days post-vaccination (data not shown). Frequency of responding CD4 and CD8 memory cells was comparable between the two aged groups following both MVA and influenza vaccinations. Young animals generated a slightly larger, albeit not statistically significant, T cell response compared to aged animals (Fig. 4c, d).
Discussion
This study examined the impact of testosterone/DHEA supplementation on immune senescence in aged male rhesus macaques. We assessed the frequency of naïve and memory T and B cell subsets, innate immune cells, plasma levels of key pro- and anti-inflammatory cytokines, and the immune response to vaccination with MVA as well as the seasonal H1N1 influenza vaccine. Our results showed that short-term physiological androgen (testosterone and DHEA) supplementation designed to mimic the circulating 24-h pattern of young animals maintained the frequency of naïve T cells, slowed the increase in plasma levels of some pro-inflammatory cytokines while accelerating the increase in the anti-inflammatory cytokine IL-1RA, and modestly improved IgG antibody response to seasonal H1N1 influenza vaccine in aged male rhesus macaques.
One of the most striking features of immune senescence is the loss of naïve T cells (Fagnoni et al. 2000), believed to be largely due to diminished thymic output (Naylor et al. 2005), increased homeostatic proliferation, and life-long exposure to chronic pathogens such as CMV (Czesnikiewicz-Guzik et al. 2008). Similarly, rhesus macaques experience a progressive loss of naïve T cells and a concomitant increase of memory T cells, especially CD8+ effector memory (EM) T cells (Haberthur et al. 2010; Asquith et al. 2012). Data presented here show that androgen supplementation was associated with a better maintenance of naïve CD4 and CD8 T cell frequencies. Our data are in contrast to previous studies that showed castration of aged male mice and prostate cancer patient results in increased thymic/bone marrow output and increased frequency of naïve T cells (Olsen and Kovacs 2001; Sutherland et al. 2005). This difference could be due to the fact that mice do not undergo age-related decline in androgen levels, and that previous clinical studies focused on cancer patients. In contrast, our study utilized healthy aged rhesus macaques which showed a normal age-related decline in physiological levels of circulating androgens.
Aging of the immune system is also associated with an increase in the production of inflammatory cytokines, especially IL-6 and TNFα (De Martinis et al. 2005). This phenomenon, often referred to as “inflammaging,” is hypothesized to contribute to the development and/or aggravation of several chronic diseases such as Alzheimer’s and osteoporosis (De Martinis et al. 2005). Both IL-6 and IL-1RA levels are increased in older macaques, consistent with the concept of inflammaging (Asquith et al. 2012). Earlier clinical studies showed that testosterone treatment of hypogonadal men led to decreased plasma levels of TNFα and IL-1β and increased IL-10 levels (Stanworth and Jones 2008). Our data showed that 6 months of androgen supplementation slows the age-related increase in plasma levels of pro-inflammatory cytokines IL-15, IL-6, and IL-1β, while the increase in the anti-inflammatory cytokine IL-1RA was higher. Together with published clinical data (Stanworth and Jones 2008), these observations suggest that amelioration of systemic inflammation following androgen supplementation could explain its beneficial effect on atherosclerosis and diabetes (Malkin et al. 2004a, 2004b).
Interestingly, and in contrast to our earlier studies (Haberthur et al. 2010; Asquith et al. 2012), we did not detect a significant difference in the frequency of naïve and memory T and B cell subsets or levels of inflammatory cytokines between young and aged macaques in this study. However, aging is a highly heterogeneous process and it is possible that the aged animals we selected were healthier than the norm (successful agers) and therefore did not display classical hallmarks of immune senescence.
Age-related changes in immunity leads to reduced immune response to infection and vaccination, which in turn contribute to increased morbidity and mortality from infectious diseases (Weinberger et al. 2008a, 2008b). Thus, we next measured the effect of androgen supplementation on the immune response following MVA and the seasonal influenza vaccinations. As described for humans, aged rhesus macaques generate a reduced B cell response to MVA and seasonal influenza vaccination compared to young animals (Cicin-Sain et al. 2010; Engelmann et al. 2011; Carroll et al. 2014). Our data show that aged controls generated lower antibody responses than those observed in the young animals following MVA and influenza vaccinations. While androgen-supplemented males generated a comparable IgG response to non-treated controls following MVA vaccination, they generated a higher IgG antibody response post influenza vaccination than the aged non-treated controls.
In summary, data presented in this manuscript suggest that short-term physiological androgen supplementation can improve some aspects of immune senescence (slow conversion of naïve to memory T cells and age-related increase in some inflammatory cytokines and improve IgG antibody response to H1N1 influenza vaccine) but not restore or reverse all aspects of immune senescence. A limitation of our study is the short duration of androgen supplementation (6 months) which is a very small window when one considers the long lifespan of rhesus macaques (34 years). Finally, the small group size may have precluded us from detecting more significant differences between treated and supplemented aged macaques. Nevertheless, these studies pave the way for longer studies that can also investigate the mechanisms underlying the reduced rate of naïve T cell loss and increased levels of inflammatory cytokines as well as the biological impact of improved IgG antibody levels following influenza vaccination.
