Abstract
As one of the important gas signal molecules, hydrogen sulfide (H2S) is associated with many important physiological processes in living organisms. Organelles, especially endoplasmic reticulum (ER), play a crucial role in the cell metabolism. Accordingly, the detection of H2S in the ER is of high interest. Toward this goal, we have described the development of the first ER-targeted fluorescent H2S probe (Na-H 2 S-ER). The new probe exhibited favorable features, such as a large turn-on fluorescence signal (45-fold fluorescence enhancement), high sensitivity and selectivity. The probe was successfully employed for imaging exogenous and endogenous H2S in the living HeLa cells. Significantly, the new probe Na-H 2 S-ER was employed to visualize H2S in the ER of living cells for the first time. In addition, the probe was also successfully used for imaging H2S in the living tissues up to a depth of 100 μm and in the living zebrafish.
Introduction
Although hydrogen sulfide (H2S) is an important industrial raw material in the sulfide industry, it is also a serious hazardous substance in the industrial process. For example, H2S could seriously corrode metallic equipment1. Early researches suggest that H2S has been regarded as a poisonous and harmful gas2. Prolonged exposure to H2S may lead to a series of malignant diseases, such as Down syndrome3, Alzheimer’s disease4, diabetes5, and liver cirrhosis6. However, recent studies show that H2S is also a gas signal molecule and plays the important physiological functions as the same as nitric oxide (NO) and carbon monoxide (CO) in organisms7,8. Endogenous H2S is involved in many physiological activities including regulating cardiovascular systems, influencing the proliferation and apoptosis of human cells, stabilizing the nervous and the immune system. Medical researches show that endogenous H2S is produced from L-cysteine by the enzyme-catalytic reaction of cystathionine-γ-lyase (CSE), cystathionine-β-synthetase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST)9–11. Although H2S is associated with different physiological processes, most of the physiological functions of H2S are still unknown. Therefore, the development of detection methods for H2S with high sensitivity and good selectivity is very valuable in complicated biological systems.
Nowadays, there are some analytical methods for H2S including colorimetry12, electrochemical assay13, gas chromatography14, and sulfide precipitation15. However, these techniques can’t realize the instantaneous monitoring of H2S. In addition, the tissues or cells samples need to be destroyed in the detection process. By comparison, the fluorescence imaging methods have many advantages, such as high selectivity and sensitivity, and undamaged samples in the detection process16–19. In fluorescence imaging, the two-photon fluorescence imaging has drawn much attention because of low photo-damage sample, deep penetration, and low background fluorescence20–22.
In the eukaryotic cells, the endoplasmic reticulum (ER) is the largest organelle with single-membrane structure23. The ER performs a variety of functions in living cells, including synthesizing, processing, and modifying protein and lipid, stabilizing intracellular Ca2+ concentrations24. Biologist studies show that the ER is closely associated with other organelles, including golgi apparatus, cell membrane, and mitochondria25–27. So far, many fluorescent probes have been reported for H2S detection in the past decade28–39. However, because of lack of the ER-specific fluorescent probes, the detection of physiological function of H2S in the ER is still very challenging.
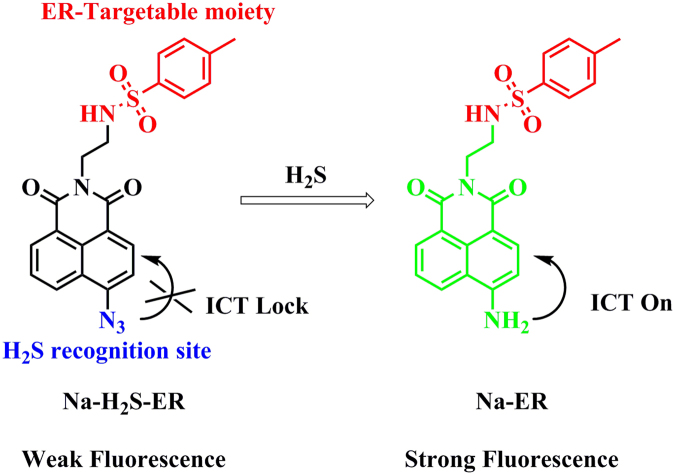
Herein, we report the first ER-targeted H2S fluorescent probe Na-H 2 S-ER. The probe contains the fluorescent platform 1,8-naphthalimide, and the H2S recognition site azide group. Combined with the methyl sulfonamide group, the fluorescent probe Na-H 2 S-ER preferentially accumulated in the ER40–43. Through the spectrum analysis, the probe Na-H 2 S-ER shows excellent selectivity and sensitivity to H2S relative to other analytes, and the probe can be used to detect H2S in cells. In addition, the probe Na-H 2 S-ER is suitable for fluorescence imaging of H2S in living tissues and zebrafish.
