Abstract
Strong epidemiological and experimental evidence indicates that hypertension has detrimental effects on the cerebral microcirculation and thereby promotes accelerated brain aging. Hypertension is an independent risk factor for both vascular cognitive impairment (VCI) and Alzheimer’s disease (AD). However, the pathophysiological link between hypertension-induced cerebromicrovascular injury (e.g., blood–brain barrier disruption, increased microvascular oxidative stress, and inflammation) and cognitive decline remains elusive. The present study was designed to characterize neuronal functional and morphological alterations induced by chronic hypertension and compare them to those induced by aging. To achieve that goal, we induced hypertension in young C57BL/6 mice by chronic (4 weeks) infusion of angiotensin II. We found that long-term potentiation (LTP) of performant path synapses following high-frequency stimulation of afferent fibers was decreased in hippocampal slices obtained from hypertensive mice, mimicking the aging phenotype. Hypertension and advanced age were associated with comparable decline in synaptic density in the stratum radiatum of the mouse hippocampus. Hypertension, similar to aging, was associated with changes in mRNA expression of several genes involved in regulation of neuronal function, including down-regulation of Bdnf, Homer1, and Dlg4, which may have a role in impaired synaptic plasticity. Collectively, hypertension impairs synaptic plasticity, reduces synaptic density, and promotes dysregulation of genes involved in synaptic function in the mouse hippocampus mimicking the aging phenotype. These hypertension-induced neuronal alterations may impair establishment of memories in the hippocampus and contribute to the pathogenesis and clinical manifestation of both vascular cognitive impairment (VCI) and Alzheimer’s disease (AD).
Keywords: Hypertension, Blood pressure, Vascular aging, Microcirculation, Inflammation, Dementia
Introduction
There is abundant evidence that hypertension has detrimental effects on the cerebral circulation and thereby causes accelerated brain aging (Girouard et al. 2006; Gorelick et al. 2011; Iadecola et al. 2009; Kazama et al. 2004; Toth et al. 2013a, 2015a). Epidemiological studies demonstrate that, in addition to increasing the incidence of ischemic and hemorrhagic stroke, hypertension-induced microvascular injury promotes premature cognitive decline (Gelber et al. 2012; Gottesman et al. 2014; Kohler et al. 2014). Importantly, hypertension is an independent risk factor for both vascular cognitive impairment (VCI) and Alzheimer’s disease (AD) (Iadecola et al. 2009; Iadecola 2014; Toth et al. 2017). Prospective studies demonstrate that patients on antihypertensive treatment have lower risk of developing cognitive impairment (Gorelick et al. 2011). Experimental studies strengthen the conclusions of the clinical studies. Accordingly, experimentally induced hypertension was shown to exert negative effects on hippocampally encoded functions of learning and in mice, mimicking the aging phenotype (Toth et al. 2013a; Csiszar et al. 2013; Toth et al. 2014a). Similar conclusions have been reached by other studies in laboratory rats and non-human primates as well (Hennigan et al. 2009; Kemper et al. 2001; Moss and Jonak 2007; Moore et al. 2002; Togashi et al. 1996). The aforementioned findings provide evidence that the presence of hypertension in laboratory animals triggers significant impairment of hippocampal function, mimicking important aspects of age-related mild cognitive impairment.
The mechanisms by which hypertension promotes cognitive decline are likely multifaceted and include both dysregulation of cerebral blood flow (Kazama et al. 2004; Girouard and Iadecola 2006) and neuronal dysfunction induced by altered local microenvironment in the cerebral tissue (Iadecola et al. 2009; Iadecola 2014). In recent years, significant progress has been made clarifying the pathophysiology of cerebromicrovascular impairment associated with hypertension. There is strong evidence that hypertension promotes microvascular rarefaction (Toth et al. 2013a), endothelial dysfunction (Girouard et al. 2007), and neurovascular uncoupling (Kazama et al. 2004), which likely lead to impaired delivery of oxygen and glucose to the activated brain regions and inadequate wash-out of by-products. Hypertension also promotes disruption of the blood–brain barrier, microglia activation, and neuroinflammation in the hippocampus (Toth et al. 2013b). Although the aforementioned microvascular alterations are likely to have secondary adverse effects on neuronal function, the effects of hypertension on the function and phenotype of hippocampal neurons are not well understood.
Long-term potentiation (LTP), defined as a long-lasting increase in synaptic efficacy following high-frequency stimulation of afferent fibers, is presumed to play an important role in the establishment and storage of stable long-term memories in the hippocampus (Lynch 2004). The findings that in animal models of aging cognitive decline is associated with impaired LTP support the concept that decreased synaptic efficacy contributes to defective memory storage (Auffret et al. 2009; Cowley et al. 2012; Griffin et al. 2006; Diogenes et al. 2011; Robillard et al. 2011; Ryan et al. 2015; Shi et al. 2015; Yang et al. 2010). Despite recent advances in our understanding of the deleterious cerebrovascular effects of high blood pressure (Girouard et al. 2006; Kazama et al. 2004; Capone et al. 2012), the effects of hypertension on hippocampal LTP remain still elusive.
A number of recent studies in laboratory rodents and primates have demonstrated that aging is not associated with loss of principal hippocampal neurons. In contrast, hippocampal synapse number seems to be decreased in the aged brain, which seems to correlate with cognitive performance (Morrison and Baxter 2012). It is less understood whether hypertension can exert aging-like effects on synaptic density in the hippocampus.
The present study was designed to test the hypothesis that hypertension promotes the acquisition of an accelerated and premature aging phenotype in the mouse hippocampus, impairing synaptic plasticity, reducing synaptic density, and/or altering hippocampal gene expression profile. To achieve that goal, we induced hypertension in young C57BL/6 mice by chronic infusion of angiotensin II followed by measurement of LTP in acute hippocampal slices, assessment of changes in synaptic densities, and measurement of changes in hippocampal expression of genes involved in regulation of synaptic function. Hypertension-induced and age-related changes in relevant endpoints were compared.
Methods
Animals
Young (3–6 month, n = 40) and aged (24 month, n = 20) male C57/BL6 mice were purchased from the National Institute on Aging and were housed 3–5/cage in the rodent barrier facility at the University of Oklahoma Health Sciences Center. All mice were maintained under specific pathogen-free barrier conditions on a 12-h light/12-h dark cycle, with access to standard rodent chow (Purina Mills, Richmond, IN) and water ad libitum as reported (Ungvari et al. 2017a). All procedures were approved by the Institutional Animal Use and Care Committee of the University of Oklahoma Health Sciences Center.
Induction of hypertension in mice
To induce hypertension, Alzet mini-osmotic pumps (Model 2006, 0.15 μl/h, 42 days; Durect Co, Cupertino, CA) were implanted into young mice. Pumps were filled either with saline vehicle or solutions of Ang II (Sigma Chemical Co., St. Louis, MO, USA) that delivered (subcutaneously) 1 μg/min/kg of Ang II for 28 days (Wakisaka et al. 2010). Pumps were placed into the subcutaneous space of ketamine/xylazine anesthetized mice through a small incision in the back of the neck that was closed with surgical sutures. All incision sites healed rapidly without the need for any medication.
Systolic blood pressure of mice in each experimental group was measured by the tail cuff method (CODA Non-Invasive Blood Pressure System, Kent Scientific Co., Torrington, CT) before and 4 weeks after the minipump implantation. At the end of the experimental period, mice were decapitated, the brains were removed, and the hippocampi were isolated.
Electrophysiological studies for synaptic function and LTP
To compare how hypertension and aging affect synaptic function, extracellular recordings were performed from acute hippocampal slices with an adopted protocol as originally described (Oka et al. 1999; Liu et al. 2014; Tarantini et al. 2015). Briefly, horizontal hippocampal slices of 325 μm thickness from mice in each cohort were prepared in ice-cold solution containing (in mmol/L) sucrose 110, NaCl 60, KCl 3, NaH2PO4 1.25, NaHCO3 28, sodium ascorbate acid 0.6, glucose 5, MgCl2 7, and CaCl2 0.5 using a HM650V vibrating microtome (Thermo Scientific). Slices were then transferred to a holding chamber (Scientific Designs, Inc.) which contained oxygenated artificial cerebrospinal fluid (aCSF) of the following composition (in mmol/L): NaCl 126, KCl 2.5, NaH2PO4 1.25, MgCl2 2, CaCl2 2, NaHCO3 26, glucose 10, pyruvic acid 2, and ascorbic acid 0.4. Slices were left to recover for at least 60 min at room temperature prior to recording in a brain slice chamber (Automate Scientific Inc., CA). Slices from the treatment and control groups were positioned on P5002A multi-electrode arrays (Alpha MED Scientific Inc., Japan) and perfused with aCSF at a rate of 2 ml/min, equilibrated with 95% O2 and 5% CO2 at 32 °C. Field excitatory postsynaptic potentials (fEPSPs) were invoked through stimulation of the performant path collaterals (0.2 msec biphasic pulse) and obtained from the dentate gyrus area. Threshold for evoking fEPSPs was determined, and the stimulus was increased incrementally (5–100 μA) until the maximum amplitude of the fEPSP was reached. All other stimulation paradigms were induced at the same half-maximal stimulus strength, defined as 50% of the stimulus used to produce the maximum fEPSP amplitude, as determined for each individual slice. After a stable baseline recording of 15 min was established, LTP was induced using high-frequency stimulation (HFS), which consisted of 100 pulses at 100 Hz applied four times with half-minute intervals. fEPSPs were monitored every 30 s for 60 min following HFS and were recorded with MED-64 system and Mobius software (Alpha MED Scientific Inc). Potentiation was calculated as the percent increase of the mean fEPSP descending slope following HFS and normalized to the mean fEPSP descending slope of baseline recordings.
