Abstract
The number of papers discussing probiotics increases tremendously that limits the possibility for primary care physicians and clinicians to stay updated. Therefore, the aim of this paper will be to summarize available evidence of probiotic use in well-defined clinical indications of importance for pediatricians. Based on currently available evidence certain probiotic strains (Lactobacillus rhamnosus GG [LGG] and Saccharomyces boulardii) have proven effect in the treatment of acute gastroenteritis and prevention of antibiotic associated diarrhea. Furthermore, LGG was proven to be effective in prevention of nosocomial diarrhea and respiratory tract infection in day care centers. In conclusion, not all probiotic strains have same efficacy for all clinical indications, therefore, only strains with proven efficacy and safety should be recommended.
Keywords: Lactobacillus, Saccharomyces, Bifidobacterium, Diarrhea, Infection
INTRODUCTION
Most widely used definition of probiotics was given by the Food and Agriculture Organization of the United Nations and the World Health Organization in 2002 [1]. That definition was accepted with minimal change by expert panel (International Scientific Association for Probiotics and Prebiotics) in 2014 stating that probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [2].
On the same document panel tried to emphasize the probiotic action, emphasizing that some of probiotics' effect can be attributed only to specific probiotic strain, but some effects can be ascribed to probiotics in general or certain species of probiotics [2]. Same recognition of clinical effectiveness was also approved and highlighted by European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Working Group (ESPGHAN WG) on pre- and probiotics. Stating that recommendations for probiotic use should always be strain specific and aim is to recommend only the strains which have proven efficacy by well-designed randomized controlled trials (RCTs).
There are many papers about probiotics produced on daily basis which makes clinical up-date on their effectiveness extremely difficult.
Therefore, the aim of this paper will be to summarize available evidence of probiotic use in well-defined clinical indication including the treatment of acute gastroenteritis, prevention of antibiotic associated diarrhea and prevention of infections in children.
TREATMENT OF ACUTE GASTROENTERITIS
Acute gastroenteritis is usually defined as decrease in the stool consistency (loose or liquid) and/or an increase in the frequency (typically >3 stools/day), with or without vomiting or fever [3]. Diarrhea typically lasts less than 7 days and not longer than 14 days [3]. The incidence of acute gastroenteritis is still high, even in Europe, and it is estimated that the incidence in small children ranges from 0.5 to 1.9 episodes/child/year [3]. Major causes are still rotavirus, which decreases in countries with high rate of rotavirus vaccination, followed by norovirus [4]. The treatment strategy aims to treat and prevent dehydration, shorten duration of diarrhea and to prevent prolonged diarrhea. Therefore, the mainstay of treatment is rehydration which in majority of children can be provided orally by using oral rehydration solutions [3]. Yet, there is still no causal treatment. One treatment option is racecadotril, enkephalinase inhibitor which was proven to be effective in shortening the diarrhea [5]. Other well-defined treatment modalities include probiotics.
Recently, ESPGHAN WG on pre- and probiotic performed systematic review and provided guidelines on the use of different probiotic strains for the treatment of acute gastroenteritis [6]. Based on available, well designed RCTs, ESPGHAN WG recommended only two probiotic strains proved to be effective in at least two RCTs; these are Lactobacillus rhamnosus GG (LGG) and Saccharomyces boulardii.
Based on the Cochrane review from 2010 [7], LGG was investigated in 11 RCTs (n=2,072) and this meta-analysis found that use of LGG reduced the duration of diarrhea for mean of 27 hours (95% confidence interval [CI], −41 to −13). Subsequent systematic review performed by Szajewska et al. [8] in 2013 identified 15 RCTs (n=2,963). This review confirmed superiority of LGG in significantly decreasing duration of diarrhea comparing to placebo (mean difference [MD], −1.05 days; 95% CI, −1.7 to −0.4; based on 11 RCTs). However, there was no influence on stool volume (MD, 8.97 mL/g; 95% CI, −86.26 to 104.2; based on 2 RCTs). Regarding the dose, ≥1010 colony-forming units (CFU) was more effective than <1010 CFU [8].
Other strain with well-proven effect is S. boulardii. The above-mentioned Cochrane review found 6 RCTs (n=606) and reported reduced risk of diarrhea lasting ≥4 days (risk ratio [RR], 0.37; 95% CI, 0.2 to 0.65) if S. boulardii was used [7]. More recent systematic review analyzing 11 RCTs (n=1,306) showed that S. boulardii significantly reduced diarrhea duration (MD, −0.99 days; 95% CI, −1.4 to −0.6) [9]. None of the studies evaluated the influence on stool volume.