Results and Discussion
Design and synthesis
Naphthylamide dye is a classical fluorescence platform and has excellent two-photon properties. We used naphthylamide and introduced the H2S identification site azide group to construct a fluorescent H2S probe, named as Na-H 2 S-ER. Attach the strong electron-withdrawing azide group to the naphthalimide fluorescent platform directly, the electronic structure of the probe was shown as “A-π-A”. This electronic structure made the fluorescent probe have a weak fluorescent emission signal. When the probe was treated with H2S, the azide group became an amine moiety, and the electronic structure of the probe become “D-π-A” because of the amine moiety was the strong electron-donating group. Due to the intramolecular charge transfer (ICT) effect, the fluorescent probe had strong fluorescent emission (Fig. 1). Sulfonylurea compounds are inhibitors of ATP-sensitive K+ channels, and thus can selectively bind to these proteins that are prominent on ER. Therefore, fluorescent analogues of sulfonylurea are usually used to label ER. As shown in Fig. S1, glyburide was used as the targeting group in ER-tracker Green and ER-tracker Red. In contrast, in ER-tracker Blue, sulfanilamide, an analogue of sulfonylurea, was used as the recognition group. Therefore, in this work, we have also used sulfanilamide as the targeting group, and constructed the probe that can label ER due to selectively binding to ATP-sensitive K+ channels. The synthetic route of the fluorescent probe was shown in Fig. S2. The new compound Na-H 2 S-ER was fully characterized by 1H NMR, 13C NMR and HR-MS.
Figure 1.
The structure of the probe Na-H 2 S-ER and the proposed recognition mechanism for H2S.
Optical properties of probe Na-H2S-ER
After synthesizing the probe, we first tested its absorption spectra with different concentration of Na2S (acknowledged as a release reagent of H2S) in PBS buffer (pH 7.4, 5% DMSO). The maximum absorption wavelength of the probe Na-H 2 S-ER was at about 385 nm (ε = 9,200 M−1 cm−1) (Fig. S3). With the increase of the introduction of the concentration of Na2S (0–20 equiv.), the absorption value of the probe at 385 nm gradually reduced, and the absorption value at 440 nm gradually increased. Similar to the absorption spectrum, the fluorescence emission spectrum of the probe Na-H 2 S-ER also changed significantly. As designed, the free probe was almost non-fluorescent because of the specific electronic structure of the probe. However, when titrated with Na2S, a significant fluorescence turn-on response at 545 nm was observed (Fig. 2). The large fluorescence enhancement (up to 45-fold) was observed when the probe was treated with Na2S (20 equiv.) for 30 min at room temperature (Fig. 2). Based on the titration experiments, the detection limit of the probe for H2S was calculated to be 7.77 × 10−6 M (Fig. S4), indicating that the fluorescent probe has a potential application value for detecting H2S due to it could detect the dynamic changes of H2S concentrations in the living system.
Figure 2.
Fluorescence spectra of the probe Na-H2S-ER (5 μM) in 10 mM PBS buffer (pH 7.4, 5% DMSO) with various concentrations of Na2S.
Mechanism
The compound Na-ER (the reaction product of the probe with Na2S) was synthesized to further verify the design strategy of the probe Na-H 2 S-ER. Na-ER was purified by the column chromatography, and the structure was determined by NMR (Figs S5 and S6) analysis and HR-MS spectrometry (Fig. S7). These results confirmed that the recognition mechanism of the probe with H2S as proposed we expected (Fig. 1).
Dynamic research
The rate of response is a basic parameter of the probe recognition capability, we then carried out the dynamic test of the probe Na-H 2 S-ER (5 μM) reacted with various concentrations of Na2S (Fig. 3). Untreated with Na2S, the fluorescence intensity of the probe had almost no change within 60 min. By contrast, the fluorescence intensity changed significantly when the addition of Na2S. The intensity essentially reached a maximum in 30 min at room temperature when the probe Na-H 2 S-ER (5 μM) reacted with Na2S (100 μM). Under the pseudo-first-order conditions, the rate constant for the probe was determined to be k = 0.0702 min−1 (Fig. S8), suggesting that the probe may be used to detect H2S for real-time imaging applications in living systems.
Figure 3.

Reaction-time profiles of the probe Na-H
2
S-ER (5 μM) in the absence [■] or presence of Na2S (10 μM  , 25 μM
, 25 μM  , 100 μM
, 100 μM  , and 150 μM
, and 150 μM  ). The fluorescence intensities at 545 nm were continuously monitored at time intervals in PBS buffer.
). The fluorescence intensities at 545 nm were continuously monitored at time intervals in PBS buffer.
Selectivity study
The high selectivity of probe makes it potentially useful for biomedical application. To investigate the selectivity, Na-H 2 S-ER (5 μM) was treated with various relevant analytes, including various anions/cations, representative amino acid, reactive oxygen/nitrogen species (ROS/RNS) in 10 mM PBS buffer (pH 7.4, 5% DMSO) (Fig. 4). The addition of the anions (Br−, NO2 −, Cl−, NO3 −, AcO−, F−, HSO3 −, SCN−, I−, S2O3 2−, SO4 2−) and cations (Na+, K+, Mg2+, Zn2+, Fe2+), ROS/RNS (H2O2, NaClO, tert-butylhydroperoxide (TBHP), di-tert-butylperoxide (DTBP), NO), representative amino acid (glycine (Gly), alanine (Ala), cysteine (Cys), homocysteine (Hcy), glutathione (GSH)), and other analytes (Vitamin C (VC), sodium citrate) at 100 μM caused almost no change of the fluorescence intensity of the probe Na-H 2 S-ER at room temperature for 30 min. By contrast, upon treatment of Na2S (100 μM) with the probe, the significant change occurred in the fluorescence intensity of the probe (about 45-fold fluorescence enhancement). These results showed that the probe has a high selectivity for H2S over the other tested analytes, suggesting that probe had the potential to detect hydrogen sulfide in living biological systems.