Immunofluorescent labeling, confocal microscopy, and synaptic density quantification
To compare how hypertension and aging affect synaptic density, a separate cohort of mice was transcardially perfused with 1× heparin containing PBS, then the brains were removed and hemisected. The left hemispheres were fixed overnight in 4% paraformaldehyde, then were cryoprotected in a series of graded sucrose solutions (10, 20, and 30% overnight) and frozen in Cryo-Gel (Electron Microscopy Sciences, Hatfield, PA). Coronal sections of 10 μm were cut through the hippocampus and stored at −20 °C. Selected sections were ~1.6 mm caudal to Bregma, representing the more rostral hippocampus. Slides were dried at 70 °C for 30 min, washed (5 min with 1× Tris-buffered saline [TBS] + 0.25% Triton X-100 plus 2 × 5 min with TBS), and treated with 1% of sodium borohydride solution for 10 min. After the second wash (2 × 5 min with 1× TBS + 0.25% Triton X-100 plus 2 × 5 min with TBS), antigen retrieval was applied with Sodium Citrate buffer pH = 6 (Sigma, W302600) at 100 °C for 20 min. After the third wash (2 × 5 min with 1× TBS + 0.25% Triton X-100 plus 2 × 5 min with TBS) and blocking in TBS (with 0.5% Triton X-100, 0.3 M glycine, and 1% fish gelatin for 3 h), sections were immunostained using the following primary antibodies in 1:20 dilution for two nights at 4 °C: mouse anti-rat MAP-2 (Lifespan Biosciences, LS-B3439; to label neuronal somata and dendrites) and rabbit anti-human synaptophysin (Lifespan Biosciences, LS-B7275; to label synaptophysin, which is an abundant presynaptic vesicle protein). The following secondary antibody was used in 1:500 dilution for 2 h at room temperature: donkey anti-rabbit IgG Alexa 647 (Life Technologies, A31573) and goat anti-mouse IgG1 Alexa 488 (Life Technologies, A21121). Sections were washed for 2 × 5 min with 1× TBS + 0.25% Triton X-100 plus 2 × 5 min with TBS. For nuclear counterstaining, Hoechst 33342 was used. Then, the sections were transferred to slides and coverslipped. Confocal images were captured using a Leica SP2 MP confocal laser scanning microscope. Specificity of the immunolabeling was confirmed by processing negative control sections, excluding the primary antibody. No immunostaining was observed in the control sections. For synaptic density quantification in each animal, six randomly selected fields from the stratum radiatum of the hippocampus (which contains septal and commissural fibers and Schaffer collateral fibers, which are the projection forward from CA3 to CA1) were analyzed in six nonadjacent sections. Six animals per group were analyzed. The sections were imaged using a ×63 oil objective and ×4 optical zoom, and the number of puncta with a diameter of ~0.4 μm was counted. Quantification of the density of synaptophysin-immunoreactive puncta, corrected by the section thickness, was performed using MetaMorph software (version 7.7.9.0). All synapse counting was performed blind to the experimental group.
Western blotting
Immunoblotting studies for the AMPA receptor subunits GluR1 (GRIA1) and GluR2 (GRIA2) and the NMDA receptor channel subunits NMDAR1 (GRIN1) and NMDAR2A (GRIN2A) in hippocampal homogenates were performed. In brief, hippocampal samples (n = 4 per experimental group) were homogenized in ice-cold RIPA buffer (Thermo Scientific, 89901) with Protease and Phosphatase Inhibitor Cocktails (1:100, Sigma-Aldrich). BCA assay was performed (Thermo Scientific, 23227) to determine protein concentration, then 2× Laemmli buffer was added (4% SDS, 20% glycerol, 0.02% bromphenol blue, 0.12 M Tris HCl, 6 M urea) to denature the proteins. Samples were then subjected to SDS-PAGE gel electrophoresis and transferred to a PVDF membrane. Proteins were visualized with Ponceau Red staining (Fisher Bioreagents, BP103). Membranes were blocked with 5% BSA (in 0.1% Tween in TBS, for 90 min, at room temperature) and overnight incubated with the following primary antibodies: rabbit anti-glutamate receptor 1 1:1000 (Abcam, ab31232), rabbit anti-glutamate receptor 2 1:1000 (Abcam, ab20673), rabbit anti-NMDAR1 1:1000 (Abcam, ab109182), and rabbit anti-NMDAR2A 1:300 (Abcam, ab169873). After incubation with the secondary antibody for 90 min at room temperature (donkey anti-rabbit IgG HRP, 1:2000, Abcam, ab16284), membranes were developed using Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare). The relative abundance of studied proteins was determined with densitometry and β-actin (mouse monoclonal, 1:7500, for 1 h, for 45 min, at room temperature, Abcam) as a loading control.
Quantitative real-time RT-PCR
To compare how hypertension and aging affect hippocampal gene expression, total RNA was isolated from frozen hippocampal samples with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (Bailey-Downs et al. 2012a). A quantitative real-time RT-PCR technique was used to analyze hippocampal mRNA expression of 90 genes known to be involved in regulation of synaptic function using validated TaqMan Gene Expression Assays (Applied Biosystems) and a Strategen MX3000 platform, as previously reported (Bailey-Downs et al. 2012a). Quantification was performed using the ΔΔCq method. The relative quantities of the reference genes Actb, Hprt1, Ywhaz, and Gapdh were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed using a one-way ANOVA followed by Tukey’s post hoc test, using Prism 5.0 for Windows (Graphpad Software, La Jolla, CA). A p value less than 0.05 was considered statistically significant (Bennis et al. 2017; Callisaya et al. 2017; Grimmig et al. 2017; Hancock et al. 2017; Kane et al. 2017; Kim et al. 2017; Konopka et al. 2017; Liu et al. 2017; Meschiari et al. 2017; Perrott et al. 2017; Sierra and Kohanski 2017; Tenk et al. 2017; Urfer et al. 2017a, b).
Results
Angiotensin II-induced hypertension impairs synaptic function, mimicking the aging phenotype
Blood pressure was significantly increased in mice receiving Ang II infusion (young: 110 ± 5 mmHg, young + Ang II: 152 ± 4 mmHg, aged: 113 ± 4 mmHg; p < 0.05 young + Ang II vs. young).
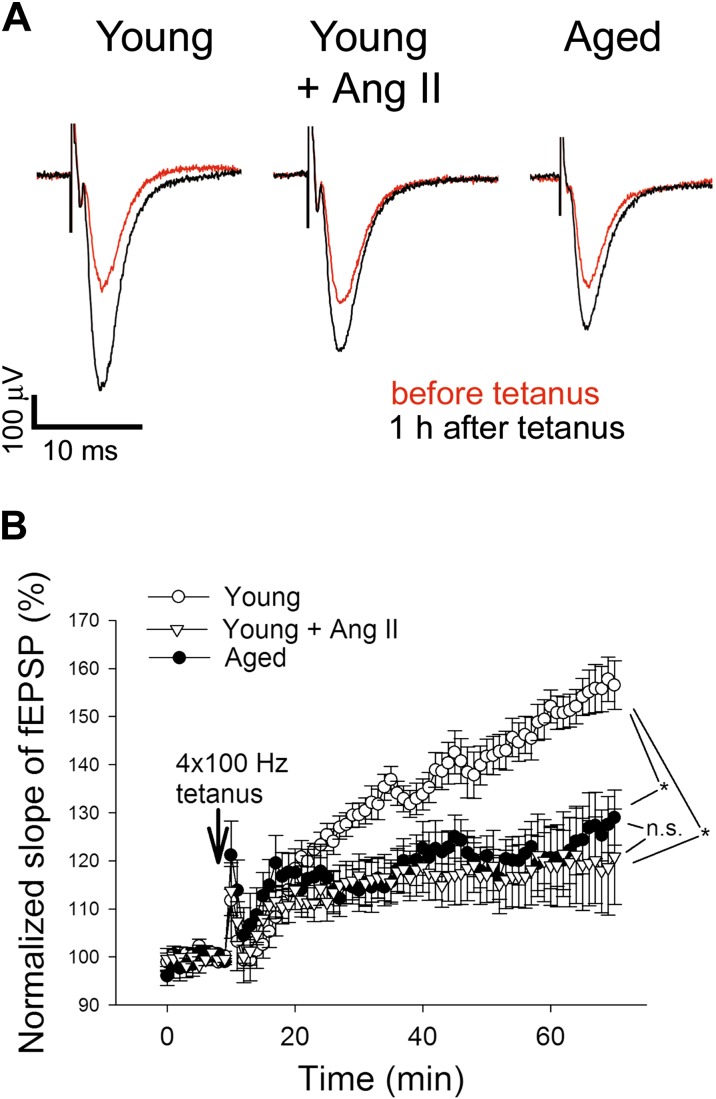
In order to characterize the effects of hypertension and compare them to those of aging on synaptic function, we measured field excitatory postsynaptic potential (EPSP) in the dentate gyrus of hippocampi in response to electrical stimulation of the perforant pathway (with 5 μA steps increased up to 100 μA). Original recordings showing field EPSPs in the dentate gyrus in response to the stimulation of the perforant pathway in each group are shown in Fig. 1a. We found that each group of mice exhibited normal basal synaptic properties. In particular, the ratio of evoked responses to the presynaptic fiber volley was similar in normotensive and hypertensive mice (p = 0.8), showing that hypertension does not affect neuronal EPSP. Following a 4x100Hz tetanic stimulation, the fEPSP slope in the dentate gyrus increased significantly less in the hypertensive young group as compared to the normotensive controls during the 60-min experimental period (Fig. 1b). In the hippocampi of aged mice, LTP was impaired and was indistinguishable from LTPs obtained in hippocampi of young hypertensive mice (Fig. 1b). Collectively, these results indicate that hypertension significantly impairs synaptic plasticity in the hippocampus, mimicking the aging phenotype.
Fig. 1.
