Abstract
Our goal was to evaluate the pain response in an LL-37 induced murine model for interstitial cystitis/painful bladder syndrome (IC/PBS). In particular, we sought to characterize the dose dependence, time-course, and relationship of LL-37 induced bladder inflammation and pain. The IC/PBS model was induced in C57Bl/6 mice by instilling 50 μL of LL-37, an immunomodulatory human cathelicidin (anti-microbial peptide), in the bladder for 1 hr. Pain responses were measured using von Frey filaments (0.04 gm to 4.0 gm) before and after LL-37 instillation. Inflammation was evaluated using tissue myeloperoxidase (MPO) assay, gross inspection, and microscopic histologic examination. The dose response experiment demonstrated a graded pain response, with higher concentrations of LL-37 challenge yielding higher pain responses across all stimuli tested. Statistical significance was seen when comparing 1.0 gm von Frey filament results at 320 μM (68 ± 8% response) vs. 0 μM (38 ± 6% response). Interestingly, pain responses did not attenuate across time but increased significantly after 5 (p=0.0012) and 7 days (p=0.0096). Comparison with MPO data suggested that pain responses could be independent of inflammation. We demonstrated within our LL-37 induced IC/PBS model pain occurs in a dose-dependent fashion, pain responses persist beyond the initial point of insult, and our dose response and time course experiments demonstrated that pain was independent of inflammation.
Keywords: LL-37, cathelicidin, bladder inflammation, bladder pain, interstitial cystitis, painful bladder syndrome
Introduction
Chronic inflammatory bladder diseases such as interstitial cystitis (IC) remain a major problem clinically. IC, or painful bladder syndrome (IC/PBS), is still an “enigma” in urology due to its elusive etiology and lack of curative therapy [1,2]. IC/PBS can cause significant pain-related discomfort at the pelvic and the supra-pubic bladder region independent of any infectious etiology. IC/PBS severely impacts patients quality of life and can be a chronic and debilitating condition [1,3-5]. Alterations of the pain pathway can lead to hypersensitivity which may never resolve, resulting in a chronic and debilitating pain disorder [5,6]. Under a chronic inflammatory setting like IC/PBS, the primary goal of treatment is pain management which improves overall patient quality of life [5,7].
There are several theories proposed for inducing IC/PBS based on clinical and experimental findings, including, but not limited to: a defective glycosaminoglycan (GAG) layer, leaky urothelium, autoimmunity, mast cell activation, and neurogenic chronic inflammation [8,9]. Unfortunately, no ideal animal model exists that can mimic all the features associated with clinical IC/PBS, largely owing to the complexity of the disease and unknown etiology. An accepted pre-clinical animal model would tremendously assist with both understanding mechanistic pain pathways associated with IC/PBS, and with the identification of therapeutic interventions that would assist physicians and scientists in developing and evaluating new potential courses of treatment.
LL-37 is the only known human cathelicidin (anti-microbial peptide) produced by the human body and acts as a natural antibiotic and immunomodulator during urinary tract infections [10,11]. Previously, we reported a profound bladder inflammation mouse model induced with LL-37 intravesical instillation [11-14]. In the present study, we quantitatively evaluated the LL-37 IC/PBS model for bladder specific pain. We hypothesized that: (i) pain responses could escalate with exogenously presented LL-37 in a dose-dependent fashion, (ii) a single exposure to LL-37 could yield prolonged pain responses, and (iii) LL-37 induced bladder injury could exacerbate pain responses independent of inflammation. Testing these hypotheses would enhance the translational potential of our model by demonstrating a more biologically based approach that yields findings which more closely mimics the human phenotype seen in IC/PBS. In addition, demonstrating that pain responses could be independent of inflammation is highly innovative and has not been demonstrated in other animal models of IC/PBS.
Materials and methods
LL-37 bladder instillation
Experiments were performed with approval and in accordance with the University of Utah Institutional Animal Care and Use Committee. Eight to 10-week-old female C57 BL/6 mice were used for both dose-dependent and time-dependent approaches. LL-37 was obtained in high-performance liquid chromatography homogenous form (peptide sequence: LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) and dissolved in nano-pure water. After establishing isoflurane anesthesia, saline wash followed by LL-37 was instilled in the mice via transurethral catheterization at a 50 µl volume for an intravesical dwell time of 1 hr [11-14]. Both saline wash and LL-37 were infused slowly to avoid distension and vesicoureteral reflux, with the syringes kept in place to ensure no leakage of fluid occurred during instillation.