Figure 4.
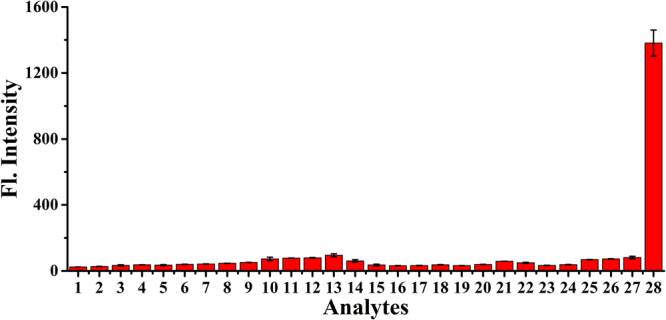
Fluorescence responses of Na-H 2 S-ER (5 μM) in the presence of various relevant analytes in 10 mM PBS buffer (pH 7.4, 5% DMSO). The concentrations of the representative analytes are: 100 μM. Legend: (1) probe; (2) NaBr; (3) NaNO2; (4) MgCl2; (5) NaNO3; (6) Zn(OAc)2; (7) ZnCl2; (8) KF; (9) NaHSO3; (10) KSCN; (11) KI; (12) Na2S2O3; (13) FeSO4; (14) N2H4; (15) TBHP; (16) DTBP; (17) H2O2; (18) NaClO; (19) NO; (20) sodium citrate; (21) glucose; (22) VC; (23) Gly; (24) Ala; (25) GSH; (26) Hcy; (27) Cys; (28) Na2S. λex/em = 440/545 nm.
The pH and photostability studies
The pH and photostability are all related to the application of the probe. We then examined the possible effects of different pH on the fluorescence changes of the probe Na-H 2 S-ER in the absence or presence of Na2S (Fig. S9). The fluorescence intensity of the probe was almost unchanged over a wide pH range of 4–10 range in the absence of Na2S, indicating that the probe was almost impervious to the effect of pH. However, when Na2S was introduced, a remarkable fluorescent signal enhancement was observed at about 545 nm, especially under the physiological conditions (pH 7.4). These results suggested that the probe can detect H2S in different pH environments, indicating that the probe has the potential value for biological applications. Furthermore, the photostability experiment was also carried out. After excited by the short wavelength UV light (365 nm), the fluorescent intensity of the probe displayed slight variation (Fig. S10), suggesting that the probe has good photostability and may have potential usefulness in living system.
Cytotoxicity and bio-imaging H2S in living HeLa cells
As a potential imaging agent, the probe Na-H 2 S-ER should have low toxicity. We carried out a cytotoxicity test for the fluorescence probe using the standard MTT assay (Fig. S11). The results showed that the probe has no remarkable cytotoxicity to the cells in low doses (0–20 μM) after 24 h of incubation, meaning that the probe can be used for imaging experiments.
Because the probe had many excellent properties, including high sensitivity and selectivity, good photostability, low cytotoxicity, and working appropriately at the physiological pH, we would carry out the fluorescence imaging experiments in living cells. Because of the 1,8-naphthalimide is the classic two-photon dye, the fluorescence imaging experiments were carried out by the one-photon (OP) mode and two-photon (TP) mode. There was no fluorescent signal was detected when the HeLa cells were only incubated with probe (5 μM) whether by OP or TP mode (Fig. S12b,d). By contrast, when the HeLa cells were pre-incubated with probe (5 μM), and then treated with Na2S (50 μM), a very strong fluorescent signals could be detected by OP and TP excitation (Fig. S12f,h), confirming that the fluorescence probe has good biocompatibility and it is able to recognize the exogenous H2S in the living cells.
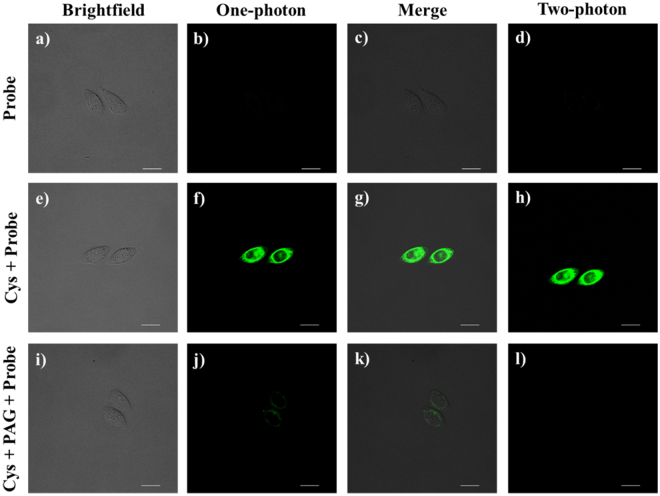
Encouraged by the results of the exogenous H2S imaging experiment, we decided to investigate the ability of the fluorescent probe Na-H 2 S-ER to identify the endogenous H2S in the living cells. Researches show that endogenous H2S can be generated by the enzymatic reaction of CSE with Cysteine (Cys) in living cells10. The cells treated with probe (5 µM) showed non-fluorescence signal (Fig. 5b,d). Under the same imaging parameters, we pre-incubated HeLa cells with Cys (100 µM) and then treated the cells with probe (5 µM), a dramatic fluorescent enhancement was observed by OP and TP modes (Fig. 5f,h). In order to further verify the increased fluorescent signals were mainly generated by the reaction between the probe and the endogenous H2S, we carried out a negative control imaging experiment. We conducted the negative control imaging experiment with propargylglycine (PAG) because it decreased the intracellular H2S concentration by inhibiting the activity of CSE44. As shown in the Fig. 5j,l, with the addition of PAG (200 µM), the fluorescence intensity of the probe decreased significantly whether by OP or TP mode. These data indicated that the probe Na-H 2 S-ER can monitor endogenous H2S in living cells.