Similar effects of hypertension and advanced aging on synaptic function in the mouse hippocampus. a Original recordings showing the effects of hypertension and advanced age on field EPSP in the dentate gyrus in response to the stimulation of the perforant pathway on hippocampal brain slices before (10 min) and 1 h after (70 min) 4x100Hz tetanus. b Long-term potentiation shown as change of fEPSP slope following a 4x100Hz (1 s) tetanic stimulus in the dentate gyrus of the hippocampus. Data are normalized to baseline responses and depicted as mean ± SEM (n = 10–12; *P < 0.05 vs. young control mice. P = 0.9 aged mice vs. young hypertensive mice during the last 5 min of the recording)
Angiotensin II-induced hypertension is associated with reduced synaptic density in the mouse hippocampus, mimicking the aging phenotype
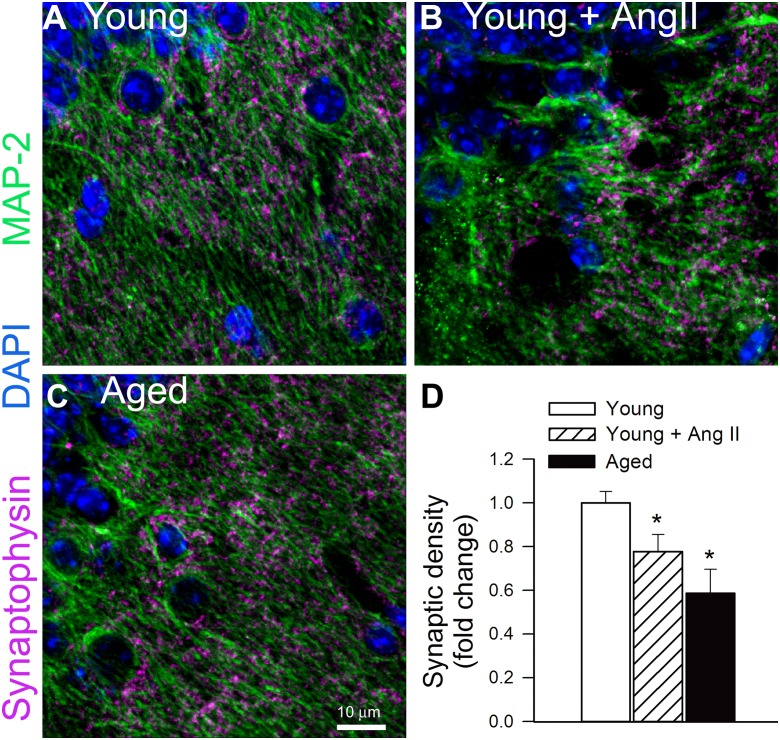
We used immunolabeling against synaptophysin, a protein localized in presynaptic vesicles, to label the density of synapses in mouse hippocampal samples. Double-immunofluorescence labeling against MAP2 demonstrated that synaptophysin-expressing presynaptic puncta were concentrated in the neuropil surrounding the MAP2-immunoreactive somata and dendrites in each group (Fig. 2a–c). We found that synaptophysin-immunoreactive puncta density was significantly lower in the stratum radiatum of the hippocampi of young hypertensive mice as compared to that in normotensive control mice (Fig. 2). Density of synaptophysin-expressing puncta was also decreased in the stratum radiatum of the hippocampi of aged control mice (Fig. 2).
Fig. 2.
Hypertension and advanced aging are associated with comparable decline in synaptic density in the mouse hippocampus. a–c Representative confocal images of synaptophysin immunoreactivity (purple) in stratum radiatum of the hippocampi of young normotensive control mice (a), young mice with angiotensin II-induced hypertension (b), and aged normotensive mice (c). Green fluorescence: MAP2 labeled somata and dendrites; blue fluorescence: nuclei. Panel c depicts summary data of relative changes in the number of synaptophysin positive presynaptic puncta (fold change). Data are mean ± SEM. *P < 0.05 vs. young normotensive controls
Effects of hypertension and aging on the hippocampal expression of postsynaptic neurotransmitter receptors
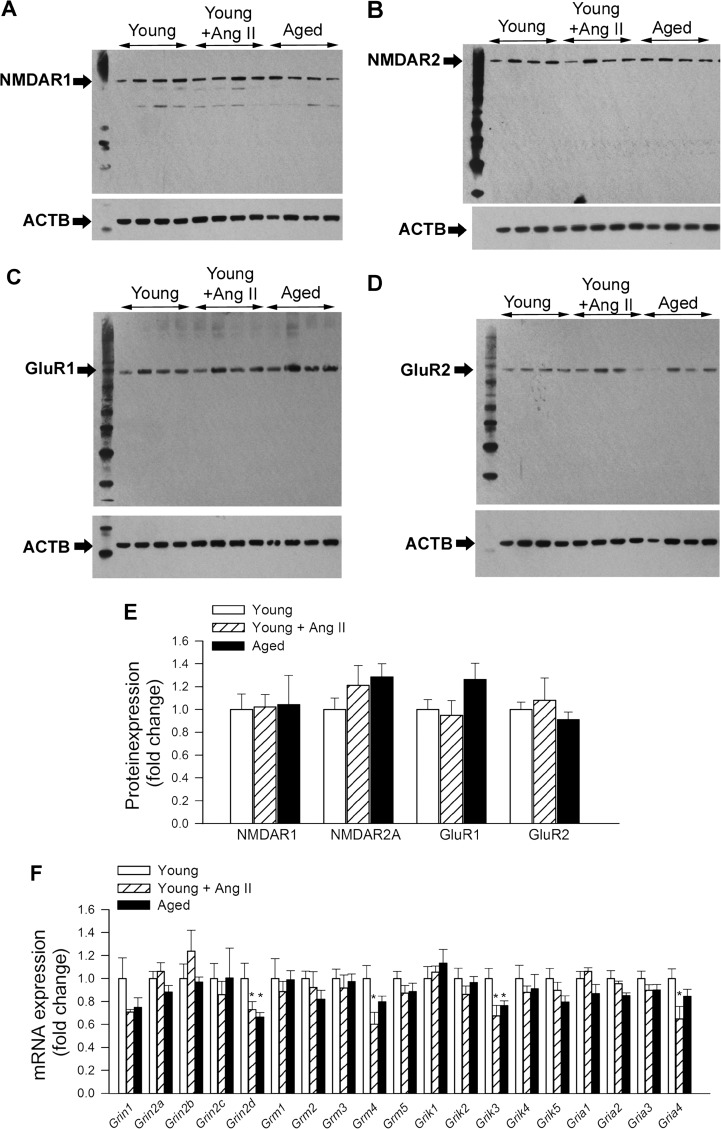
Decreased expression of postsynaptic neurotransmitter receptors may contribute to altered LTP under various pathophysiological conditions. In the present study, neither hypertension nor aging was associated with changes in protein expression of GluR1, GluR2, NMDAR1, and NMDAR2 (Fig. 3a–e). The effects of hypertension and aging on the mRNA expression of various postsynaptic neurotransmitter receptors in the mouse hippocampus are shown in Fig. 3f. Both Grin2d and Grik3 mRNA levels were equally reduced by hypertension and advanced age.
Fig. 3.
Effects of aging and hypertension on the expression of postsynaptic neurotransmitter receptors. a–d Original Western blots showing expression of NMDAR1, NMDAR2, GluR1, and GluR2 in young normotensive control mice, young mice with angiotensin II-induced hypertension, and aged normotensive mice. Summary data are shown in e. d qPCR data showing mRNA expression of neurotransmitter receptors. Data are mean ± SEM (n = 6 in each group). *P < 0.05 vs. Young; # P < 0.05 vs. Young + AngII
Effects of hypertension and aging on the hippocampal expression of genes relevant for regulation of neuronal function
Effects of hypertension and aging on the hippocampal expression of genes relevant for regulation of synaptic transmission were assessed by qPCR. As shown in Table 1, we found that both hypertension and aging were associated with similar changes in mRNA expression of several genes involved in regulation of neuronal function, including down-regulation of Bdnf, Homer1, and Dlg4, which may have a role in impaired synaptic plasticity.
Table 1.
Effects of aging and hypertension on the hippocampal expression of genes relevant for regulation of neuronal function, including synaptic transmission, development of LTP, and potentially the pathogenesis of cognitive decline. mRNA expression was analyzed by qPCR. Arrows indicate the direction of gene expression changes observed in hippocampi of aged mice and hypertensive young mice, as compared to young control mice. Parentheses indicate trends that do not reach statistical significance. (n = 5 in each group)
| Symbol | Gene name | Description/function | Change with advanced age | Change with hypertension (young) |
|---|---|---|---|---|
| Bdnf | Brain-derived neurotrophic factor | • Member of the neurotrophin family of growth factors • Promotes neuronal survival and synapse formation • Promotes LTP (Pozzo-Miller et al. 1999) • Declines in aging (Chapman et al. 2012) |
↓ | ↓ |
| Igf1 | Insulin-like growth factor 1 (IGF-1), somatomedin C | • Exerts multifaceted neuroprotective effects, including preservation of normal LTP responses in rodent models (Maher et al. 2006; Bozdagi et al. 2013; Martin et al. 2012; Trejo et al. 2007) • Multifaceted anti-aging effects (Bailey-Downs et al. 2012a; Sonntag et al. 2013; Podlutsky et al. 2017; Ashpole et al. 2015; Bailey-Downs et al. 2012b; Mitschelen et al. 2011; Tarantini et al. 2016a, b; Toth et al. 2015b, 2014b; Ungvari et al. 2010, 2011) • Circulating IGF-1 declines with advanced age (Sonntag et al. 2013) • paracrine IGF-1 contributes to neuroprotection |
→ | ↓ |
| Igf1r | Insulin-like growth factor 1 receptor | • Mediated neuroprotective effectsof IGF-1 | → | → |
| Vamp1 | Vesicle-associated membrane protein 1, synaptobrevin 1 | • VAMP1 (synaptobrevin 1) is a member of the SNARE complex (Schoch et al. 2001). • Synaptobrevins/VAMPs, syntaxins, and SNAP25 are the main components of a protein complex involved in the docking and/or fusion of synaptic vesicles with the presynaptic membrane (Deak et al. 2004). • Altered VAMP1 expression may contribute to Alzheimer’s pathophysiology (Sevlever et al. 2015) |
↓ | → |
| Vamp2 | Vesicle-associated membrane protein 2 | • Encodes a member of the SNARE complex. • Mediates Ca2 + −triggered exocytosis (Deak et al. 2006) • Contributes to AMPAR exocytosis during LTP (Jurado et al. 2013) |
↓ | → |
| Vamp3 | Vesicle-associated membrane protein 3 | • Encodes a member of the SNARE complex. • Generally expressed v-SNARE, present in hippocampal astrocytes as well (Schubert et al. 2011) but not in neurons (Schoch et al. 2001) |
→ | → |
| Vamp4 | Vesicle-associated membrane protein 4 | • Encodes a SNARE protein structurally homologous to synaptobrevin 2 • Selectively maintains bulk Ca(2+)-dependent asynchronous neurotransmission (Raingo et al. 2012) |
→ | → |
| Snap25 | Synaptosomal-associated protein 25 | • Encodes a synaptosomal protein, which plays a role in a number of neuronal functions including axonal growth, dendrite formation, fusion of synaptic vesicles with membrane and the expression of LTP in the hippocampus (Hou et al. 2004; Roberts et al. 1998) | → | → |
| Rab3a | RAB3A, | • Member of small GTPase family • Cooperates with synapsin II in the regulation of synaptic activity in hippocampus (Feliciano et al. 2013) • Associates with recycling synaptic vesicles and modulates their trafficking (Star et al. 2005) • Required for brain-derived neurotrophic factor-induced synaptic plasticity (Thakker-Varia et al. 2001) |
(↓) | (↓) |
| Rab3b | RAB3B | • Required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning (Tsetsenis et al. 2011) • Regulates synaptic vesicle trafficking (Schluter et al. 2006) |
(↓) | (↓) |
| Rab3c | RAB3C | • regulates synaptic vesicle trafficking (Schluter et al. 2006) | (↓) | (↓) |
| Rab3d | RAB3D | • Regulates synaptic vesicle trafficking (Schluter et al. 2006) | ↑ | ↑ |
| Unc13b | unc-13 homolog B; Munc-13 | • Regulates priming step of synaptic vesicle exocytosis • Regulates hippocampal synaptic transmission and plasticity (Lipstein et al. 2012; Breustedt et al. 2010; Augustin et al. 1999) |
→ | → |
| Stxbp1 | Syntaxin binding protein 1; MUNC18–1; UNC18 | • Munc18–1 binding to the neuronal SNARE complex controls synaptic vesicle priming (Deak et al. 2009) • Munc18–1 is essential for vesicular neurotransmitter release (Verhage et al. 2000) |
(↓) | (↓) |
| Syt1 | Synaptotagmin I | • Synaptotagmins are integral membrane proteins of synaptic vesicles that serve as Ca(2+) sensors. Calcium binding to synaptotagmin I triggers neurotransmitter release at the synapse (Fernandez-Chacon et al. 2001) • Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP (Wu et al. 2017) |
↓ | ↓ |
| Syt2 | Synaptotagmin II | • Synaptotagmins are integral membrane proteins of synaptic vesicles that serve as Ca(2+) sensors | → | ↓ |
| Syp | Synaptophysin; major synaptic vesicle protein p38 | • Interacts with synaptobrevin • Elimination of synaptophysin in mice results in impaired object novelty recognition and reduced spatial learning (Schmitt et al. 2009) • Essential functions in synaptic plasticity (Janz et al. 1999) |
↓ | ↓ |
| Syngr1 | Synaptogyrin-1 | • Essential functions in synaptic plasticity (Janz et al. 1999) | (↓) | (↓) |
| Vti1b | Vesicle transport through interaction with t-SNAREs homolog 1B | • The SNARE Vti1b is localized to small synaptic vesicles and participates in SNARE complex | → | → |
| Rims1 | Regulating synaptic membrane exocytosis 1 | • Regulates vesicle release • Required for LTP (Castillo et al. 2002) |
→ | → |
| Rims2 | Regulating synaptic membrane exocytosis 2 | • Regulates vesicle release (Kaeser et al. 2012) | → | → |
| Stx1a | Syntaxin 1A | • Regulates synaptic vesicle exocytosis • Regulates monoaminergic transmissions (Mishima et al. 2014) |
→ | → |
| Stx1b | Syntaxin 1B | • Regulates synaptic vesicle exocytosis (Mishima et al. 2014) • Upregulated in the dentate gyrus after induction of LTP (Richter-Levin et al. 1998; Helme-Guizon et al. 1998) • Dysregulated in aged rat after LTP induction (Davis et al. 2000) |