Bladder pain assays
To quantify and analyze pain levels, each mouse was placed in a clear plexiglass chamber measuring 6 × 6 × 12 cm3 with the top covered with a clear lid and the floor consisting of a perforated metal grid (1 cm × 1 cm) to allow for the pain test to be administered. Each mouse was placed in their individual chamber and allowed to acclimate to their surroundings for at least 15 minutes before stimulation. Von Frey filaments (Touch-Test, Stoelting Co, IL, USA) at five different forces (0.04 g, 0.16 g, 0.4 g, 1.0 g, and 4.0 g) were used to stimulate within the pelvic region observing for bladder pain [15]. One-second application to the supra-pubic region for a total of 10 applications were applied for each filament force with at least 3-second intervals between stimuli. Positive responses included: sharp retraction of the abdomen, immediate licking in the region of filament stimulation, and jumping. Both positive and negative responses were recorded as data points. The pain response for each animal for each stimuli level was reported as the percentage of 10 applications with a positive result. Tester was blinded to the treatment each group received. Pain assays were performed prior to instillation (baseline) and prior to animal sacrifice/tissue harvesting.
Dose-dependent bladder pain model
To test the correlation between levels of pain and LL-37 concentration, adult female C57BL/6 mice were placed under anesthesia, catheterized, then challenged with 50 μL of LL-37 for 1 hr. Instillations were performed at six different logarithmic concentrations of LL-37, 10, 20, 40, 80, 160, 320 μM, with controls consisting of sterile saline (n=5 for each concentration). Mice were sacrificed after 5 days. Baseline pain assessments were performed with the von Frey filaments on all mice prior to LL-37 instillation (baseline), and post-installation pain assessments were performed prior to sacrifice 5 days post-instillation. After sacrifice, the bladders were harvested for further analysis.
Time-dependent bladder pain model
To test if LL-37 induced injury yields prolonged pain, mice were challenged with 50 μL of 320 μM LL-37 for 1 hr. Baseline pain response was recorded for all mice prior to LL-37 instillation. Pain assays and tissue harvest were performed after 1, 2, 3, 5 and 7 days post-installation (n=5 for each time point).
Tissue collection, gross images, and tissue MPO assay
Bladders were removed by transecting the urethra and dissecting away connective tissues, then split longitudinally and imaged at 10 × magnification. One section was fixed in 4% paraformaldehyde, and the other was processed for analysis of tissue MPO. Tissues were processed, and MPO assays were performed [11-14]. The fixed tissue was sectioned, stained with hematoxylin and eosin (H&E), and imaged.
Statistical analysis of data
Data was tested for normality using a Shapiro-Wilk test and used to determine if parametric or nonparametric statistical tests would be used to analyze each set of data. Statistical methods used to analyze the data from these experiments included the use of Wilcoxon signed-rank when the data was assumed to be non-normal and pairs of samples were compared, a paired t-test to make comparisons between two data sets, the Kruskal-Wallis test was used for non-parametric data when three or more groups were compared, and one-way ANOVA with a Bonferroni adjustment when multiple groups were compared. The statistical significance of linear relationships was determined by testing the line against the null hypothesis that slope coefficient was zero (no effect). Graphs were prepared and statistical analysis was performed in GraphPad Prism 5.0. A p-value <0.05 was used as the threshold for statistical significance for all data.
Results
Baseline pain responses
Baseline pain responses were as expected, having a logarithmic form with diminishing returns becoming notable at 1.0 g of stimulus. This trend was visible in the data seen in the data for intact pre-instillation mice in the baseline groups (see Figures 1 and 2). This pattern was typical for pain response curves.
Figure 1.