Figure 5.
Fluorescence imaging of the endogenous H2S in the HeLa cells. (a) The brighfield image of the HeLa cells treated with Na-H 2 S-ER (5 μM); (b) The OP Fluorescence image of a; (c) The merge image of (a and b–d) The TP Fluorescence image of a; (e) The brighfield image of the HeLa cells treated with Cys (100 μM) and Na-H 2 S-ER (5 μM); (f) The OP Fluorescence image of (e,g) The merge image of (e and f,h) The TP Fluorescence image of (e,i) The brighfield image of the HeLa cells treated with Cys (100 μM), PAG (200 μM) and Na-H 2 S-ER (5 μM); (j) The OP Fluorescence image of (i,k) The merge image of (i and j,l) The TP Fluorescence image of (i). The OP mode was excitated at 488 nm and emission collection was from 500–550 nm. The TP mode was excitated at 760 nm and emission collection was from 500–550 nm. Scale bar: 500 μm. Scale bar: 20 μm.
Colocalization imaging experiments
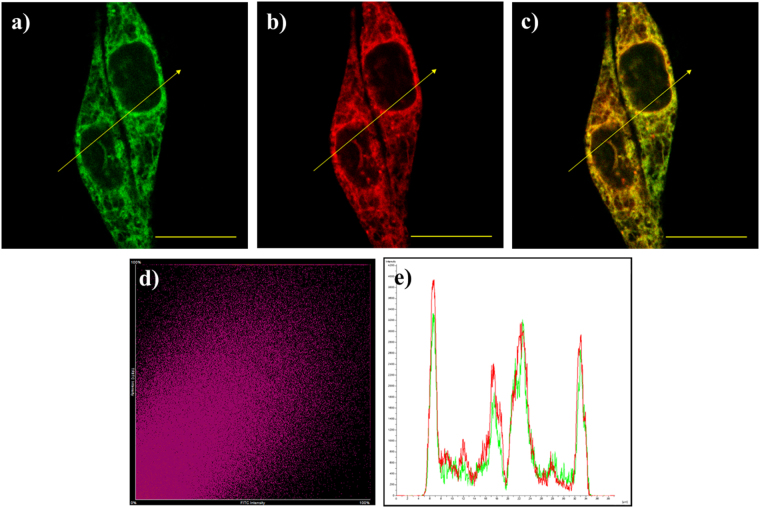
Encouraged by the above promising results of above imaging experiments, we decided to further verify the sub-cellular distribution of Na-H 2 S-ER with exogenous H2S. Because the methyl sulfonamide group is an effective targeting group for ER, the Hela cells were co-incubated with Na-H 2 S-ER, Na2S and the ER-Tracker TM Red (a commercial available ER Tracker) for the colocalization study (Fig. 6). The HeLa cells showed strong fluorescent signals in the green channel (λex = 488 nm; λem = 500–550 nm) in the presence of Na2S. Meanwhile, a significant fluorescence was detected in the red channel (λex = 561 nm; λem = 570–620 nm) because of the gathering of ER Tracker. The merged image showed that the well overlap between the green fluorescence and the red fluorescence (Fig. 6c), and the Pearson’s colocalization coefficient was 0.91 and the Mander’s overlap coefficient was 0.92 (Fig. 6d). The intensity profile of linear regions of interest across HeLa cells in the two channels also varies in close synchrony (Fig. 6e). These data showed that the probe has the prominent property of ER-targeted in the living cells.
Figure 6.
The images of the living HeLa cells co-incubated with the probe Na-H 2 S-ER (5 μM), Na2S (50 μM), and ER-Tracker TM Red. (a) The fluorescence image of the green channel; (b) The fluorescence image of the red channel; (c) The merged image of (a and b,d) Intensity scatter plot of the green and red channels. (e) Intensity profile of linear region of interest across in the HeLa cells co-stained with ER-Tracker Red and green channel of Na-H 2 S-ER. Scale bar: 20 μm.
