Abstract
The aryl hydrocarbon receptor (AhR) is a transcription factor implicated in several pathways known to be relevant in lymphomagenesis. Aim of our study was to explore the link between AhR activation and risk of lymphoma subtypes. We used a Dual-Luciferase Assay® and a luminometer to detect the activation of the luciferase gene, in HepG2 cells transfected with a specific reporter systems, by a 50 ml serum aliquot of cases of diffuse large B cell lymphoma (N = 108), follicular lymphoma (N = 85), chronic lymphocytic leukemia (N = 72), multiple myeloma (N = 80), and Hodgkin lymphoma (N = 94) and 357 controls who participated in the multicentre Italian study on gene-environment interactions in lymphoma etiology (ItGxE). Risk of each lymphoma subtype associated with AhR activation was calculated with polytomous logistic regression adjusting by age, gender, and study centre. The overall prevalence of AhR activation ranged 13.9-23.6% by subtype, and it varied by study area (8-39%). Risk associated with AhR activation was moderately elevated for follicular lymphoma (OR = 1.56, 95% CI 0.86, 2.80) and chronic lymphocytic leukemia (OR = 1.56, 95% CI 0.83, 2.96). Despite our inconclusive findings about the association with risk of lymphoma subtypes, we showed that the Dual-Luciferase Assay can be reliably and easily applied in population-based studies to detect AhR activation.
Keywords: Aryl hydrocarbon receptor, lymphoma, molecular epidemiology, dual luciferase assay, case-control study
Introduction
The International Agency for Research on Cancer (IARC) includes several organochlorines, such as tetra-chloro-dibenzo-dioxin (TCDD), and coplanar, dioxin-like polychlorobiphenyls, among the causing agents of non Hodgkin lymphomas, which effect appears to be mediated by their interaction with the aryl hydrocarbon receptor (AhR) [1,2]. AhR is a transcription factor implicated in several pathways, such as activation of environmental carcinogens, immune suppression, oxidative stress, and apoptosis [3]; such mechanisms are known to play a role in lymphomagenesis. Recent research has extended the knowledge of AhR function from the toxic effects of dioxins and other environmental pollutants to its biological roles, including the contribution by AhR-responsive elements in retrotransposons to the functional structure of the genome, its role in conferring growth advantage to human brain tumors, in neurotoxicology, in the circadian rhythm, and in several immune system functions [4]. Besides, numerous other agents capable of linking and activating the AhR include internal agents, such as bilirubin and low density lipoproteins, and nutrients, such as indole-3-carbinole, and caffeine [5]. When inactive, the AhR can be found in the cytosol. Free state hydrophobic molecules in the surrounding tissue can cross the cell membrane and penetrate the cytosol, where they are captured mainly by the AhR. Following the link, the activated receptor migrates to the nucleus where it forms a heterodimer with a structurally similar protein, the aryl hydrocarbon receptor nuclear translocator (ARNT), which in turn links to a specific DNA sequence, the xenobiotic response element (XRE). XRE is an enhancer, which can promote the transcription of specific target genes, such as CYP1A1 and other member of cytochrome P450 superfamily [6,7].
Activation of the AhR might also be involved in other pathways that have been linked to risk of cancer, including inhibition of tumor suppressor genes, such as p53 and BRCA1, promoting cell transformation and angiogenesis, affecting cell survival, proliferation, and differentiation, and interfering with the signal pathway of estrogen receptors and inflammation [3]. Different AhR ligands might exhibit agonistic or antagonistic activity in the carcinogenic process, suggesting this as a potential target for new anti-tumoral treatments [4,8].
Therefore, we investigated the association between AhR activation and risk of lymphoma, B-cell lymphoma, and the major lymphoma subtypes, using the biological samples from the participants in the multicentre Italian study on gene-environment interactions in lymphoma etiology (ItGxE).