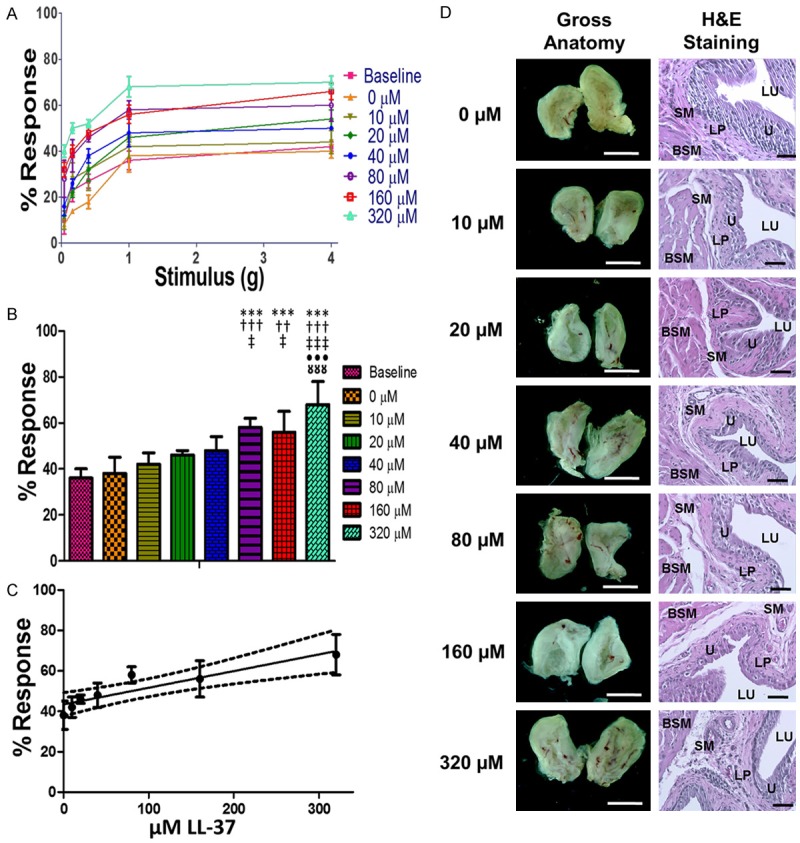
Results from dose dependence assays. A. Response traces for mice at baseline and five days after the administration of 50 µL of 0, 10, 20, 40, 80, 160, and 320 µM LL-37 intravesically. B. The referred pain response observed at 1g stimulation to the lower abdomen. C. A linear regression of the pain response at 1.0 g of stimulation for escalating doses of LL-37. The solid line on the graph is the least squares regression line for the data, R2=0.84, while the dashed lines indicate the 95% confidence interval. The line is statistically significant with respect to the null hypothesis that there is no correlation between the dose of LL-37 and the pain response in the animals, P=0.004. D. Images of the gross anatomy at 10 × magnification and H&E stained tissue sections taken at 200 × magnification. Pain response increased as the LL-37 dose increased. *** indicates a P<0.001 with respect to baseline. Respectively, †††, †† indicate a P<0.001 and P<0.005 with respect to 0 µM LL-37. Respectively, ‡‡‡, ‡ indicate a P<0.001 and P<0.05 with respect to 10 µM LL-37. ••• indicates a P<0.001 with respect to 20 µM LL-37. □□□ indicates a P<0.001 with respect to 40 µM LL-37. The white scale bars in images of gross anatomy are 500 µm and the black scale bars in histology images are 50 µm. LU, lumen of the bladder. U, urothelium. SM, submucosa. LP, lamina propria. BSM, bladder smooth muscle layer.
Figure 2.
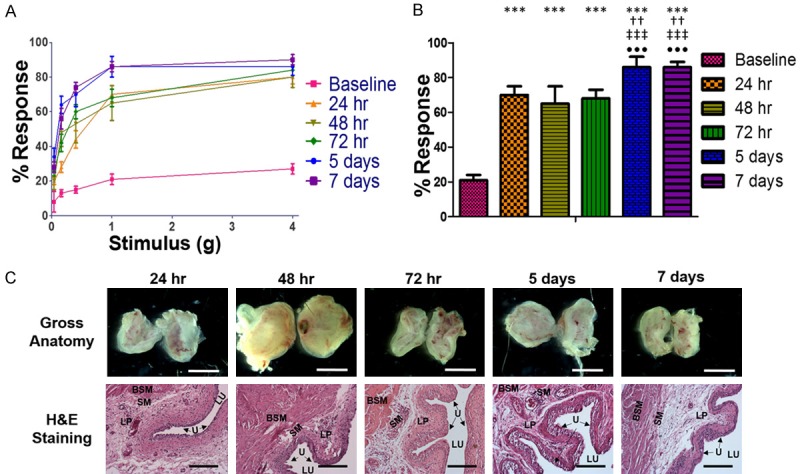
Results from time dependence assays. A. Response traces for mice at baseline and 24 hr, 48 hr, 72 hr, 5 days, and 7 days after the administration of 50 µL of 320 µM LL-37 intravesically. B. The referred pain response observed at 1 g stimulation to the lower abdomen. C. Images of the gross anatomy at 10 × magnification and H&E stained tissue sections taken at 200 × magnification. Pain response increased within 24 hours and achieved and reached peak values after 5 days. *** indicates a P<0.001 with respect to baseline. †† indicate a P<0.005 with respect to 24 hr. ‡‡‡ indicates a P<0.001 with respect to 48 hr. ••• indicates a P<0.001 with respect to 72 hr. The white scale bars in images of gross anatomy are 500 µm and the black scale bars in histology images are 50 µm. LU, lumen of the bladder. U, urothelium. SM, submucosa. LP, lamina propria. BSM, bladder smooth muscle layer.