Two-photon fluorescence imaging in living tissues
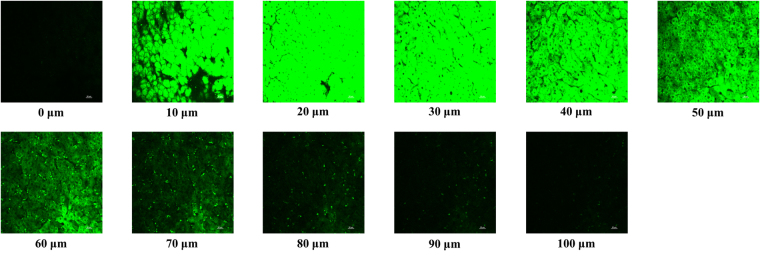
The beneficial results of the living cell researches led us to further apply Na-H 2 S-ER to trace the added H2S in the living liver tissue. Similar to the cell imaging experiments, the liver tissue slices only incubated with Na-H 2 S-ER exhibited non-fluorescence (Fig. S13). By contrast, the liver tissue slices treated with the probe and then incubated with added Na2S exhibits strong fluorescent signals was observed up to a depth of 100 µm (Fig. 7). Thus, the data showed that the probe is able to detect the addition of H2S in liver tissue slides.
Figure 7.
Fluorescence imaging of added H2S in living liver tissues. The liver tissue slices pretreated with Na-H 2 S-ER (30 μM) for 30 min, and then treated with Na2S (150 μM) for another 30 min. Excitation was at 760 nm and emission collection was from 500–550 nm. Scale bar: 20 μm.
Bio-imaging H2S in living zebrafish
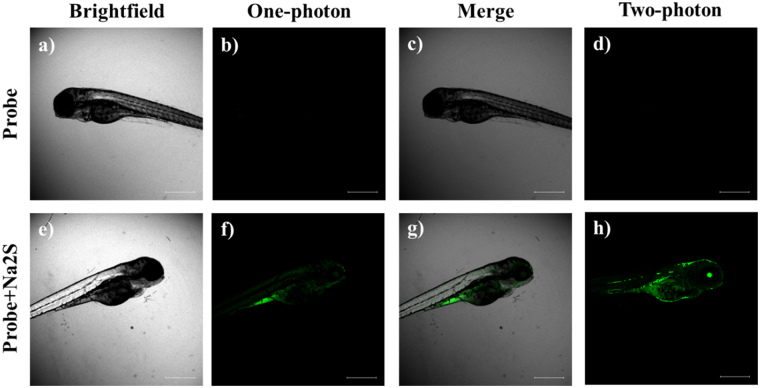
As a model of good vertebrate animals, zebrafish have been widely used for the study of human genetic diseases and developmental biology45,46. However, the study of H2S fluorescence imaging using zebrafish as the biological sample is still scarce. Based on the excellent features of Na-H 2 S-ER, we carried out the fluorescence imaging experiment in the living zebrafish. The zebrafish with feeding Na-H 2 S-ER were still alive, suggesting that Na-H 2 S-ER has the potential for biological imaging (Fig. 8a,e). The zebrafish treated only with the probe Na-H 2 S-ER displayed almost no fluorescence (Fig. 8b,d). However, the zebrafish was fed with both the probe and Na2S showed bright fluorescence (Fig. 8f,h), suggesting that Na-H 2 S-ER is capable of imaging added H2S in living zebrafish.
Figure 8.
Fluorescence imaging of H2S in living zebrafish. (a) The brighfield image of the zebrafish treated with Na-H 2 S-ER (10 μM); (b) The OP Fluorescence image of (a,c) The merge image of (a and b,d) The TP Fluorescence image of (a,e) The brighfield image of the zebrafish treated with Na-H 2 S-ER (10 μM) and Na2S (50 μM); (f) The OP Fluorescence image of (e,g) The merge image of (e and f,h) The TP Fluorescence image of e. The OP mode was excitated at 488 nm and emission collection was from 500–550 nm. The TP mode was excitated at 760 nm and emission collection was from 500–550 nm. Scale bar: 500 μm.
Conclusions
We had engineered the first ER-targeted two-photon H2S fluorescent probe Na-H 2 S-ER. The fluorescence probe contains the fluorescent platform 1,8-naphthalimide and the responding site azide group. When introduced of the H2S to Na-H 2 S-ER, a large fluorescent emission signal at 545 nm was observed because of the azide group reduced by H2S to the amine moieties. The probe Na-H 2 S-ER possessed potential biological imaging value because it has many excellent characteristics, including large fluorescent enhancement signals, high sensitivity and selectivity, low cytotoxicity, and working appropriately at the physiological pH. Remarkably, this probe could be applied to monitor the dynamic changes of H2S in ER of the living cells because it possesses the excellent ER-targeted feature. Importantly, the probe has successfully carried out the tissue and zebrafish imaging experiments. We believe that Na-H 2 S-ER as a powerful molecular tool for monitoring H2S in biological samples.