Methods
Study setting
The Italian study on gene-environment interactions in lymphoma etiology is a multicentre case-control study conducted in six Italian areas: Novara (eastern Piedmont), Verona, Florence, Perugia, Bari and Taranto in the Apulia region, and central southern Sardinia. Cases are adult patients (aged 20-74) diagnosed with lymphoma along the study period (2012-2016). The clinical diagnosis of lymphoma was reviewed by the pathologists in each study centre using the 2008 World Health Organization (WHO) classification of Lymphoma. All lymphoma subtypes, including B-cell and T cell lymphomas, and Hodgkin lymphoma were included. In all study centers but Sardinia, controls were patients admitted to hospitals in the same area where cases were recruited for non neoplastic diseases. Cases affected by infectious diseases and suffering from immune system disorders were also non eligible to serve as controls. In Sardinia, controls were a random sample of the resident population within the territory of the local health units referring to the two main Hematology hospital departments in central-southern Sardinia. Controls were frequency matched to the cases by gender, 5-year age group, and local health unit of residence; due to the high proportion of refusals and untraced subjects among the population controls, we oversampled these controls in specific age and gender groups. Following the Helsinki protocol for studies involving human subjects, all study subjects gave a written consent to the use of their biological samples before participation, in which they acknowledged that their identity could not be identified via the papers, and that samples would have been fully anonymized. The study protocol included an in-person interview, conducted by trained interviewers at the hospital or the residence home, gathering information on demographics, lifestyle habits (smoking, alcohol, diet, leisure time physical activity), health history, and a detailed occupational history. At the end of the interview, subjects were requested to donate a 40 ml blood sample to investigate genetic and epigenetic determinants of disease, as well as biomarkers.
Dual Luciferase assay
To test AhR activation, we used a Dual-Glo® Luciferase Assay System (Promega Corporation, Madison, WI, U.S.A.), which is based on the response of hepatocellular carcinoma (Hep G2) cells [HEPG2] (ATCC® HB8065™, Manassas, VA, U.S.A.), purchased and received in January 2014. Cells were incubated in 5% CO2 at the temperature of 37°C under regularly controlled conditions, in an incubator with a regular maintenance program, and maintained in D-minimum essential medium (D-MEM) (Sigma-Aldrich, Milan, Italy), supplemented with 10% (v/v) heat inactivated FBS with 1% penicillin/streptomycin and 1% L-glutamin, according to the manufacturer instructions. Cells viability was 98%, and it was checked by optical microscopy, with no evidence of deviation from the expected growth process or Mycoplasma contamination. Before use in the experiment, they underwent three passages: 1. after thawing they stayed for 24 hours in a 25 mm flask; 2. then they were splitted in 75 mm flasks; and 3. used after two days. Briefly, HepG2 cells were seeded at a density of 1 x 104 cells/well in 24-well plates. After 24 hour, following the manufacturer instructions, the HepG2 cells medium was replaced with a fresh medium and the cells were transfected using the FuGENE HD Transfection Reagent (Promega Corporation, U.S.A.) with plasmids containing the reporter system, the pGL4.43 [luc2p/XRE/HYGRO] vector (1 mg/ml), which carried the XRE enhancer sequence for the luciferase gene, and the pGL4.73 [hRLucSV40] vector (100 ng/ml), an internal control of the efficacy of transfection with a strong promoter, such as the SV40, that drives transcription of the reporter gene.
Twenty four hours later, the medium was replaced with a fresh medium Opti-MEM (Life Technologies, Paisley, UK) and the cells were exposed to serum sample 10% (v/v) heat-inactivated by incubation at 65° for 30 min and tetra-chloro-dibenzo-dioxin (TCDD, Sigma-Aldrich, Italy) was added to the cells at increasing concentrations in the 10-12-10-8 M range. The lowest TCDD dose capable of activating the system was detected at 1 x 10-9 M; such concentration was used as the positive standard. Three wells were designated to one treatment condition.
Following AhR induction, the culture cells were lysed and the presence of the luciferase protein in the 20 ml of lysate was measured with a luminometer. The response to the positive standard and to the activating molecules in the serum samples of study subjects was detected with 100 ml Stop & Glo® Reagent using a luminescence reader (Victor x5 Perkin Helmer, Waltham MA, USA). The luminescence is translated into a numeric value, directly correlated with the luciferase expression, which in turn is proportional to the AhR activity. Such reading is then referred to the internal control of transfection efficacy (the vector containing the non-induced luciferase gene), the positive control (10-9 M TCDD), and the negative control (the cells without the serum sample). All mandatory laboratory health and safety procedures had been complied with in conducting the above described assays.
Statistical analysis
Overall, serum samples from 692 lymphoma cases and 486 controls, including population and hospital controls, were available for study. We excluded from analysis samples from one centre (Perugia, 60 cases and 41 controls) and another 60 case samples and 88 control samples for technical problems in storing, preserving, or labeling, and we conducted the analysis on the remaining 572 cases and 357 controls.
To predict risk of the most represented lymphoma subtypes, including diffuse large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukaemia, multiple myeloma, and Hodgkin lymphoma, we used a polytomous regression model with AhR activation as the binary risk factor, and age (continuous), gender, and study centre as the adjusting covariates. The Cochrane Q test was used to test heterogeneity in findings across the five lymphoma subtypes. Risk of all lymphomas combined overall, and of the B-cell lymphoma subgroup was calculated with an unconditional regression model including the same adjusting covariates. All the analyses were conducted with SPSS® version 12.1.