Dose-dependence of LL-37 induced pain
Response assays demonstrated that increasing concentrations of LL-37 increased pain responses over baseline in a dose-dependent manner (see Figure 1C). Pain assays for 50 μL of deionized water after intravesical instillation produced a parallel response curve compared to baseline (see Figure 1A). At 320 μM, the highest concentration tested, instillation produced the greatest enhancement to the pain response (see Figure 1B). The other concentrations of LL-37 challenge, 10, 20, 40, 80, 160 μM, yielded pain response curves that displayed a regularity of increasing pain with increasing concentrations of LL-37, for all von Frey filament forces (Figure 1). There was a statistically significant difference (p=0.005, Wilcoxon signed-rank statistical method) when comparing the 1.0-gram von Frey filament at 320 μM LL-37 challenge with a 68 ± 8% stimulus response versus a 38 ± 6% stimulus response for the 1.0-gram filament at 0μM LL-37 challenge (Table 1).
Table 1.
Response rate with increasing concentrations of LL-37
| % Response Rate (Mean ± St. Dev.) | |||||
|---|---|---|---|---|---|
| Stimulation (g) | 0.04 | 0.16 | 0.40 | 1.00 | 4.00 |
| Baseline | 10 ± 6 | 23 ± 5 | 27 ± 3 | 36 ± 4 | 42 ± 2 |
| 0 µM | 8 ± 1 | 14 ± 1 | 18 ± 3 | 38 ± 7 | 40 ± 3 |
| 10 µM | 10 ± 4 | 28 ± 7 | 32 ± 9 | 42 ± 5 | 44 ± 5 |
| 20 µM | 12 ± 1 | 22 ± 2 | 32 ± 5 | 46 ± 2 | 54 ± 4 |
| 40 µM | 16 ± 4 | 26 ± 2 | 38 ± 3 | 48 ± 6 | 50 ± 1 |
| 80 µM | 28 ± 8 | 38 ± 7 | 46 ± 2 | 58 ± 4 | 60 ± 7 |
| 160 µM | 32 ± 7 | 40 ± 6 | 48 ± 5 | 56 ± 9 | 66 ± 8 |
| 320 µM | 40 ± 3 | 50 ± 5 | 52 ± 4 | 68 ± 10 | 70 ± 6 |
LL-37 induced bladder inflammation occurred in a dose-dependent fashion 5 days after insult. Gross examination of bisected bladders from the 0 µM control group showed no signs of inflammation. In the 320 µM there were signs of hemorrhage and tissue edema, with a graded response for groups with lesser doses (see Figure 1D). There was erythema observed in all groups subjected to 20 µM and higher of LL-37 challenge as indicated by the zones of blush observed within the lumen of the bladder. The visually observed levels of inflammation were consistent within each treatment group. Edema was evident in the histology for groups that received greater than 40 µM of LL-37 (see Figure 1D).
Time-dependent pain assay
In the time course pain assays, the mice demonstrated an increased and prolonged pain response with bladders challenged with a single dose of 320 µM LL-37 compared to baseline (see Figure 2A and 2B, Table 2). When comparing the average baseline to all other time points for the 1.0 g stimuli, a minimum of 44% increase in pain response was demonstrated. The 1.0 g stimuli at 24 h, 48 h, and 72 h time points demonstrated 70 ± 5%, 65 ± 10%, and 68 ± 5% response rates respectively (p=0.0093, 0.1123, 0.0125, respectively using the Paired T-test, Table 2). The greatest response rates at the 1.0 g stimulus were observed at 5 and 7 days, both demonstrating a response rate of 86 ± 6% (p=0.0121 and 0.0014, respectively).