Methods
Synthesis of the probe Na-H2S-ER
The synthesis of compound 2, 4 was referred to the previous literature41. 472 mg compound 4 (1 mmol) and 325 mg sodium azide (5 mmol) were added to 5 mL of anhydrous DMF. The reaction mixture was heated at 50 °C overnight under the nitrogen atmosphere. Then 50 mL H2O was added to the reaction system, then extracted with dichloromethane (20 mL × 3). The organic phase was washed with H2O, dried with MgSO4. The crude product was purified by flash chromatography to afford a yellow solid Na-H 2 S-ER 249 mg with a yield of 57%. 1H NMR (400 MHz, DMSO-d 6) δ 8.49 (d, J = 7.2 Hz, 1 H), 8.42 (m, 2 H), 7.86 (t, J = 8.0 Hz, 1 H), 7.76 (m, 2 H), 7.58 (d, J = 8.0 Hz, 2 H), 7.23d, J = 8.0 Hz, 2 H), 4.09 (t, J = 8.0 Hz, 2 H), 3.08 (q, J = 5.6 Hz, 2 H), 2.26 (s, 3 H)13; C NMR (100 MHz, DMSO-d 6) δ 163.75, 163.30, 143.23, 142.89, 138.14, 131.96, 131.88, 129.93, 128.68, 128.75, 127.71, 126.80, 123.96, 122.70, 118.72, 116.36, 21.32; HR-MS calculated for C21H17N5O4S [M + H]+ m/z 436.1074, found 436.1070.
Synthesis of the compound Na-ER
A mixture of compound Na-H 2 S-ER 87 mg (0.2 mmol) was dissolved in 95% EtOH (1 mL), and then 80 mg Na2S·9H2O (1 mmol) was added. The suspension was stirred at room temperature for 1 h. Subsequently, the mixture was concentrated under vacuum, and the resulting residue was purified by silica gel column chromatography (CH2Cl2/MeOH as the eluent) to afford 67 mg orange solid of the compound Na-ER with a yield of 83%. 1H NMR (400 MHz, DMSO-d 6) δ 8.61 (d, J = 8.0 Hz, 1 H), 8.38 (d, J = 6.8 Hz, 1 H), 8.15 (d, J = 8.0 Hz, 1 H), 7.71 (t, J = 6.0 Hz, 1 H), 7.64 (t, J = 8.0 Hz, 1 H), 7.59 (d, J = 8.0 Hz, 2 H), 7.46 (s, 2 H), 7.23 (d, J = 8.0 Hz, 2 H), 6.83 (d, J = 8.0 Hz, 1 H), 4.07 (t, J = 6.4 Hz, 2 H), 3.02 (q, J = 6.4 Hz, 2 H), 2.27 (s, 3 H); 13C NMR (100 MHz, DMSO-d 6) δ 164.32, 163.38, 153.17, 142.86, 138.06, 134.36, 131.38, 130.26, 129.89, 129.72, 126.84, 124.35, 122.25, 119.81, 108.57, 108.04, 40.79, 39.20, 21.33; HR-MS calculated for C21H19N3O4S [M + H]+ m/z 410.1169, found 410.1162.
Spectral analysis
Unless otherwise noted, all the measurements were made according to the following procedure. The concentration of the probe stock solution was 1.0 mM in DMSO, and the analytes stock solutions were prepared in the ultrapure water at the appropriate concentration. In 5 mL volumetric flask, the test solution was prepared by placing 25 μL probe stock solution and 225 μL DMSO, requisite amount of analyte stock solution, then adjusted the final volume to 5 mL with PBS buffer. The spectrum tests were recorded with a 1 cm standard quartz cell at room temperature. The absorption spectra were obtained on a Shimadzu UV-2700 Power spectrometer. The photoluminescent spectra were recorded with a HITACHI F4600 fluorescence spectrophotometer. The excitation wavelength was 440 nm, the excitation slit widths were 5 nm, and the emission slit widths were 5 nm.
Cytotoxicity assay
The HeLa cells were seeded up to appropriate density in a 96-well plate. Then the cells were incubated with a series of concentrations of Na-H 2 S-ER (0–20 μM) at 37 °C. After 24 h, the cells were with PBS buffer, then added MTT (5 mg/mL, 10 μL) and further incubated for 4 h. After removed the culture medium, DMSO (100 μL) was added into the dishes to dissolve the formazan crystal product. Then the absorbance of the solution was measured at 570 nm by the microplate reader.
ODsample denotes the cells incubated with various concentrations of the probe, ODcontrol denotes the cells without the probe, ODblank denotes the wells containing only the culture medium.
Bio-imaging of exogenous H2S in living HeLa cells
The HeLa cells were seeded up to appropriate density into a 35 mm glass-bottom culture dishes (Nest). Then the cells further incubated with Na-H 2 S-ER (5 μM) for another 20 min at 37 °C. Then the cells were washed with PBS buffer (pH = 7.4) three times, and the cells incubated with Na2S (50 μM) for more 30 min. Finally, the cells were washed three times with PBS buffer. The imaging experiments were recorded through a Nikon A1MP confocal microscopy inverted fluorescence microscopy equipped with a cooled CCD camera. The OP fluorescence emission was collected at 500–550 nm upon excitation at 488 nm with a femtosecond pulse, and the TP fluorescence emission was collected at 500–550 nm upon excitation at 760 nm with a femtosecond pulse.
Bio-imaging of endogenous H2S in living HeLa cells
For the endogenous H2S group, the cells further incubated with Cys (100 μM) for another 30 min at 37 °C, then washed with PBS three times and further incubated with Na-H 2 S-ER (5 μM) for another 20 min. For negative control group, the cells incubated with Cys (100 μM) and PAG (200 μM) for 30 min, then further incubated with Na-H 2 S-ER (5 μM) for another 20 min. Finally, the cells were washed three times with PBS buffer. Then the imaging experiments were carried out.