Finally, strain Lactobacillus reuteri ATCC 55730 had proven moderate clinical effect in treating acute gastroenteritis in children; however, this strain was found to carry transferable resistance trait for antibiotic resistance and was replaced by a new strain, L. reuteri DSM 17938 [10]. This, new strain L. reuteri DSM 17938 was investigated by 3 RCTs; two RCTs (n=196) were analyzed in systematic review from 2014 and showed significantly reduced diarrhea duration (MD, −32 hours; 95% CI, −41 to −24) [11]. Subsequently, one more RCT was published including 64 infants and children, showing similar results in the reduction of diarrhea duration [12].
Generally, after reviewing these results ESPGHAN WG on pre- and probiotics recommended the use of the following probiotic strains as an adjunct to rehydration therapy: LGG (quality of evidence: low; recommendation: strong), S. boulardii (quality of evidence: low; recommendation: strong) and L. reuteri DSM 17938 (quality of evidence: very low; recommendation: weak) [6].
It should be emphasized once again that systematic review of the literature did not found enough evidence (or evidence was negative) to recommend other probiotic strains.
For clinicians, it is of importance to know that probiotics have been proven mostly in watery (mainly viral) diarrhea and that their efficacy is more pronounced on the duration of diarrhea (study showed ability to shorten diarrhea for 1 day) than in stool volume [3,6]. Furthermore, when recommended they should be recommended only as an adjunct to rehydration and it is better to use them in the early course of disease [6].
PREVENTION OF ANTIBIOTIC ASSOCIATED DIARRHEA
Antibiotic-associated diarrhea (AAD) is a common complication of antibiotic therapy, defined as diarrhea that occurs in relation to antibiotic treatment with the exclusion of other etiologies [13]. It is more commonly caused by antibiotics that target anaerobic bacteria (e.g. clindamycin, penicillin, amoxicillin and clavulanic acid etc.) which cause significant disruption of the enteric microbiome [14,15]. Clinically, AAD may present as mild diarrhea, but it can also present as fulminant pseudomembranous colitis caused by Clostridium difficile [13]. Measures which can prevent AAD are limited mainly to reduction in antibiotic use, type of antibiotic prescribed and the use of probiotics.
Due to large number of studies and different recommendations available ESPGHAN WG on pre- and probiotics preformed systematic review with meta-analysis with aim to provide evidence based guidelines for every specific probiotic strain in the prevention of AAD [13]. This systematic review found only two probiotic strains with enough evidence (efficacy proven in more than 2 well-designed RCTs); these strains are LGG and S. boulardii [13].
LGG was investigated in 5 RCTs (n=445) and administration in children reduced the risk of AAD from 23% to 9.6% (RR, 0.48; 95% CI, 0.26 to 0.89), regardless of the reason for which antibiotics and probiotics were used [13]. However, only one trial [16] evaluated the effect of LGG in the prevention of C. difficile-associated diarrhea in children and found no effect.
Similarly, S. boulardii used in children reduced the risk of AAD based on 6 RCTs (n=1,653) from 20.9% to 8.8% (RR, 0.43; 95% CI, 0.30 to 0.60) [13]. Furthermore, the administration of S. boulardii reduced the risk of C. difficile-associated diarrhea in children (2 RCTs, n=579; RR, 0.25; 95% CI, 0.08 to 0.73) [13].
However, there is a constant discussion whether probiotics should be used every time antibiotic is prescribed. The reasons for their routine use are the proven effect and the fact that AAD can be serious illness [13,17]. On contrary, reasons not to use them is usually related to their costs and fact that AAD is usually self-limited mild disease. There are certain groups of patients that would benefit the most from probiotic use including children of younger age, hospitalized children and children who experienced AAD (especially C. difficile-associated diarrhea) before [17]. Once again, the recent review identified only two strains to be effective in prevention of AAD; these are LGG (quality of evidence: moderate, recommendation: strong) and S. boulardii (quality of evidence: moderate, recommendation: strong) [13]. For prevention of C. difficile-associated diarrhea only S. boulardii showed efficacy (quality of evidence: moderate, recommendation: conditional) [13].
There is always a question when to administer probiotic in order not to be killed by antibiotic; there are no scientific evidence for that. However, some probiotic strains (like S. boulardii) are resistant to antibiotics used for bacterial infections. On the other hand, other strains (like LGG) were effective in RCTs when used for AAD, therefore their administration should follow the same scheme like in RCTs.
PREVENTION OF INFECTIONS
Infectious diseases are the most important cause of morbidity in children where respiratory and gastrointestinal (GI) infections encounter for majority of them [18]. Recurrent respiratory tract infections are common problem in preschool age, mainly due to the presence of unfavorable environmental conditions including early socialization in daycare centers and the physiologic immaturity of the immune system [19]. There are two major settings where children acquire respiratory and GI infections and those are hospital and day care centers.