Table 2.
Response rate over time for mice instilled with 320 µM of LL-37
| % Response Rate (Mean ± St. Dev.) | |||||
|---|---|---|---|---|---|
| Stimulation (g) | 0.04 | 0.16 | 0.40 | 1.00 | 4.00 |
| Baseline (n=5) | 8 ± 6 | 13 ± 2 | 15 ± 2 | 21 ± 3 | 27 ± 3 |
| 24 hr (n=5) | 20 ± 2 | 28 ± 3 | 44 ± 5 | 70 ± 5 | 80 ± 4 |
| 48 hr (n=5) | 20 ± 5 | 48 ± 8 | 53 ± 11 | 65 ± 10 | 80 ± 6 |
| 72 hr (n=5) | 26 ± 3 | 42 ± 5 | 60 ± 4 | 68 ± 5 | 84 ± 3 |
| 5 days (n=5) | 34 ± 5 | 64 ± 5 | 70 ± 7 | 86 ± 6 | 86 ± 5 |
| 7 days (n=5) | 28 ± 3 | 56 ± 6 | 74 ± 3 | 86 ± 3 | 90 ± 3 |
Gross evaluation of bisected bladders showed signs of inflammation beginning at 24 hr and lasting through the end of the trial. However, the greatest response was observed after 48 hr as indicated by edema, erythema, and hemorrhage. The observed levels of inflammation decreased from 72 hr to 7 days, but did not return to a healthy appearance due to persistent erythema and edema. The histology showed a similar pattern of peaking at 48 hours, but partially resolving by days 5 and 7 (see Figure 2C). Similar features including edema and inflammatory cell infiltrate (polymorphonuclear leukocytes) were seen as the 320 µM group from Figure 1.
Quantifying inflammation with MPO ELISA
After the pain assays were performed, bladder tissues were harvested and measured for MPO concentrations using ELISA. MPO levels for the dose response experiment demonstrated minimal detection of MPO at lower concentrations. The 0 μM to 160 μM LL-37 challenged bladders all demonstrated MPO levels ~7-8 ng MPO/mL, respectively. Significant elevation of MPO was detected for the 320 μM LL-37 challenged bladders, reaching 41 ± 13 ng MPO/mL demonstrating over a 5-fold increase in MPO activity when compared to all other groups (see Figure 3A). Statistical significance was found when comparing 320 μM LL-37 versus all other groups (p<1.6e-10, ANOVA with Bonferroni p-value adjustment).
Figure 3.
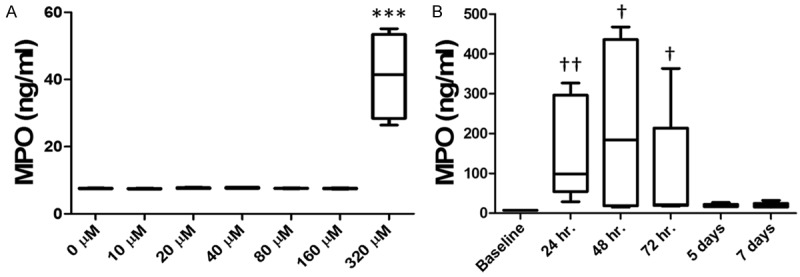
Quantification of inflammatory response via MPO ELISA assay. (A) MPO concentration in bladder tissues at baseline and five days after the administration of 50 µL of 0, 10, 20, 40, 80, 160, and 320 µM LL-37 intravesically. 320 µM was very highly statistically elevated compared to baseline and all other dose administered within (A). *** indicates a P<0.001. (B) MPO concentration in the bladder at baseline and 24 hr, 48 hr, 72 hr, 5 days, and 7 days after the administration of 50 µL of 320 µM LL-37 intravesically. MPO levels peaked 48 hours after LL-37 challenge. MPO levels peaked 48 hours after LL-37 challenge, and was significant 24, 48 and 72 hours after administration. †, †† indicate a significance of P<0.05 and P<0.005 with respect to baseline, respectively. Significance was determined using a Kruskal-Wallis test.