Electronic supplementary material
Gating strategy of rhesus macaque PBMC. (A) Flow cytometric gating strategy to delineate T and B cell populations. (B) Flow cytometric gating strategy to delineate innate cell populations. (C) Flow cytometric analysis of T cell cytokine response to stimulation with MVA and H1N1 antigens (example show is following stimulation with PMA/ionomycin). (PDF 1337 kb)
Frequency of circulating naïve and memory B cell populations. The frequencies (mean ± SEM) of (A) total B cells; (B) naïve (IgD+ CD27-); (C) marginal zone (MZ)-like (IgD+ CD27+); (D) class-switched memory (IgD− CD27+); (E) and double-negative (Other; IgD− CD27−) B cells were measured in PBMC samples by flow cytometry. (PDF 273 kb)
Frequencies of circulating monocytes, dendritic cells, and natural killer cells. The frequencies (means ± SEM) of (A) dendritic cells (DCs; CD3− CD20- CD14− HLA-DR+); (B) monocytes (CD3− CD20- CD14+ HLA-DR−); (C) natural killer cells (NK cells; CD3− CD20- CD8α +); (D) myeloid DCs (mDCs; CD123− CD11c+); and (E) plasmacytoid DCs (pDCs; CD123+ CD11c−) in PBMC were measured by flow cytometry (*, P < 0.05 supplemented macaques compared to aged controls). (PDF 278 kb)
Acknowledgements
The authors thank the veterinarians and the husbandry staff at the Oregon National Primate Research Center for sample collection and expert care of the animals. We also thank Norma Mendoza for her help in performing ELISA to measure IgG titers. This work was supported by R21AG-043896, NIH R01AG-036670, OD-010426, and OD-011092.
Authors’ contributions
Designed and directed the project: IM and HU; performed experiments: MR, IM, and RW; analyzed data and the prepared figures: MR and IM; wrote the manuscript: MR, IM, and HU.
Compliance with ethical standards
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Office of Animal Welfare, and the US Department of Agriculture. All animal work was approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee.
All procedures were carried out under ketamine anesthesia in the presence of veterinary staff, and all efforts were made to minimize animal suffering. White blood cell counts were obtained using a complete blood count machine (Hemavet; Drew Scientific Group, Waterbury, CT).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-017-9979-5) contains supplementary material, which is available to authorized users.
References
- Asquith M, Haberthur K, Brown M, Engelmann F, Murphy A, Al-Mahdi Z and Messaoudi I (2012) “Age-dependent changes in innate immune phenotype and function in rhesus macaques (Macaca mulatta).” Pathobiol Aging Age Relat Dis 2 [DOI] [PMC free article] [PubMed]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56(6):1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Carroll TD, Matzinger SR, Barry PA, McChesney MB, Fairman J, Miller CJ. Efficacy of influenza vaccination of elderly rhesus macaques is dramatically improved by addition of a cationic lipid/DNA adjuvant. J Infect Dis. 2014;209(1):24–33. doi: 10.1093/infdis/jit540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, Raskind MA, Johnson M, Craft S. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64(2):290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64(12):2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Lewinsohn DA, Nikolich-Zugich J. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184(12):6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Bhasin S, Matsumoto AM, Parsons JK, Gill TM, Molitch ME, Farrar JT, Cella D, Barrett-Connor E, Cauley JA, Cifelli D, Crandall JP, Ensrud KE, Gallagher L, Zeldow B, Lewis CE, Pahor M, Swerdloff RS, Hou X, Anton S, Basaria S, Diem SJ, Tabatabaie V, Ellenberg SS, Snyder PJ. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab. 2016;101(8):3096–3104. doi: 10.1210/jc.2016-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM and Goronzy JJ (2008) T cell subset-specific susceptibility to aging. Clin Immunol [DOI] [PMC free article] [PubMed]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED. Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol. 2001;13(4):553–558. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- Engelmann F, Barron A, Urbanski H, Neuringer M, Kohama SG, Park B, Messaoudi I. Accelerated immune senescence and reduced response to vaccination in ovariectomized female rhesus macaques. Age (Dordr) 2011;33(3):275–289. doi: 10.1007/s11357-010-9178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95(9):2860–2868. [PubMed] [Google Scholar]
- Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF, Jr, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, McElhaney J, Pawelec G, Cohen AA, Morais JA, Dupuis G, Baehl S, Camous X, Witkowski JM, Larbi A. Frailty, inflammation and immunosenescence. Interdiscip Top Gerontol Geriatr. 2015;41:26–40. doi: 10.1159/000381134. [DOI] [PubMed] [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Haberthur K, Engelman F, Barron A, Messaoudi I. Immune senescence in aged nonhuman primates. Exp Gerontol. 2010;45(9):655–661. doi: 10.1016/j.exger.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- High KP. Infection as a cause of age-related morbidity and mortality. Ageing Res Rev. 2004;3(1):1–14. doi: 10.1016/j.arr.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Holmang S, Marin P, Lindstedt G, Hedelin H. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate. 1993;23(2):99–106. doi: 10.1002/pros.2990230203. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108(2):325–332. doi: 10.1037/0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Josset L, Engelmann F, Haberthur K, Kelly S, Park B, Kawoaka Y, Garcia-Sastre A, Katze MG, Messaoudi I. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza a virus. J Virol. 2012;86(20):11115–11127. doi: 10.1128/JVI.01571-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, Jones TH, Channer KS. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871–876. doi: 10.1136/hrt.2003.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L, Hamprecht K, Pawelec G (2017) The role of CMV in immunosenescence. The Ageing Immune System and Health. V. Bueno. J. M. Lord and T. A. Jackson. Cham, Springer International Publishing: 53–68