→ | → |
| Stx2 | Syntaxin 2 | • Regulates synaptic vesicle exocytosis | → | → |
| Stx3 | Syntaxin 3 | • Regulates synaptic vesicle exocytosis | → | → |
| Syn1 | Synapsin I | • Regulates synaptogenesis and neurotransmitter release • upregulated after induction of LTP (Morimoto et al. 1998) • In contrast, normal LTP hippocampal slices prepared from mice lacking synapsins (Spillane et al. 1995) |
↓ | (↓) |
| Syn2 | Synapsin II | • Regulates synaptogenesis and neurotransmitter release | ↓ | (↓) |
| Syn3 | Synapsin III | • Regulates synaptogenesis and neurotransmitter release | ↓ | → |
| Dlg1 | Discs, large homolog 4 (Drosophila); SAP-97 |
• Member of the membrane-associated guanylate kinase (MAGUK) family • Located in the postsynaptic density of neurons • Involved in the trafficking AMPAR during synaptic plasticity |
→ | → |
| Dlg4 | Discs, large homolog 4 (Drosophila); PSD-95 (postsynaptic density protein 95); SAP-90 (synapse-associated protein 90) |
• Member of the membrane-associated guanylate kinase (MAGUK) family • Located in the postsynaptic density of neurons • Is involved in anchoring synaptic proteins • Plays an important role in synaptic plasticity and the stabilization of synaptic changes during LTP (Carlisle et al. 2008) • SD-95 mutant mice exhibit clear deficits in AMPA receptor-mediated transmission (Carlisle et al. 2008) |
↓ | ↓ |
| Pclo | Piccolo (presynaptic cytomatrix protein); PCH3 | • Plays a critical role in synaptic plasticity and in hippocampus-dependent learning in mice (Ibi et al. 2010) • Extracellular levels of glutamate in the hippocampus under stimulated conditions are controlled by Piccolo (Ibi et al. 2010) |
→ | → |
| Sv2a | Synaptic vesicle glycoprotein 2 a | • SV2A knockout mice and SV2A/SV2B double KO mice, but not SV2B KO animals, start to experience severe seizures and weight loss 7 days after birth and die at about P14-P23 (Venkatesan et al. 2012) • SV2A (+/−) mice exhibit an anxiety-like phenotype (Lamberty et al. 2009) |
→ | → |
| Sv2b | Synaptic vesicle glycoprotein 2 b | • V2B KO mice showed normal learning and memory abilities (Detrait et al. 2014) • SV2B could be a key modulator of amyloid toxicity at the synaptic site (Detrait et al. 2014) |
→ | → |
| Bsn | Bassoon Presynaptic Cytomatrix Protein; Neuronal Double Zinc Finger Protein | • Bassoon is important for organizing the presynaptic active zone during the maturation of glutamatergic synapses (Lanore et al. 2010) • Plays an essential role in the regulated neurotransmitter release from glutamatergic synapses |
→ | → |
| Cttn | Cortactin | • Neuronal growth cone migration and targeting are essential processes for the formation of a neural network during embryonic development (Kurklinsky et al. 2011). • The large GTPase dynamin and an interacting actin-binding protein, cortactin, have been localized to the growth cone (Kurklinsky et al. 2011) • Regulates dendritic spine formation (Racz and Weinberg 2004) |
→ | → |
| Dnm1 | Dynamin 1 | • Dynamin is a large GTPase crucial for endocytosis and sustained neurotransmission (Fan et al. 2016) | → | → |
| Grn | Granulin; GRN, CLN11, GEP, GP88, PCDGF, PEPI, PGranulin | • Regulates neurogenesis • Mutations in the GRN gene have been implicated in frontotemporal lobar degeneration |
→ | → |
| Gap43 | Growth-associated protein 43; neuromodulin | • Expressed at high levels in neuronal growth cones • Role in plasticity, axonal regeneration, LTP • Down-regulated in aged rats (Ma et al. 2014) |
(↓) | ↓ |
| Rtn4 | Reticulon 4; neurite outgrowth inhibitor or Nogo | • Nogo negatively regulates synaptic plasticity (Raiker et al. 2010; Delekate et al. 2011; Zemmar et al. 2014) | → | → |
| Cltc | Clathrin | • Depletion of clathrin results in defects in synaptic vesicle reformation (Kononenko et al. 2014) | → | → |
| Agrn | Agrin | • Plays a role in synaptogenesis (McCroskery et al. 2009) • Synapse loss in cortex of agrin-deficient mice (Ksiazek et al. 2007) |
→ | → |
| Tardbp | Transactive response DNA-binding protein 43 (TDP-43) | • Neuronal activity response factor in the dendrites of hippocampal neurons • Hyperphosphorylated TDP-43 proteinopathy has recently been described in aging and in association with cognitive impairment (AD) |
↓ | ↓ |
| Nptx2 | Neuronal pentraxin 2 | • Synaptic protein that is related to C-reactive protein • Nptx2 is involved in excitatory synapse formation |
→ | ↓ |
| Arc | Activity-regulated cytoskeletal-associated protein | • Plasticity protein • Immediate-early gene (IEG) family • Arc is necessary for normal memory function • Type I mGluRs induces LTP controlled by Arc signaling in the hippocampus (Wang et al. 2016) • BDNF-induced LTP is associated with Arc activation (Kuipers et al. 2016) • Arc expression is reduced in aging (Penner et al. 2011; Fletcher et al. 2014) |
(↓) | (↓) |
| Homer1 | Homer1; Vesl; PSD-Zip45 | • LTP-induced immediate early gene • Two major splice variants, short-form (Homer1a) and long-form (Homer1b and c) • Homer1c plays a role in synaptic plasticity and the stabilization of synaptic changes during LTP (Meyer et al. 2014) |
↓ | ↓ |
| Cplx2 | Complexin 2 | • Complexins I and II are highly homologous, evolutionarily conserved proteins that regulate SNAP receptor function during exocytosis • LTP is impaired in the hippocampus of Cplx2−/− mice (Gibson et al. 2005; Huang et al. 2000) |
↓ | → |
| Cplx1 | Complexin 1 | • Complexins I and II are highly homologous, evolutionarily conserved proteins that regulate SNAP receptor function during exocytosis | ↓ | ↓ |
| Snca | Synuclein, alpha | • Plays a role in maintaining a supply of synaptic vesicles in presynaptic terminals by clustering synaptic vesicles • Knockout mice exhibit impaired hippocampal learning and memory (Kokhan et al. 2012) |
↓ | → |
| Dnajc5 | DnaJ homolog subfamily C member 5 | • Role in stimulated exocytosis, regulates synaptic transmission | → | → |
| Camk2a | Calcium/calmodulin-dependent protein kinase II alpha | • Required for hippocampal LTP and spatial learning (Lamsa et al. 2007) | ↓ | → |
| Camk2b | Calcium/calmodulin-dependent protein kinase II, beta | • αCaMKII and βCaMKII act in concert, but with distinct functions, to regulate hippocampal synaptic plasticity and learning (Borgesius et al. 2011) | ↓ | → |
| Cask | Calcium/calmodulin-dependent serine protein kinase 3 (MAGUK family) | • Role in synaptic transmembrane protein anchoring and ion channel trafficking (Atasoy et al. 2007) | → | → |
| Ppp1ca | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit | • Suppressor of learning and memory | → | → |
| Ppp1cc | Protein Phosphatase 1 Catalytic Subunit Gamma | • Involved in regulation of ionic conductance and long-term synaptic plasticity • May play an important role in dephosphorylating the postsynaptic density-associated Ca(2+)/calmodulin-dependent protein kinase II |
→ | → |
| Ppp2ca | Protein Phosphatase 2 Catalytic Subunit Alpha | • Role in regulation of LTP | ↓ | → |
| Ppp3ca | Protein phosphatase 3 catalytic subunit alpha | ↓ | → | |
| Prkca | Protein kinase C alpha | • Role in LTP | → | → |
| Slc1a2 | Solute carrier family 1 (glial high affinity glutamate transporter), member 2; EAAT2 | • Clears the excitatory neurotransmitter glutamate from the extracellular space at synapses (responsible for over 90% of glutamate reuptake) | → | → |
| Slc1a3 | Solute carrier family 1 (glial high affinity glutamate transporter), member 3; GLutamate ASpartate Transporter (GLAST);Excitatory Amino Acid Transporter 1 (EAAT1) | • Clears glutamate from the extracellular space • Highly expressed in astrocytes |
→ | → |
| Trpc1 | Transient receptor potential cation channel, subfamily C, member 1 | • Non-specific ion channel abundantly expressed in hippocampus and prefrontal cortex | → | → |
| Trpc4 | Transient receptor potential cation channel, subfamily C, member 4 | • Role in neurotransmitter release (Munsch et al. 2003) | → | → |
| Trpc5 | Transient receptor potential cation channel, subfamily C, member 5 | → | → | |
| Trpc6 | transient receptor potential cation channel, subfamily C, member 6 | • Impaired spatial cognition and synaptic plasticity was reported to associate with down-regulation of TRPC6 in rodent models of stress (Liu et al. 2015). • Upregulated in cerebral vessels in young mice with angiotensin II-induced hypertension (Toth et al. 2013a) |
→ | (↑) |
| Itpr1 | Inositol 1,4,5-trisphosphate receptor type 1 | • Act as receptor and channel in release of calcium from intracellular stores by IP3. | → | → |
| Cacna2d1 | Voltage-dependent calcium channel subunit alpha-2/delta-1 | → | → | |
| Cacna2d2 | Calcium channel, voltage-dependent, alpha 2/delta subunit 2 | • Critical role of calcium signaling in molecular processes underlying memory (Villela et al. 2016) | ↓ | ↓ |
| Cacna1b | Voltage-dependent N-type calcium channel subunit alpha-1B | → | → |
Discussion
The results of this study suggest that previously documented hypertension-induced cognitive deficits in mice are associated with impairment of LTP in the hippocampus, decreased synaptic density, and dysregulation of expression of genes involved in regulation of neuronal function, all of which mimic important aspects of the aging phenotype. These findings are translationally relevant, as there is strong evidence extant linking LTP in the hippocampal formation to induction and storage of memories (Lynch 2004; Neves et al. 2008). The available evidence suggests that hypertension-induced impairment of LTP and cognitive decline does not depend on the experimental model used. For example, genetically hypertensive strain of Wistar rat also exhibit impaired LTP in perforant path-granule cell synapses associated with impairments in long-term recognition memory (Hennigan et al. 2009). It is predicted that if similar synaptic dysfunction is also manifested in the hippocampi of hypertensive humans, it would likely contribute to the development of vascular cognitive impairment.