The study was approved by the Ethics Committee of the University Hospital of Cagliari (approval date: 26/05/2009, number 269/09/CE), and acknowledged by the local Ethics Committee in each study centre.
Results
Table 1 shows basic characteristics of the study population, and the rate of AhR activation by case-control status and by the most represented lymphoma subtypes. Mean age of the participating subject was 55.8 years (sd 15.19); the male:female ratio among the cases was 1.25, consistent with the known higher prevalence of lymphomas among males. As expected, mean age and male:female ratio varied substantially by lymphoma histotypes. AhR activation rate was highest for CLL (23.6%) and FL (23.5%) cases, although not significant in respect to the rest of the lymphoma cases (p value 0.40 and 0.45 respectively).
Table 1.
Main characteristics of the study population, and rate of AhR activation by case-control status and by major lymphoma subtypes
| N | Gender | Age | AhR + | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M | F | M/F | Mean | sd | N | % | ||
| Controls | 357 | 190 | 167 | 1.14 | 54.5 | 14.74 | 63 | 17.6 |
| DLBCL | 108 | 63 | 45 | 1.40 | 58.0 | 15.38 | 15 | 13.9 |
| Follicular Lymphoma | 85 | 41 | 44 | 0.93 | 57.3 | 11.77 | 20 | 23.5 |
| Chronic Lymphocytic Leukaemia | 72 | 47 | 25 | 1.88 | 63.5 | 12.17 | 17 | 23.6 |
| Multiple Myeloma | 60 | 32 | 28 | 1.14 | 63.9 | 10.32 | 13 | 21.7 |
| Other B-cell Lymphoma | 80 | 52 | 28 | 1.86 | 61.1 | 11.17 | 17 | 21.2 |
| B-cell Lymphoma (total) | 405 | 235 | 170 | 1.38 | 60.3 | 12.82 | 82 | 20.2 |
| Hodgkin Lymphoma | 94 | 43 | 51 | 0.84 | 37.9 | 14.74 | 19 | 20.2 |
| T-cell Lymphoma | 22 | 14 | 8 | 1.75 | 57.5 | 13.05 | 5 | 22.7 |
| Unspecified Lymphoma subtype | 51 | 32 | 19 | 1.68 | 59.2 | 14.28 | 8 | 15.7 |
| All cases | 572 | 326 | 246 | 1.33 | 55.8 | 15.19 | 114 | 19.9 |
AhR activation rate did not vary by age or gender (data not shown), while it showed a substantial variation by study centre (Figure 1), with the highest rate among participants from the city of Bari and Nuoro, and the lowest among participants from Florence and Novara, with around 8% of the plasma samples from either centre inducing AhR activation.
Figure 1.
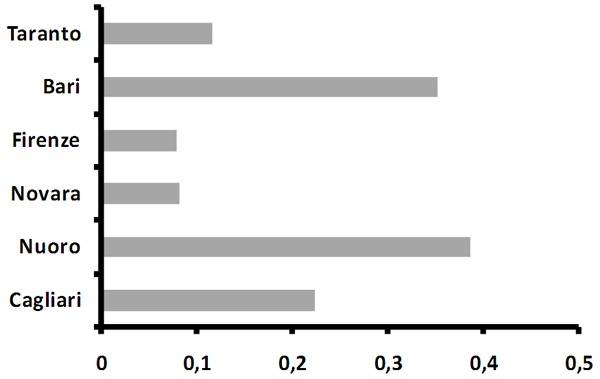
Rate of AhR activation by study centre.
Table 2 shows the risk of lymphoma and its major subtypes associated with AhR activation. The highest increase in risk was observed for follicular lymphoma (OR = 1.56; 95% CI 0.87-2.80), and for CLL (OR = 1.56, 95% CI 0.83-2.96), while risk of DLBCL was inverse (OR = 0.79, 95% CI 0.43-1.46); however no heterogeneity in risk was detected (Q = 3.03, 4 degrees of freedom, p = 0.552). Risk was marginally elevated for lymphoma overall (OR = 1.21, 95% CI 0.86-1.70), and for the B-cell lymphoma subgroup (OR = 1.23, 95% CI 0.84-1.78).
Table 2.