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Effects of androgens on T and B lymphocyte development. Immunol Res. 2001;23(2–3):281–288. doi: 10.1385/IR:23:2-3:281. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90(3):1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, Amory JK, Nelson PS, Wu JD. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290(5):E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER, 3rd, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS, I. Testosterone Trials Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PJ, Ellenberg SS, Farrar JT. Testosterone treatment in older men. N Engl J Med. 2016;375(1):90. doi: 10.1056/NEJMc1603852. [DOI] [PubMed] [Google Scholar]
- Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clin Interv Aging. 2008;3(1):25–44. doi: 10.2147/cia.s190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strindhall J, Ernerudh J, Mörner A, Waalen K, Löfgren S, Matussek A, Bengner M. Humoral response to influenza vaccination in relation to pre-vaccination antibody titres, vaccination history, cytomegalovirus serostatus and CD4/CD8 ratio. Infectious Diseases. 2016;48(6):436–442. doi: 10.3109/23744235.2015.1135252. [DOI] [PubMed] [Google Scholar]
- Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175(4):2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- Swerdloff R, Wang C. Testosterone treatment of older men—why are controversies created? J Clin Endocrinol Metab. 2011;96(1):62–65. doi: 10.1210/jc.2010-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male. 2003;6(1):13–17. doi: 10.1080/tam.6.1.13.17. [DOI] [PubMed] [Google Scholar]
- Tivesten A, Moverare-Skrtic S, Chagin A, Venken K, Salmon P, Vanderschueren D, Savendahl L, Holmang A, Ohlsson C. Additive protective effects of estrogen and androgen treatment on trabecular bone in ovariectomized rats. J Bone Miner Res. 2004;19(11):1833–1839. doi: 10.1359/JBMR.040819. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Sorwell KG. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age (Dordr) 2012;34(5):1111–1121. doi: 10.1007/s11357-011-9352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Sorwell KG, Garyfallou VT, Garten J, Weiss A, Renner L, Neuringer M, Kohama SG. Androgen supplementation during aging: development of a physiologically appropriate protocol. Rejuvenation Res. 2014;17(2):150–153. doi: 10.1089/rej.2013.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25(3):389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Viallard JF, Marit G, Mercie P, Leng B, Reiffers J, Pellegrin JL. Polycythaemia as a complication of transdermal testosterone therapy. Br J Haematol. 2000;110(1):237–238. doi: 10.1046/j.1365-2141.2000.02072-3.x. [DOI] [PubMed] [Google Scholar]
- Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89(5):2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- Webb CM, Adamson DL, de Zeigler D, Collins P. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol. 1999;83(3):437–439. doi: 10.1016/S0002-9149(98)00880-7. [DOI] [PubMed] [Google Scholar]
- Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100(16):1690–1696. doi: 10.1161/01.CIR.100.16.1690. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46(7):1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Vaccines: biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46(7):1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Lofgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127(8):695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy of rhesus macaque PBMC. (A) Flow cytometric gating strategy to delineate T and B cell populations. (B) Flow cytometric gating strategy to delineate innate cell populations. (C) Flow cytometric analysis of T cell cytokine response to stimulation with MVA and H1N1 antigens (example show is following stimulation with PMA/ionomycin). (PDF 1337 kb)
Frequency of circulating naïve and memory B cell populations. The frequencies (mean ± SEM) of (A) total B cells; (B) naïve (IgD+ CD27-); (C) marginal zone (MZ)-like (IgD+ CD27+); (D) class-switched memory (IgD− CD27+); (E) and double-negative (Other; IgD− CD27−) B cells were measured in PBMC samples by flow cytometry. (PDF 273 kb)
Frequencies of circulating monocytes, dendritic cells, and natural killer cells. The frequencies (means ± SEM) of (A) dendritic cells (DCs; CD3− CD20- CD14− HLA-DR+); (B) monocytes (CD3− CD20- CD14+ HLA-DR−); (C) natural killer cells (NK cells; CD3− CD20- CD8α +); (D) myeloid DCs (mDCs; CD123− CD11c+); and (E) plasmacytoid DCs (pDCs; CD123+ CD11c−) in PBMC were measured by flow cytometry (*, P < 0.05 supplemented macaques compared to aged controls). (PDF 278 kb)