The mechanisms by which hypertension impairs synaptic plasticity and reduces the number of synapses in the hippocampus are likely multifaceted. Hypertension is known to promote disruption of the blood–brain barrier, microglia activation, and neuroinflammation in the hippocampus (Toth et al. 2013a, 2014a), which are known to alter the local microenvironment in the hippocampus and impair normal synaptic function (Di Filippo et al. 2013; Hao et al. 2016; Kyrargyri et al. 2015; Liu et al. 2012; Min et al. 2009; Perry and O'Connor 2010; Riazi et al. 2015). Importantly, circulating angiotensin II is too large to enter the hippocampus by crossing an intact blood–brain barrier in healthy subjects. However, when hypertension disrupts the blood–brain barrier, it provides a means by which circulating angiotensin II could enter the brain parenchyma and affect neuronal function directly. Thus, it cannot be excluded that angiotensin II-induced activation of neuronal signaling pathways, such as p38 MAP kinase-dependent pathways (Dai et al. 2016), also contribute to functional impairment of hippocampal neurons. However, direct angiotensin II effects on LTP in our experiments is rather unlikely as hippocampal slices were incubated and perfused without angiotensin II during the electrophysiological assays and the angiotensin II effect was reported to be reversible within 3 h (Wayner et al. 1995). Hypertension-related factors (including inflammatory mediators and other factors released by activated microglia) may alter LTP induction by eliciting changes in intrinsic neuronal properties or baseline synaptic transmission. There are several steps in this postsynaptic transduction cascade that contribute to LTP, which may be altered in hypertension. While changes in hippocampal expression of AMPA receptors and NMDA receptors were not evident, we have identified several factors important for neuronal health whose expression was significantly altered by hypertension (Table 1). The findings that hypertension decreases hippocampal expression of BDNF and IGF-1 are particularly interesting, as both growth factors exert multifaceted neuroprotective effects, including preservation of normal LTP responses. There is strong evidence that blockade of BDNF or knockdown of IGF-1 results in impaired LTP (Montalbano et al. 2013) and/or memory impairment (Sonntag et al. 2013). Further, both BDNF (von Bohlen 2010) and IGF-1 (Deak and Sonntag 2012; Poe et al. 2001; Ramsey et al. 2005; Shi et al. 2005) can increase the number of synapses. Deficits in LTP in the genetically hypertensive rat were also demonstrated to associate with decreased expression of BDNF and its receptors in the dentate gyrus (Hennigan et al. 2009). Importantly, a decline in IGF-1 signaling is also considered as an important evolutionarily conserved mechanism of brain aging (Sonntag et al. 2013; Ashpole et al. 2017). In that regard, it is interesting that treatment that increases IGF-1 levels can improve learning and memory function both in vertebrate (Ramsey et al. 2004; Thornton et al. 2000) and invertebrate models (Lymnaea stagnalis) (Pirger et al. 2014) of aging. Our studies provide additional evidence in support of the concept that synaptic plasticity in the hippocampus and neocortex significantly changes with aging (Lynch 2004; Griffin et al. 2006; Liu et al. 2012; Deak and Sonntag 2012; Lynch 2010). It is remarkable that hypertension-induced changes in synaptic plasticity and synaptic density in young mice were comparable to the age-dependent changes in capacity for LTP induction in the same mouse strain. Our present and previous studies (Csiszar et al. 2013) also reveal complex hypertension-induced changes in hippocampal gene expression profile, which mimic various aspects of the aging phenotype and may contribute to synaptic dysfunction. Among them, both hypertension and aging significantly decreased expression of synaptophysin I, an abundant synaptic vesicle protein that accounts for 7% of the total vesicle protein (Janz et al. 1999). Synaptophysin I interacts with vesicular SNARE synaptobrevins, and its genetic depletion (in the presence of concomitant decrease in synaptogyrin I expression) results in significant synaptic dysfunction (Janz et al. 1999). Additional potential factors identified in this study that may similarly contribute to both hypertension- and aging-induced synaptic dysfunction in mice include altered expression of synaptic scaffold proteins like PSD-95 and Homer-1. Further studies are evidently needed to elucidate the functional role of these phenotypic changes.
On the basis of previous findings (Toth et al. 2013a, 2015a; Springo et al. 2015; Toth et al. 2013c) and the present results, it seems to be likely that advanced aging and comorbid hypertension have synergistic effects on hippocampal neuronal function. Additional studies are warranted to determine whether the effects of hypertension are indeed exacerbated in aged mice. Clinical studies suggest that hypertension-induced cognitive decline develops gradually, leaving a time window for therapeutic intervention for prevention (Gottesman et al. 2014). For example, treatment of hypertension resulted in a 19 and 50% reduction in dementia incidence in the elderly in the PROGRESS (PROGRESS.Collaborative.Group 2001) and Syst-Eur (Forette et al. 1998) studies, respectively. Further preclinical studies should determine whether hypertension-induced impairment of synaptic plasticity and decline in synapse density are also reversible with antihypertensive medication. In addition to causing phenotypic and functional alterations in hippocampal neurons, hypertension also impairs their blood supply by dysregulating cerebral blood flow (Toth et al. 2017; Faraco and Iadecola 2013), promoting microvascular rarefaction (Toth et al. 2013a) and microvascular injury (Toth et al. 2013a, 2015a). The large pyramidal neurons in the hippocampus have high metabolic demand, which render these neurons especially sensitive to impaired supply of oxygen and nutrients through the hippocampal microvasculature. Thus, hypertension-induced cognitive decline is likely a result of these complex and interrelated microvascular factors and secondary neuronal pathologies. During the past decade, it has been well established that high blood pressure significantly increases oxidative stress in the brain (Girouard et al. 2006; Kazama et al. 2004; Girouard et al. 2007; Kazama et al. 2003; Poulet et al. 2006). It is presumed that increased production of ROS plays an important role disruption of the blood–brain barrier, microvascular injury, and neurodegeneration. Further studies are warranted to elucidate the role of increased oxidative stress (Deepa et al. 2017) in impairments of LTP and to assess the protective effects of antioxidant treatments against hypertension-induced synaptic dysfunction.
There is growing epidemiological evidence that synaptic dysfunction contributes to the pathogenesis of AD and that hypertension worsens the clinical outcome of Alzheimer’s disease (Israeli-Korn et al. 2010; Guo et al. 2001). Recent studies on experimental models of Alzheimer’s disease also support the validity of the vascular hypothesis of AD by demonstrating that induction of hypertension in mice by transverse aortic coarctation exacerbates cognitive impairment (Carnevale and Lembo 2011; Carnevale et al. 2012a, b). Our findings combined with these observations highlight a novel mechanism by which hypertension may exacerbate the symptoms of AD and provide additional support for aggressive blood pressure management for neuroprotection in patients at risk for AD (Trenkwalder 2006; Khachaturian et al. 2006).
Limitations of the study
A number of important limitations of the present study need to be considered. First, we do not have data on protein expression of most of the investigated target genes. Second, as many proteins involved in regulation of synaptic transmission and LTP are known to be modulated at the posttranslational level, further studies are needed to investigate the effects of aging and hypertension on synaptic proteins both at the translational and at the posttranslational levels as well. Third, there is evidence linking neuroinflammation to cognitive decline in hypertension (Carnevale et al. 2012a) and strong data suggest that aging is also associated with increased sterile inflammation in the brain (Shobin et al. 2017), which is exacerbated by hypertension (Toth et al. 2013a). However, the specific mechanisms by which inflammatory mediators impair synaptic function in aged hypertensive subjects remain elusive.