Risk of the most represented lymphoma subtypes associated with AhR activation. Risks for the group of B-cell Lymphoma (all subtypes), and for all lymphomas combined are also shown
| N | AhR + (N, %) | OR (95% CI) | |
|---|---|---|---|
| Controls | 357 | 63, 17.6 | Ref |
| Diffuse large B-cell Lymphoma | 108 | 15, 13.9 | 0.79 (0.43-1.46) |
| Follicular Lymphoma | 85 | 20, 23.5 | 1.56 (0.87-2.80) |
| Chronic Lymphocytic Leukaemia | 72 | 17, 23.6 | 1.56 (0.83-2.96) |
| Multiple Myeloma | 60 | 13, 21.7 | 1.37 (0.68-2.76) |
| Hodgkin Lymphoma | 94 | 19, 20.2 | 1.12 (0.59-2.13) |
| B-cell Lymphoma (all subtypes) | 405 | 82, 20.2 | 1.23 (0.84-1.78) |
| Lymphoma (all subtypes) | 572 | 114, 19.9 | 1.21 (0.86-1.70) |
Discussion
Our results show that the Dual Luciferase assay is a reliable, simple and inexpensive method to detect AhR activation. We used this assay to explore risk of lymphoma subtypes associated with AhR activation; our explorative findings do not provide clear evidence of an association with risk of any of the B-cell lymphoma subtypes most frequently represented in our study. We are not aware of previous reports exploring this hypothesis; however, results showing a significant increase in risk of non Hodgkin lymphoma, consistent across all NHL subtypes, among Canadian carriers of AhR gene variants, with a significant interaction between such gene polymorphisms and an elevated serum concentration of persistent organochlorines [9] would suggest that such a hypothesis is worth considering in future larger studies.
The overall rate of AhR activation in our study population has been found to be 19%, with a wide range by study area (8-39%). We have not identified the reasons for such a wide variation. Technical issues would be unlikely, as the positive and negative standards as well as the transfection quality control were always included, and their results confirmed the expectation. Besides, samples from the two areas with extreme rates were selected for replicating the analysis, showing a 100% replication of the original finding. Chance, unknown sources of bias, or geographical variation in relevant exposures might be alternative explanations. We adjusted our risk estimates by study centre. Also, a sensitivity analysis excluding one centre at the time confirmed the results (not shown in the Tables).
Another limitation in interpreting our findings is related to the retrospective study design we used. We were careful in identifying the cases and we elicited donation of a blood sample before starting chemotherapy; however, we cannot exclude that in one or more centers a fraction of blood withdrawals might have been performed subsequent to chemotherapy, or that other therapies might have been initiated before diagnosis, capable of activating the AhR, which would be one of the possible unknown sources of bias. A prospective follow-up of a large pooled set of controls showing AhR activation vs those not showing it would help in solving the uncertainty in interpreting our retrospective results.
In conclusion, although with inconclusive findings, our study paves the way to further attempts to test whether AhR activation would be an important step in the development of specific xenobiotic-responsive B-cell lymphoma subtypes.
Acknowledgements
This work was supported by the Italian Association for Cancer Research [IG grant N. 2011/11855]; and the Italian Ministry of University, Education and Research [PRIN grant N. 20092ZELR2].
Disclosure of conflict of interest
None.
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans Volume 100F. Lyon, France. IARC; 2012. Review of human carcinogens. 2,3,7,8-tetrachlorodibenzo-para-dioxin, 2,3,4,7,8-pentachlorodibenzo furan, and 3,3’,4,4’,5-pentachlorobiphenyl; pp. 339–378. [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans, Volume 107. Lyon, France. IARC; 2016. Polychlorinated biphenyls and polybrominated biphenyls; pp. 41–440. [PMC free article] [PubMed] [Google Scholar]
- 3.Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biochem Biophys Acta. 2013;1836:197–210. doi: 10.1016/j.bbcan.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Feng Y, Fu H, Xie HQ, Jiang JX, Zhao B. The Aryl Hydrocarbon Receptor: a key bridging molecule of external and internal chemical signals. Environ Sci Technol. 2015;49:9518–9531. doi: 10.1021/acs.est.5b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friends and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochem Biophys Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 7.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- 8.Safe S, Lee SO, Jin UH. Role of the Aryl Hydrocarbon Receptor in Carcinogenesis and Potential as a Drug Target. Toxicol Sci. 2013;135:1–16. doi: 10.1093/toxsci/kft128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng CH, Janoo-Gilani R, Sipahimalani P, Gallagher RP, Gascoyne RD, Connors JM, Weber JP, Lai AS, Leach S, Le ND, Brooks-Wilson AR, Spinelli JJ. Interaction between organochlorines and the AHR gene, and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2010;21:11–22. doi: 10.1007/s10552-009-9429-5. [DOI] [PubMed] [Google Scholar]