MPO data for the time course experiment demonstrated higher MPO levels for 24, 48, and 72 hrs. Minimal MPO levels were detected for the 5 and 7 day time points with concentrations of 19 and 19.5 ng MPO/mL respectively (see Figure 3B). Statistical significance was found when comparing 24, 48, and 72 hr. to baseline.
Discussion
IC/PBS is a significant clinical issue with rising medical costs, increasing social burden, and is conservatively estimated to afflict over 1 million patients in the United States [3]. IC/PBS adversely impacts quality of life, with more than half of diagnosed patients reporting daily or persistent pain [16]. In some refractory IC/PBS cases, simple cystectomy is required in order to relieve the severe chronic pain related symptoms [17]. Therefore, pain management is at the forefront in the development of IC/PBS therapeutics. However, the etiology behind IC/PBS is still unknown and no one theory has been able to explain IC/PBS. Current treatment regimens are only effective for a small percentage of patients and depend on empirical evidence from the clinic [18].
Minimal understanding of the pathophysiological forces driving IC/PBS and the lack of efficacious therapeutics makes IC/PBS a difficult disorder to model and has previously lacked an effective animal model that accurately mimics the symptomology of the disease. IC/PBS patients demonstrate a prominent hypersensitivity to subtle mechanical forces in the suprapubic region [19]. We measured the referred pain response in a mouse IC/PBS model, using flexible von Frey filaments applied to the mouse suprapubic region [15]. Our current study demonstrated a tailorable and predictable dose response pain profile, with higher concentrations of LL-37 challenge yielding higher pain responses. The ability to control bladder specific pain responses is a powerful tool, especially for the evaluation of potential palliative treatments for IC/PBS.
Inflammation alone is insufficient to explain the pain present in IC/PBS. Frequently, the degree of pain in IC/PBS patients is independent from the observed level of inflammation or the extent of pathological changes in bladder tissue [9]. The LL-37 induced IC/PBS model mimics clinical observations where patient pain and discomfort is independent of inflammation. This strengthens the potential clinical applicability of the LL-37 IC/PBS model. We identified peak pain responses after only a single 320 μM LL-37 challenge to be present 5 and 7 days after initial challenge. However, MPO concentration, an index of tissue inflammation severity, had returned to near baseline prior to day 5. The phenomenon of pain being independent from inflammation is intriguing both within the LL-37 model and clinically, and merits future investigation. We propose that future investigations with LL-37, an endogenous human antimicrobial peptide that in excess can upregulate both pain and inflammatory responses in preclinical animal models, may also shed light on why numerous IC/PBS patients in the clinic experience pain without ulcerative urothelial lesions or other signs of inflammation in the bladder.
A primary limitation of this study is the short period, 7 days, and thus a representation of acute cystitis. Additionally, only MPO was used as a quantitative indication of inflammation. Other inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), could have important roles in both pain and inflammation observed in response to LL-37.
Future work will extend the study period and alter the dosing regimen to include multiple exposures to allow for a chronic IC/PBS investigative approach. Additionally, future work will also seek to quantify multiple inflammatory signals, in addition to MPO, to increase our understanding of LL-37 induced inflammation.
Conclusions
This study successfully demonstrated the ability to tune pain responses based on LL-37 doses and that the pain response matches clinical observations for IC/PBS-associated pain. Additionally, LL-37 appears to elicit pain responses that escalates over an extended period after an acute insult, independently from inflammation, as is frequently observed clinically for IC/PBS. These findings substantiate the physiologic and symptomatic relevance of the LL-37 induced bladder inflammation model to further investigate IC/PBS.
Acknowledgements
NIH NIDDK R01 grant DK100868 (SO), Primary Children’s Hospital Integrated Science Award (SO), NIH K12 grant UL1RR025764 (SO), Nanotechnology Training Program Fellowship (MMJ), National Science Foundation Graduate Research Fellowship Grant 1256065 (MMJ).
Disclosure of conflict of interest
GDP is a founder and holds equity in GlycoMira Therapeutics; SO & JAA are holders of equity in GlycoMira Therapeutics. JAA is a consultant for Medtronic, Inc. and Spirox.