Conclusion
In conclusion, the pathological alterations in synaptic plasticity and the hippocampal gene expression signature observed in hypertensive mice in the present study provides important clues for subsequent studies to elucidate the mechanisms by which hypertension may contribute to the pathogenesis and clinical manifestation of VCI and AD. Further studies are evidently needed to determine whether pharmacological treatments that confer microvascular protection, anti-oxidative, and/or anti-inflammatory effects will attenuate hypertension-induced alterations in synaptic plasticity and neuronal gene expression preventing/delaying cognitive decline.
Acknowledgements
This work was supported by grants from the American Heart Association (to ST, ZT, MNVA, AC and ZU), the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; P30 AG050911), the NIA-supported Oklahoma Nathan Shock Center (to ZU and AC; 3P30AG050911-02S1), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC), the Oklahoma Center for the Advancement of Science and Technology (to AC, FD, ZU), the Oklahoma IDeA Network for Biomedical Research Excellence (to AC and FD), the Presbyterian Health Foundation (to AC, AY, ZU, FD), and the Reynolds Foundation (to ZU and AC). The paper was published as part of the “Translational Geroscience” initiative of the Journal of the American Aging Association (Ungvari et al. 2017a; Bennis et al. 2017; Callisaya et al. 2017; Grimmig et al. 2017; Hancock et al. 2017; Kane et al. 2017; Kim et al. 2017; Konopka et al. 2017; Liu et al. 2017; Meschiari et al. 2017; Perrott et al. 2017; Sierra and Kohanski 2017; Tenk et al. 2017; Urfer et al. 2017a, b; Ashpole et al. 2017; Deepa et al. 2017; Shobin et al. 2017; Podlutsky et al. 2017; Ungvari et al. 2017b). The authors acknowledge the support from the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), which aimed to facilitate the understanding of the interaction of processes of aging and chronic diseases (Sierra and Kohanski 2017).
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Zsuzsanna Tucsek and M. Noa Valcarcel-Ares contributed equally to this manuscript.
Contributor Information
Ferenc Deak, Email: Ferenc-Deak@ouhsc.edu.
Zoltan Ungvari, Email: zoltan-ungvari@ouhsc.edu.
References
- Ashpole NM, Herron JC, Mitschelen MC, Farley JA, Logan S, Yan H, Ungvari Z, Hodges EL, Csiszar A, Ikeno Y, Humphrey MB, Sonntag WE (2015) Igf-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res 31(2):443–454 [DOI] [PMC free article] [PubMed]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE (2017) Igf-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience 39(2):129–145 [DOI] [PMC free article] [PubMed]
- Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, Kavalali ET, Sudhof TC. Deletion of cask in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104:2525–2530. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffret A, Gautheron V, Repici M, Kraftsik R, Mount HT, Mariani J, Rovira C. Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal ca 1 neurons of a presenilin 1 transgenic mouse model of Alzheimer's disease. J Neurosci. 2009;29:10144–10152. doi: 10.1523/JNEUROSCI.1856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of igf-1 decreases vascular oxidative stress resistance by impairing the nrf 2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and igf-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39:51–59. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgesius NZ, van Woerden GM, Buitendijk GH, Keijzer N, Jaarsma D, Hoogenraad CC, Elgersma Y. Betacamkii plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting alphacamkii to synapses. J Neurosci. 2011;31:10141–10148. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Tavassoli T, Buxbaum JD. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol Autism. 2013;4:9. doi: 10.1186/2040-2392-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breustedt J, Gundlfinger A, Varoqueaux F, Reim K, Brose N, Schmitz D. Munc13-2 differentially affects hippocampal synaptic transmission and plasticity. Cereb Cortex. 2010;20:1109–1120. doi: 10.1093/cercor/bhp170. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Launay CP, Srikanth VK, Verghese J, Allali G, Beauchet O (2017) Cognitive status, fast walking speed and walking speed reserve-the gait and alzheimer interactions tracking (gait) study. Geroscience 39(2):231–239 [DOI] [PMC free article] [PubMed]
- Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin ii hypertension. J Neurosci. 2012;32:4878–4886. doi: 10.1523/JNEUROSCI.6262-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Fink AE, Grant SG, O'Dell TJ. Opposing effects of psd-93 and psd-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol. 2008;586:5885–5900. doi: 10.1113/jphysiol.2008.163469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale D, Lembo G. 'Alzheimer-like' pathology in a murine model of arterial hypertension. Biochem Soc Trans. 2011;39:939–944. doi: 10.1042/BST0390939. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Mascio G, Ajmone-Cat MA, D'Andrea I, Cifelli G, Madonna M, Cocozza G, Frati A, Carullo P, Carnevale L, Alleva E, Branchi I, Lembo G, Minghetti L. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging. 2012;33:205 e219–205 e229. doi: 10.1016/j.neurobiolaging.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. Rim1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, Patterson SL. Aging and infection reduce expression of specific brain-derived neurotrophic factor mrnas in hippocampus. Neurobiol Aging. 2012;33:832 e831–832 e814. doi: 10.1016/j.neurobiolaging.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley TR, O'Sullivan J, Blau C, Deighan BF, Jones R, Kerskens C, Richardson JC, Virley D, Upton N, Lynch MA. Rosiglitazone attenuates the age-related changes in astrocytosis and the deficit in ltp. Neurobiol Aging. 2012;33:162–175. doi: 10.1016/j.neurobiolaging.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, Deak F, Sonntag WE, Ungvari Z. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in beta-amyloid generation and alzheimer's disease. Am J Physiol Heart Circ Physiol. 2013;305:H1120–H1130. doi: 10.1152/ajpheart.00288.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai HL, Hu WY, Jiang LH, Li L, Gaung XF, Xiao ZC. P38 mapk inhibition improves synaptic plasticity and memory in angiotensin ii-dependent hypertensive mice. Sci Rep. 2016;6:27600. doi: 10.1038/srep27600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Salin H, Helme-Guizon A, Dumas S, Stephan A, Corbex M, Mallet J, Laroche S. Dysfunctional regulation of alphacamkii and syntaxin 1b transcription after induction of ltp in the aged rat. Eur J Neurosci. 2000;12:3276–3282. doi: 10.1046/j.1460-9568.2000.00193.x. [DOI] [PubMed] [Google Scholar]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (igf)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- Deak F, Shin OH, Kavalali ET, Sudhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Xu Y, Chang WP, Dulubova I, Khvotchev M, Liu X, Sudhof TC, Rizo J. Munc18-1 binding to the neuronal snare complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A (2017) A new mouse model of frailty: the cu/zn superoxide dismutase knockout mouse. Geroscience 39(2):187–198 [DOI] [PMC free article] [PubMed]
- Delekate A, Zagrebelsky M, Kramer S, Schwab ME, Korte M. Nogoa restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc Natl Acad Sci U S A. 2011;108:2569–2574. doi: 10.1073/pnas.1013322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrait E, Maurice T, Hanon E, Leclercq K, Lamberty Y. Lack of synaptic vesicle protein sv2b protects against amyloid-beta(2)(5)(−)(3)(5)-induced oxidative stress, cholinergic deficit and cognitive impairment in mice. Behav Brain Res. 2014;271:277–285. doi: 10.1016/j.bbr.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampa C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de Iure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis. 2013;52:229–236. doi: 10.1016/j.nbd.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Diogenes MJ, Costenla AR, Lopes LV, Jeronimo-Santos A, Sousa VC, Fontinha BM, Ribeiro JA, Sebastiao AM. Enhancement of ltp in aged rats is dependent on endogenous bdnf. Neuropsychopharmacology. 2011;36:1823–1836. doi: 10.1038/npp.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Funk L, Lou X. Dynamin 1- and 3-mediated endocytosis is essential for the development of a large central synapse in vivo. J Neurosci. 2016;36:6097–6115. doi: 10.1523/JNEUROSCI.3804-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano P, Andrade R, Bykhovskaia M. Synapsin ii and rab3a cooperate in the regulation of epileptic and synaptic activity in the ca1 region of the hippocampus. J Neurosci. 2013;33:18319–18330. doi: 10.1523/JNEUROSCI.5293-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin i functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fletcher BR, Hill GS, Long JM, Gallagher M, Shapiro ML, Rapp PR. A fine balance: regulation of hippocampal arc/arg3.1 transcription, translation and degradation in a rat model of normal cognitive aging. Neurobiol Learn Mem. 2014;115:58–67. doi: 10.1016/j.nlm.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in europe (syst-eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/S0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- Gelber RP, Launer LJ, White LR. The honolulu-asia aging study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res. 2012;9:664–672. doi: 10.2174/156720512801322618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson HE, Reim K, Brose N, Morton AJ, Jones S. A similar impairment in ca3 mossy fibre ltp in the r6/2 mouse model of huntington's disease and in the complexin ii knockout mouse. Eur J Neurosci. 2005;22:1701–1712. doi: 10.1111/j.1460-9568.2005.04349.x. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin ii attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin ii. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Grimmig B, Kim SH, Nash K, Bickford PC, Douglas SR. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Qiu C, Viitanen M, Fastbom J, Winblad B, Fratiglioni L. Blood pressure and dementia in persons 75+ years old: 3-year follow-up results from the kungsholmen project. J Alzheimers Dis. 2001;3:585–591. doi: 10.3233/JAD-2001-3609. [DOI] [PubMed] [Google Scholar]
- Hancock SE, Friedrich MG, Mitchell TW, Truscott RJ, Else PL. The phospholipid composition of the human entorhinal cortex remains relatively stable over 80 years of adult aging. Geroscience. 2017;39:73–82. doi: 10.1007/s11357-017-9961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Dey A, Yu X, Stranahan AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun. 2016;51:230–239. doi: 10.1016/j.bbi.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helme-Guizon A, Davis S, Israel M, Lesbats B, Mallet J, Laroche S, Hicks A. Increase in syntaxin 1b and glutamate release in mossy fibre terminals following induction of ltp in the dentate gyrus: a candidate molecular mechanism underlying transsynaptic plasticity. Eur J Neurosci. 1998;10:2231–2237. doi: 10.1046/j.1460-9568.1998.00232.x. [DOI] [PubMed] [Google Scholar]