Bio-imaging of added H2S in living tissue
4 weeks old Kunming mice were purchased from Shandong University Laboratory Animal Centre (Shandong, China). Unless otherwise noted, all procedures for this study were approved by the Animal Ethical Experimentation Committee of Shandong University according to the requirements of the National Act on the use of experimental animals (China). The mice were killed by cervical vertebra dislocation, the liver tissues were cut into about 500 μm in size. For the control group, the liver tissue slices treated with Na-H 2 S-ER (30 μM) for 30 min. For the experimental group, the liver tissue slices pretreated with Na-H 2 S-ER (30 μM) for 30 min, and then treated with Na2S (150 μM) for another 30 min. Then the imaging experiments were carried out.
Bio-imaging of exogenous H2S in living zebrafish
Wild type zebrafish were purchased from the Nanjing EzeRinka Biotechnology Co., Ltd. All procedures for this study were approved by the Animal Ethical Experimentation Committee of Shandong University according to the requirements of the National Act on the use of experimental animals (China). For the control group, the zebrafish were fed with Na-H 2 S-ER (10 μM) for 30 min. For the experimental group, the zebrafish were fed with Na-H 2 S-ER (10 μM) for 30 min, and then treated with Na2S (50 μM) for another 30 min. Then the imaging experiments were carried out.
Electronic supplementary material
Acknowledgements
This work was financially supported by NSFC (21472067, 21672083), Taishan Scholar Foundation (TS201511041), and the startup fund of University of Jinan (309–10004).
Author Contributions
W. Lin and Y. Tang conceived the idea and directed the work. Y. Tang and A. Xu designed the experiments and performed the organic synthesis. Y. Tang, G. Xu and S. Gao performed the spectral measurements. Y. Tang and Y. Ma performed the bioimaging experiments. All authors contributed to data analysis, manuscript writing and participated in research discussions.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13325-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ma H, et al. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000;42:1669–1683. doi: 10.1016/S0010-938X(00)00003-2. [DOI] [Google Scholar]
- 2.Olas B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta. 2015;439:212–218. doi: 10.1016/j.cca.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Kamoun P, Belardinelli M-C, Chabli A, Lallouchi K, Chadefaux-Vekemans B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet., Part A. 2003;116A:310–311. doi: 10.1002/ajmg.a.10847. [DOI] [PubMed] [Google Scholar]
- 4.Eto K, Asada T, Arima K, Makifuchi T, Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 5.Jain SK, et al. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid. Redox Signaling. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang W, Yang G, Jia X, Wu L, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005;569:519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorucci S, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 8.Fukuto JM, et al. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pietri R, Román-Morales E, López-Garriga J. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid. Redox Signaling. 2011;15:393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez D, et al. A new chromo-chemodosimeter selective for sulfide anion. J. Am. Chem. Soc. 2003;125:9000–9001. doi: 10.1021/ja0347336. [DOI] [PubMed] [Google Scholar]
- 13.Searcy DG, Peterson MA. Hydrogen sulfide consumption measured at low steady state concentrations using a sulfidostat. Anal. Biochem. 2004;324:269–275. doi: 10.1016/j.ab.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell TW, Savage JC, Gould DH. High-performance liquid chromatography detection of sulfide in tissues from sulfide-treated mice. J. Appl. Toxicol. 1993;13:389–394. doi: 10.1002/jat.2550130605. [DOI] [PubMed] [Google Scholar]
- 15.Ishigami M, et al. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signaling. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 16.Lackowicz, J. R. Principles of fluorescence spectroscopy (ed. Lackowicz, J. R.) (Springer US, 2006).
- 17.Tang Y, et al. Development of fluorescent probes based on protection-deprotection of the key functional groups for biological imaging. Chem. Soc. Rev. 2015;44:5003–5015. doi: 10.1039/C5CS00103J. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Dong B, Tang Y, Lin W. A Unique “Integration” Strategy for the Rational Design of Optically Tunable Near-Infrared Fluorophores. Acc. Chem. Res. 2017;50:1410–1422. doi: 10.1021/acs.accounts.7b00087. [DOI] [PubMed] [Google Scholar]
- 19.He L, Dong B, Liu Y, Lin W. Fluorescent chemosensors manipulated by dual/triple interplaying sensing mechanisms. Chem. Soc. Rev. 2016;45:6449–6461. doi: 10.1039/C6CS00413J. [DOI] [PubMed] [Google Scholar]
- 20.Göppert-Mayer M. Über Elementarakte mit zwei Quantensprüngen. Ann. Phys. 1931;401:273–294. doi: 10.1002/andp.19314010303. [DOI] [Google Scholar]
- 21.Kaiser W, Garrett CGB. Two-photon excitation in CaF2: Eu2+ Phys. Rev. Lett. 1961;7:229–231. doi: 10.1103/PhysRevLett.7.229. [DOI] [Google Scholar]