References
- 1.Chapple CR, Kelleher CJ, Evans CJ, Kopp Z, Siddiqui E, Johnson N, Mako M. A narrative review of patient-reported outcomes in overactive bladder: what is the way of the future? Eur Urol. 2016;70:799–805. doi: 10.1016/j.eururo.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 2.Dimitrakov J, Kroenke K, Steers WD, Berde C, Zurakowski D, Freeman MR, Jackson JL. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922–1929. doi: 10.1001/archinte.167.18.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrico DJ, Sherer KL, Peters KM. The relationship of interstitial cystitis/painful bladder syndrome to vulvodynia. Urol Nurs. 2009;29:233–238. [PubMed] [Google Scholar]
- 4.Sairanen J, Leppilahti M, Tammela TL, Paananen I, Aaltomaa S, Taari K, Ruutu M. Evaluation of health-related quality of life in patients with painful bladder syndrome/interstitial cystitis and the impact of four treatments on it. Scand J Urol Nephrol. 2009;43:212–219. doi: 10.1080/00365590802671031. [DOI] [PubMed] [Google Scholar]
- 5.Davis NF, Gnanappiragasam S, Thornhill JA. Interstitial cystitis/painful bladder syndrome: the influence of modern diagnostic criteria on epidemiology and on internet search activity by the public. Transl Androl Urol. 2015;4:506–511. doi: 10.3978/j.issn.2223-4683.2015.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshimura N, Oguchi T, Yokoyama H, Funahashi Y, Yoshikawa S, Sugino Y, Kawamorita N, Kashyap MP, Chancellor MB, Tyagi P, Ogawa T. Bladder afferent hyperexcitability in bladder pain syndrome/interstitial cystitis. Int J Urol. 2014;21(Suppl 1):18–25. doi: 10.1111/iju.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamarre NS, Bjorling DE. Treatment of painful bladder syndrome/interstitial cystitis with botulinum toxin A: why isn’t it effective in all patients? Transl Androl Urol. 2015;4:543–554. doi: 10.3978/j.issn.2223-4683.2015.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57:47–55. doi: 10.1016/s0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- 9.Killinger KA, Boura JA, Peters KM. Pain in interstitial cystitis/bladder pain syndrome: do characteristics differ in ulcerative and non-ulcerative subtypes? Int Urogynecol J. 2013;24:1295–1301. doi: 10.1007/s00192-012-2003-9. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen KL, Dynesen P, Larsen P, Jakobsen L, Andersen PS, Frimodt-Møller N. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated escherichia coli urinary tract infections. Infect Immun. 2014;82:1572–1578. doi: 10.1128/IAI.01393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oottamasathien S, Jia W, Roundy LM, Zhang J, Wang L, Ye X, Hill AC, Savage J, Lee WY, Hannon AM, Milner S, Prestwich GD. Physiological relevance of LL-37 induced bladder inflammation and mast cells. J Urol. 2013;190:1596–1602. doi: 10.1016/j.juro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roundy LM, Jia W, Zhang J, Ye X, Prestwich GD, Oottamasathien S. LL-37 induced cystitis and the receptor for advanced glycation end-products (RAGE) pathway. Adv Biosci Biotechnol. 2013;4:1–8. doi: 10.4236/abb.2013.48a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WY, Savage JR, Zhang J, Jia W, Oottamasathien S, Prestwich GD. Prevention of anti-microbial peptide LL-37-induced apoptosis and ATP release in the urinary bladder by a modified glycosaminoglycan. PLoS One. 2013;8:e77854. doi: 10.1371/journal.pone.0077854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oottamasathien S, Jia W, McCoard L, Slack S, Zhang J, Skardal A, Job K, Kennedy TP, Dull RO, Prestwich GD. A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides. J Urol. 2011;186:1684–1692. doi: 10.1016/j.juro.2011.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudick CN, Schaeffer AJ, Klumpp DJ. Pharmacologic attenuation of pelvic pain in a murine model of interstitial cystitis. BMC Urol. 2009;9:1. doi: 10.1186/1471-2490-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koziol JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7–20. [PubMed] [Google Scholar]
- 17.Rössberger J, Fall M, Jonsson O, Peeker R. Long-term results of reconstructive surgery in patients with bladder pain syndrome/interstitial cystitis: subtyping is imperative. Urology. 2007;70:638–642. doi: 10.1016/j.urology.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Hanno PM, Erickson D, Moldwin R, Faraday MM. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 19.Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26:253–259. doi: 10.1097/GCO.0000000000000083. [DOI] [PubMed] [Google Scholar]