- Hennigan A, Callaghan CK, Kealy J, Rouine J, Kelly AM. Deficits in ltp and recognition memory in the genetically hypertensive rat are associated with decreased expression of neurotrophic factors and their receptors in the dentate gyrus. Behav Brain Res. 2009;197:371–377. doi: 10.1016/j.bbr.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Hou Q, Gao X, Zhang X, Kong L, Wang X, Bian W, Tu Y, Jin M, Zhao G, Li B, Jing N, Yu L. Snap-25 in hippocampal ca1 region is involved in memory consolidation. Eur J Neurosci. 2004;20:1593–1603. doi: 10.1111/j.1460-9568.2004.03600.x. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Ujihara H, Takahashi S, Kaba H, Yagi T, Inoue S. Involvement of complexin ii in synaptic plasticity in the ca1 region of the hippocampus: the use of complexin ii-lacking mice. Jpn J Pharmacol. 2000;84:179–187. doi: 10.1254/jjp.84.179. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Hypertension and dementia. Hypertension. 2014;64:3–5. doi: 10.1161/HYPERTENSIONAHA.114.03040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nitta A, Ishige K, Cen X, Ohtakara T, Nabeshima T, Ito Y. Piccolo knockdown-induced impairments of spatial learning and long-term potentiation in the hippocampal ca1 region. Neurochem Int. 2010;56:77–83. doi: 10.1016/j.neuint.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Israeli-Korn SD, Masarwa M, Schechtman E, Abuful A, Strugatsky R, Avni S, Farrer LA, Friedland RP, Inzelberg R. Hypertension increases the probability of alzheimer's disease and of mild cognitive impairment in an arab community in northern israel. Neuroepidemiology. 2010;34:99–105. doi: 10.1159/000264828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz R, Sudhof TC, Hammer RE, Unni V, Siegelbaum SA, Bolshakov VY. Essential roles in synaptic plasticity for synaptogyrin i and synaptophysin i. Neuron. 1999;24:687–700. doi: 10.1016/S0896-6273(00)81122-8. [DOI] [PubMed] [Google Scholar]
- Jurado S, Goswami D, Zhang Y, Molina AJ, Sudhof TC, Malenka RC. Ltp requires a unique postsynaptic snare fusion machinery. Neuron. 2013;77:542–558. doi: 10.1016/j.neuron.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Fan M, Sudhof TC. Rim genes differentially contribute to organizing presynaptic release sites. Proc Natl Acad Sci U S A. 2012;109:11830–11835. doi: 10.1073/pnas.1209318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AE, Gregson E, Theou O, Rockwood K, Howlett SE (2017) The association between frailty, the metabolic syndrome, and mortality over the lifespan. Geroscience 39(2):221–229 [DOI] [PMC free article] [PubMed]
- Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin ii attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2003;285:H1890–H1899. doi: 10.1152/ajpheart.00464.2003. [DOI] [PubMed] [Google Scholar]
- Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin ii impairs neurovascular coupling in neocortex through nadph oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Blatt GJ, Killiany RJ, Moss MB. Neuropathology of progressive cognitive decline in chronically hypertensive rhesus monkeys. Acta Neuropathol. 2001;101:145–153. doi: 10.1007/s004010000278. [DOI] [PubMed] [Google Scholar]
- Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Antihypertensive medication use and incident alzheimer disease: the cache county study. Arch Neurol. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience. 2017;39:83–92. doi: 10.1007/s11357-017-9960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Baars MA, Spauwen P, Schievink S, Verhey FR, van Boxtel MJ. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension. 2014;63:245–251. doi: 10.1161/HYPERTENSIONAHA.113.02096. [DOI] [PubMed] [Google Scholar]
- Kokhan VS, Afanasyeva MA, Van'kin GI. Alpha-synuclein knockout mice have cognitive impairments. Behav Brain Res. 2012;231:226–230. doi: 10.1016/j.bbr.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Kononenko NL, Puchkov D, Classen GA, Walter AM, Pechstein A, Sawade L, Kaempf N, Trimbuch T, Lorenz D, Rosenmund C, Maritzen T, Haucke V. Clathrin/ap-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron. 2014;82:981–988. doi: 10.1016/j.neuron.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, Zhang Q, Peelor FF 3rd, Melby CL, Hamilton KL, Miller BF (2017) Influence of nrf 2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience 39(2):175–186 [DOI] [PMC free article] [PubMed]
- Ksiazek I, Burkhardt C, Lin S, Seddik R, Maj M, Bezakova G, Jucker M, Arber S, Caroni P, Sanes JR, Bettler B, Ruegg MA. Synapse loss in cortex of agrin-deficient mice after genetic rescue of perinatal death. J Neurosci. 2007;27:7183–7195. doi: 10.1523/JNEUROSCI.1609-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers SD, Trentani A, Tiron A, Mao X, Kuhl D, Bramham CR. Bdnf-induced ltp is associated with rapid arc/arg3.1-dependent enhancement in adult hippocampal neurogenesis. Sci Rep. 2016;6:21222. doi: 10.1038/srep21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurklinsky S, Chen J, McNiven MA. Growth cone morphology and spreading are regulated by a dynamin-cortactin complex at point contacts in hippocampal neurons. J Neurochem. 2011;117:48–60. doi: 10.1111/j.1471-4159.2011.07169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrargyri V, Vega-Flores G, Gruart A, Delgado-Garcia JM, Probert L. Differential contributions of microglial and neuronal ikkbeta to synaptic plasticity and associative learning in alert behaving mice. Glia. 2015;63:549–566. doi: 10.1002/glia.22756. [DOI] [PubMed] [Google Scholar]
- Lamberty Y, Detrait E, Leclercq K, Michel A, De Ryck M. Behavioural phenotyping reveals anxiety-like features of sv2a deficient mice. Behav Brain Res. 2009;198:329–333. doi: 10.1016/j.bbr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Irvine EE, Giese KP, Kullmann DM. Nmda receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on ca(2+)/calmodulin-dependent kinases. J Physiol. 2007;584:885–894. doi: 10.1113/jphysiol.2007.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanore F, Blanchet C, Fejtova A, Pinheiro P, Richter K, Balschun D, Gundelfinger E, Mulle C. Impaired development of hippocampal mossy fibre synapses in mouse mutants for the presynaptic scaffold protein bassoon. J Physiol. 2010;588:2133–2145. doi: 10.1113/jphysiol.2009.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipstein N, Schaks S, Dimova K, Kalkhof S, Ihling C, Kolbel K, Ashery U, Rhee J, Brose N, Sinz A, Jahn O. Nonconserved ca(2+)/calmodulin binding sites in munc13s differentially control synaptic short-term plasticity. Mol Cell Biol. 2012;32:4628–4641. doi: 10.1128/MCB.00933-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wu Z, Hayashi Y, Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012;216:133–142. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]
- Liu CC, Tsai CW, Deak F, Rogers J, Penuliar M, Sung YM, Maher JN, Fu Y, Li X, Xu H, Estus S, Hoe HS, Fryer JD, Kanekiyo T, Bu G. Deficiency in lrp 6-mediated wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer's disease. Neuron. 2014;84:63–77. doi: 10.1016/j.neuron.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu C, Qin X, Zhu M, Yang Z. The change of spatial cognition ability in depression rat model and the possible association with down-regulated protein expression of trpc6. Behav Brain Res. 2015;294:186–193. doi: 10.1016/j.bbr.2015.07.062. [DOI] [PubMed] [Google Scholar]
- Liu X, Bhatt T, Wang S, Yang F, Pai YC. Retention of the "first-trial effect" in gait-slip among community-living older adults. Geroscience. 2017;39:93–102. doi: 10.1007/s11357-017-9963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang Z, Kang L, Geng D, Wang Y, Wang M, Cui H. Repetitive transcranial magnetic stimulation (rtms) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp Gerontol. 2014;58:256–268. doi: 10.1016/j.exger.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–1571. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- Martin ED, Sanchez-Perez A, Trejo JL, Martin-Aldana JA, Cano Jaimez M, Pons S, Acosta Umanzor C, Menes L, White MF, Burks DJ. Irs-2 deficiency impairs nmda receptor-dependent long-term potentiation. Cereb Cortex. 2012;22:1717–1727. doi: 10.1093/cercor/bhr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Bailey A, Lin L, Daniels MP. Transmembrane agrin regulates dendritic filopodia and synapse formation in mature hippocampal neuron cultures. Neuroscience. 2009;163:168–179. doi: 10.1016/j.neuroscience.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience. 2017;39:7–18. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82:430–443. doi: 10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Min SS, Quan HY, Ma J, Han JS, Jeon BH, Seol GH. Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal ca1 area. Neurosci Lett. 2009;456:20–24. doi: 10.1016/j.neulet.2009.03.079. [DOI] [PubMed] [Google Scholar]
- Mishima T, Fujiwara T, Sanada M, Kofuji T, Kanai-Azuma M, Akagawa K. Syntaxin 1b, but not syntaxin 1a, is necessary for the regulation of synaptic vesicle exocytosis and of the readily releasable pool at central synapses. PLoS One. 2014;9:e90004. doi: 10.1371/journal.pone.0090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschelen M, Yan H, Farley JA, Warrington JP, Han S, Herenu CB, Csiszar A, Ungvari Z, Bailey-Downs LC, Bass CE, Sonntag WE. Long-term deficiency of circulating and hippocampal insulin-like growth factor i induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. doi: 10.1016/j.neuroscience.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbano A, Baj G, Papadia D, Tongiorgi E, Sciancalepore M. Blockade of bdnf signaling turns chemically-induced long-term potentiation into long-term depression. Hippocampus. 2013;23:879–889. doi: 10.1002/hipo.22144. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Rosene DL, Prusty S, Hollander W, Moss MB. Impairment of executive function induced by hypertension in the rhesus monkey (Macaca mulatta) Behav Neurosci. 2002;116:387–396. doi: 10.1037/0735-7044.116.3.387. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Sato K, Sato S, Yamada N, Hayabara T. Time-dependent changes in rat hippocampal synapsin i mrna expression during long-term potentiation. Brain Res. 1998;783:57–62. doi: 10.1016/S0006-8993(97)01154-2. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss MB, Jonak E. Cerebrovascular disease and dementia: a primate model of hypertension and cognition. Alzheimers Dement. 2007;3:S6–15. doi: 10.1016/j.jalz.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Munsch T, Freichel M, Flockerzi V, Pape HC. Contribution of transient receptor potential channels to the control of gaba release from dendrites. Proc Natl Acad Sci U S A. 2003;100:16065–16070. doi: 10.1073/pnas.2535311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Oka H, Shimono K, Ogawa R, Sugihara H, Taketani M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J Neurosci Methods. 1999;93:61–67. doi: 10.1016/S0165-0270(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Penner MR, Roth TL, Chawla MK, Hoang LT, Roth ED, Lubin FD, Sweatt JD, Worley PF, Barnes CA. Age-related changes in arc transcription and DNA methylation within the hippocampus. Neurobiol Aging. 2011;32:2198–2210. doi: 10.1016/j.neurobiolaging.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrott KM, Wiley CD, Desprez PY, Campisi J (2017) Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience 39(2):161–173 [DOI] [PMC free article] [PubMed]
- Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirger Z, Naskar S, László Z, Kemenes G, Reglődi D, Kemenes I (2014) Reversal of age related learning deficiency by the vertebrate pituitary adenylate cyclase activating polypeptide (pacap) and insulin-like growth factor-1 (igf-1) in a novel invertebrate model of aging: the pond snail (Lymnaea stagnalis). J Gerontol Biol Med Sci 69(11):1331–1338 [DOI] [PMC free article] [PubMed]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z (2017) The gh/igf-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience 69(11):1331–1338 [DOI] [PMC free article] [PubMed]
- Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area ca3 of hippocampus. Neuroscience. 2001;107:231–238. doi: 10.1016/S0306-4522(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Poulet R, Gentile MT, Vecchione C, Distaso M, Aretini A, Fratta L, Russo G, Echart C, Maffei A, De Simoni MG, Lembo G. Acute hypertension induces oxidative stress in brain tissues. J Cereb Blood Flow Metab. 2006;26:253–262. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of bdnf knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROGRESS.Collaborative.Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. The subcellular organization of cortactin in hippocampus. J Neurosci. 2004;24:10310–10317. doi: 10.1523/JNEUROSCI.2080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingo J, Khvotchev M, Liu P, Darios F, Li YC, Ramirez DM, Adachi M, Lemieux P, Toth K, Davletov B, Kavalali ET. Vamp4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat Neurosci. 2012;15:738–745. doi: 10.1038/nn.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-igf-1 action at excitatory synapses in the ca1 region of rat hippocampus. J Neurophysiol. 2005;94:247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA, Pittman QJ. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci. 2015;35:4942–4952. doi: 10.1523/JNEUROSCI.4485-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Thomas KL, Hunt SP, Bliss TV. Dissociation between genes activated in long-term potentiation and in spatial learning in the rat. Neurosci Lett. 1998;251:41–44. doi: 10.1016/S0304-3940(98)00476-5. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Morris BJ, O'Shaughnessy CT. Involvement of two isoforms of snap-25 in the expression of long-term potentiation in the rat hippocampus. Neuroreport. 1998;9:33–36. doi: 10.1097/00001756-199801050-00007. [DOI] [PubMed] [Google Scholar]
- Robillard JM, Gordon GR, Choi HB, Christie BR, Mac Vicar BA. Glutathione restores the mechanism of synaptic plasticity in aged mice to that of the adult. PLoS One. 2011;6:e20676. doi: 10.1371/journal.pone.0020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MM, Guevremont D, Luxmanan C, Abraham WC, Williams JM. Aging alters long-term potentiation--related gene networks and impairs synaptic protein synthesis in the rat hippocampus. Neurobiol Aging. 2015;36:1868–1880. doi: 10.1016/j.neurobiolaging.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Schluter OM, Basu J, Sudhof TC, Rosenmund C. Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J Neurosci. 2006;26:1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience. 2009;162:234–243. doi: 10.1016/j.neuroscience.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. Snare function analyzed in synaptobrevin/vamp knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Schubert V, Bouvier D, Volterra A. Snare protein expression in synaptic terminals and astrocytes in the adult hippocampus: a comparative analysis. Glia. 2011;59:1472–1488. doi: 10.1002/glia.21190. [DOI] [PubMed] [Google Scholar]
- Sevlever D, Zou F, Ma L, Carrasquillo S, Crump MG, Culley OJ, Hunter TA, Bisceglio GD, Younkin L, Allen M, Carrasquillo MM, Sando SB, Aasly JO, Dickson DW, Graff-Radford NR, Petersen RC, Deak F, Belbin O. Genetically-controlled vesicle-associated membrane. Protein 1 expression may contribute to alzheimer's pathophysiology and susceptibility. Mol Neurodegener. 2015;10:18. doi: 10.1186/s13024-015-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in ca1 of rat hippocampus. Cereb Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, Frost JL, Le KX, Li S, Dodart JC, Caldarone BJ, Stevens B, Lemere CA. Complement c3-deficient mice fail to display age-related hippocampal decline. J Neurosci. 2015;35:13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobin E, Bowley MP, Estrada LI, Heyworth NC, Orczykowski ME, Eldridge SA, Calderazzo SM, Mortazavi F, Moore TL, Rosene DL (2017) Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. Geroscience 39(2):199–220 [DOI] [PMC free article] [PubMed]
- Sierra F, Kohanski R. Geroscience and the trans-nih geroscience interest group, gsig. Geroscience. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in cns and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane DM, Rosahl TW, Sudhof TC, Malenka RC. Long-term potentiation in mice lacking synapsins. Neuropharmacology. 1995;34:1573–1579. doi: 10.1016/0028-3908(95)00107-H. [DOI] [PubMed] [Google Scholar]
- Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star EN, Newton AJ, Murthy VN. Real-time imaging of rab3a and rab5a reveals differential roles in presynaptic function. J Physiol. 2005;569:103–117. doi: 10.1113/jphysiol.2005.092528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. Igf-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering mirna-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 2016;38:239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tucsek Z, Valcarcel-Ares M, Toth P, Gautam T, Giles C, Ballabh P, Wei Y, Wren J, Ashpole N, Sonntag W, Ungvari Z, Csiszar A. Circulating igf-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenk J, Rostas I, Furedi N, Miko A, Solymar M, Soos S, Gaszner B, Feller D, Szekely M, Petervari E, Balasko M. Age-related changes in central effects of corticotropin-releasing factor (crf) suggest a role for this mediator in aging anorexia and cachexia. Geroscience. 2017;39:61–72. doi: 10.1007/s11357-017-9962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB. Rab3a is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci. 2001;21:6782–6790. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton PL, Ingram RL, Sonntag WE. Chronic [d-ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci. 2000;55:B106–B112. doi: 10.1093/gerona/55.2.B106. [DOI] [PubMed] [Google Scholar]
- Togashi H, Kimura S, Matsumoto M, Yoshioka M, Minami M, Saito H (1996) Cholinergic changes in the hippocampus of stroke-prone spontaneously hypertensive rats. Stroke 27:520–525 discussion 525–526 [DOI] [PubMed]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin ii-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z (2013b) Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33(11):1732–1742 [DOI] [PMC free article] [PubMed]
- Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-hete, trpc channels, and bkca in dysregulation of pressure-induced ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z (2014a) Igf-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34(12):1887–97 [DOI] [PMC free article] [PubMed]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Igf-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. Igf-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolos M, LeRoith D, Nunez A, Torres-Aleman I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007;12:1118–1128. doi: 10.1038/sj.mp.4002076. [DOI] [PubMed] [Google Scholar]
- Trenkwalder P. The study on cognition and prognosis in the elderly (scope)—recent analyses. J Hypertens Suppl. 2006;24:S107–S114. doi: 10.1097/01.hjh.0000220415.99610.22. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Younts TJ, Chiu CQ, Kaeser PS, Castillo PE, Sudhof TC. Rab3b protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc Natl Acad Sci U S A. 2011;108:14300–14305. doi: 10.1073/pnas.1112237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, Yan H, Mitschelen M, Farley J, Sonntag WE, Csiszar A. Vasoprotective effects of life span-extending peripubertal gh replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal gh treatment. J Gerontol A Biol Sci Med Sci. 2011;66:501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fülöp GÁ, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A (2017b) Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. GeroScience 39(1):33–42 [DOI] [PMC free article] [PubMed]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M (2017a) A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience 39(2):117–127 [DOI] [PMC free article] [PubMed]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience. 2017;39:43–50. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan K, Alix P, Marquet A, Doupagne M, Niespodziany I, Rogister B, Seutin V. Altered balance between excitatory and inhibitory inputs onto ca1 pyramidal neurons from sv2a-deficient but not sv2b-deficient mice. J Neurosci Res. 2012;90:2317–2327. doi: 10.1002/jnr.23111. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Villela D, Suemoto CK, Pasqualucci CA, Grinberg LT, Rosenberg C. Do copy number changes in cacna2d2, cacna2d3, and cacna1d constitute a predisposing risk factor for alzheimer's disease? Front Genet. 2016;7:107. doi: 10.3389/fgene.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O (2010) Involvement of bdnf in age-dependent alterations in the hippocampus. Front Aging Neurosci 2 [DOI] [PMC free article] [PubMed]
- Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30:56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ardiles AO, Yang S, Tran T, Posada-Duque R, Valdivia G, Baek M, Chuang YA, Palacios AG, Gallagher M, Worley P, Kirkwood A. Metabotropic glutamate receptors induce a form of ltp controlled by translation and arc signaling in the hippocampus. J Neurosci. 2016;36:1723–1729. doi: 10.1523/JNEUROSCI.0878-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Polan-Curtain J, Armstrong DL. Dose and time dependency of angiotensin II inhibition of hippocampal long-term potentiation. Peptides. 1995;16:1079–1082. doi: 10.1016/0196-9781(95)00089-3. [DOI] [PubMed] [Google Scholar]
- Wu D, Bacaj T, Morishita W, Goswami D, Arendt KL, Xu W, Chen L, Malenka RC, Sudhof TC (2017) Postsynaptic synaptotagmins mediate ampa receptor exocytosis during ltp. Nature 544(7650):316–321 [DOI] [PMC free article] [PubMed]
- Yang YJ, Wu PF, Long LH, Yu DF, Wu WN, Hu ZL, Fu H, Xie N, Jin Y, Ni L, Wang JZ, Wang F, Chen JG. Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and nmda receptor function. Aging Cell. 2010;9:709–721. doi: 10.1111/j.1474-9726.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- Zemmar A, Weinmann O, Kellner Y, Yu X, Vicente R, Gullo M, Kasper H, Lussi K, Ristic Z, Luft AR, Rioult-Pedotti M, Zuo Y, Zagrebelsky M, Schwab ME. Neutralization of nogo-a enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. J Neurosci. 2014;34:8685–8698. doi: 10.1523/JNEUROSCI.3817-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]