- 22.Tang Y, Kong X, Xu A, Dong B, Lin W. Development of a two-photon fluorescent probe for imaging of endogenous formaldehyde in living tissues. Angew. Chem., Int. Ed. 2016;55:3356–3359. doi: 10.1002/anie.201510373. [DOI] [PubMed] [Google Scholar]
- 23.Quinn P, Griffiths G, Warren G. Density of newly synthesized plasma membrane proteins in intracellular membranes II. Biochemical studies. J. Cell Biol. 1984;98:2142–2147. doi: 10.1083/jcb.98.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 2001;205:149–214. doi: 10.1016/S0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- 25.Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated Lipid Transfer between the Endoplasmic Reticulum and the Golgi Complex Requires the VAP Proteins and Is Essential for Golgi-mediated Transport. Mol. Biol. Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichler H, et al. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur. J. Biochem. 2001;268:2351–2361. doi: 10.1046/j.1432-1327.2001.02116.x. [DOI] [PubMed] [Google Scholar]
- 27.Friedman JR, Voeltz GK. The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol. 2011;21:709–717. doi: 10.1016/j.tcb.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin VS, Chen W, Xian M, Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015;44:4596–4618. doi: 10.1039/C4CS00298A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hai Z, Bao Y, Miao Q, Yi X, Liang G. Pyridine–biquinoline–metal complexes for sensing pyrophosphate and hydrogen sulfide in aqueous buffer and in cells. Anal. Chem. 2015;87:2678–2684. doi: 10.1021/ac504536q. [DOI] [PubMed] [Google Scholar]
- 30.Singha S, et al. Toward a selective, sensitive, fast-responsive, and biocompatible two-Photon probe for hydrogen sulfide in live cells. Anal. Chem. 2015;87:1188–1195. doi: 10.1021/ac503806w. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, et al. A fluorescent chemodosimeter for live-cell monitoring of aqueous sulfides. Anal. Chem. 2016;88:1434–1439. doi: 10.1021/acs.analchem.5b04194. [DOI] [PubMed] [Google Scholar]
- 32.Li HD, et al. A fluorescent probe for H2S in vivo with fast response and high sensitivity. Chem. Commun. 2015;51:16225–16228. doi: 10.1039/C5CC06612C. [DOI] [PubMed] [Google Scholar]
- 33.Cao J, et al. Chemiluminescent probes for imaging H2S in living animals. Chem. Sci. 2015;6:1979–1985. doi: 10.1039/C4SC03516J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, et al. Exceptional time response, stability and selectivity in doubly-activated phenyl selenium-based glutathione-selective platform. Chem. Sci. 2015;6:5435–5439. doi: 10.1039/C5SC02090E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammers MD, et al. A bright fluorescent probe for H2S enables analyte-responsive, 3D imaging in live zebrafish using light sheet fluorescence microscopy. J. Am. Chem. Soc. 2015;137:10216–10223. doi: 10.1021/jacs.5b04196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pak YL, et al. Mitochondria-targeted reaction-based fluorescent probe for hydrogen sulfide. Anal. Chem. 2016;88:5476–5481. doi: 10.1021/acs.analchem.6b00956. [DOI] [PubMed] [Google Scholar]
- 37.Karakuş E, Üçüncü M, Emrullahoğlu M. Electrophilic cyanate as a recognition motif for reactive sulfur species: selective fluorescence detection of H2S. Anal. Chem. 2016;88:1039–1043. doi: 10.1021/acs.analchem.5b04163. [DOI] [PubMed] [Google Scholar]
- 38.Gong D, et al. A phenylselenium-substituted BODIPY fluorescent turn-off probe for fluorescence imaging of hydrogen sulfide in living cells. Anal. Chem. 2017;89:1801–1807. doi: 10.1021/acs.analchem.6b04114. [DOI] [PubMed] [Google Scholar]
- 39.Lv J, et al. Enhanced response speed and selectivity of fluorescein-based H2S probe via the cleavage of nitrobenzene sulfonyl ester assisted by ortho aldehyde groups. Biosens. Bioelectron. 2017;87:96–100. doi: 10.1016/j.bios.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Xiao H, et al. Simultaneous fluorescence imaging of hydrogen peroxide in mitochondria and endoplasmic reticulum during apoptosis. Chem. Sci. 2016;7:6153–6159. doi: 10.1039/C6SC01793B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y, et al. A turn-on fluorescent probe for endogenous formaldehyde in the endoplasmic reticulum of living cells. Methods Appl. Fluoresc. 2017;5:024005. doi: 10.1088/2050-6120/aa6773. [DOI] [PubMed] [Google Scholar]
- 42.Xiao, H. et al. Ratiometric photoacoustic imaging of endoplasmic reticulum polarity in injured liver tissues of diabetic mice. Chem. Sci., 10.1039/C7SC02330H (2017). [DOI] [PMC free article] [PubMed]
- 43.Xu S, et al. Visualization of endoplasmic reticulum aminopeptidase 1 under different redox conditions with a two-photon fluorescent probe. Anal. Chem. 2017;89:7641–7648. doi: 10.1021/acs.analchem.7b01561. [DOI] [PubMed] [Google Scholar]
- 44.Sun Q, et al. Structural basis for the inhibition mechanism of human cystathionine γ-Lyase, an enzyme responsible for the production of H2S. J. Biol. Chem. 2009;284:3076–3085. doi: 10.1074/jbc.M805459200. [DOI] [PubMed] [Google Scholar]
- 45.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 46.Curado S, Stainier DYR, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.