Prevention of infections in day care centers
Children who attend daycare centers have 2-3 times more infections than children who stay at home, they have more outpatient doctor and emergency room visits and increased usage of prescribed antibiotics [20]. Furthermore, they cause a substantial economic burden not only for child's family, but healthcare in general; their costs are estimated to be $1.8 billion per year in the United States [21]. Taking all that into account, together with possible complications, respiratory tract and GI infections are important health care problem for pediatricians who are facing a real task to discriminate the children who are at higher risk and try to offer preventive measures. These preventive measures usually include good hand hygiene, absenteeism of ill child from daycare center in order to prevent spreading of infection and vaccination for influenza and rotavirus [22]. However, all those measures often are ineffective leaving a place for possible new modalities, like probiotics. In the last two decades, there have been an increasing number of trials investigating the role of probiotics on the prevention of common infections in children.
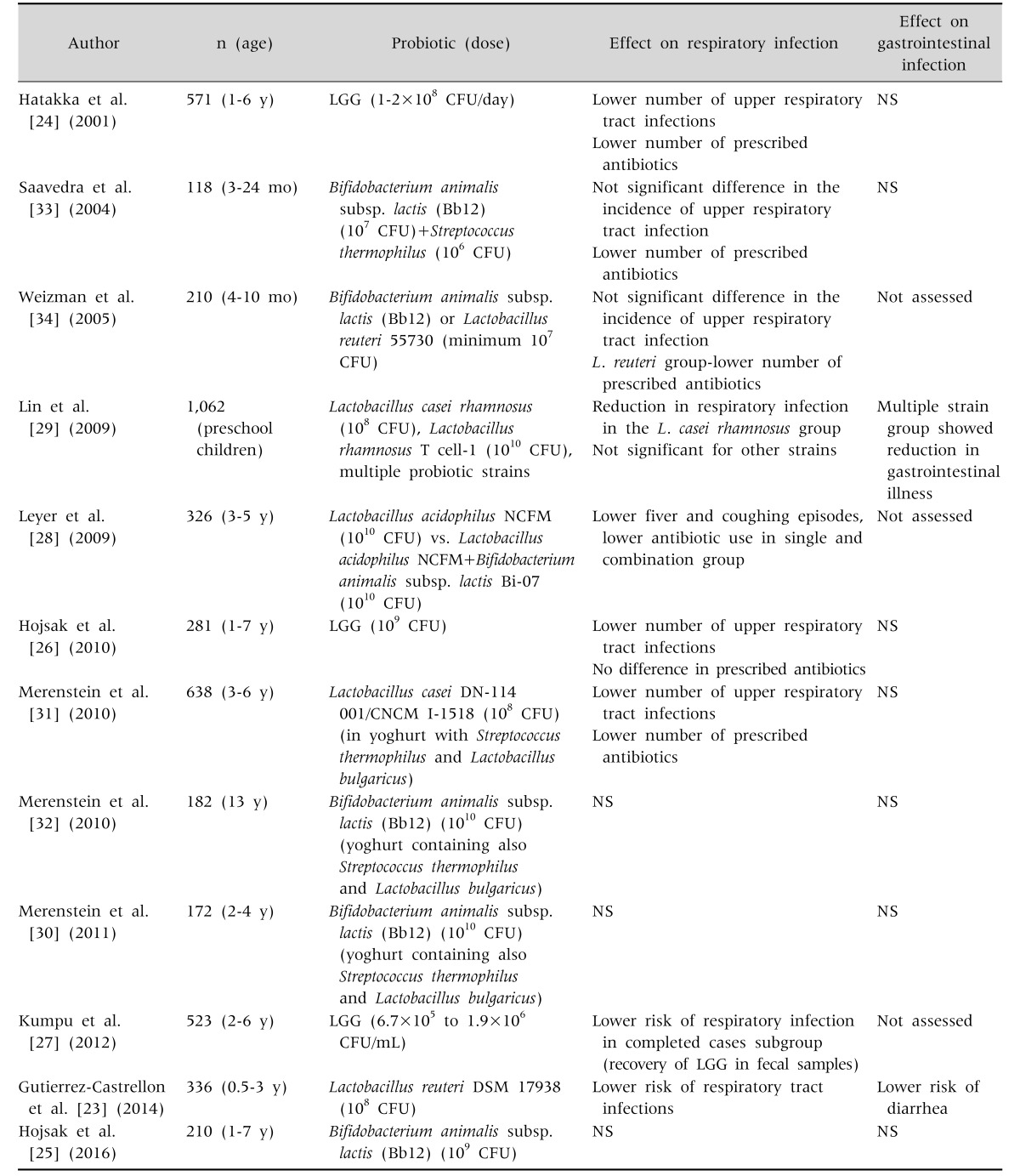
As presented in Table 1, there are several trials which evaluated probiotics in the prevention of respiratory tract infection in children attending daycare centers [23,34]. Interestingly, majority of studies beyond infancy found positive effect on the lowering of respiratory tract infections [23,24,26,27,28,29,31]. Recent meta-analysis reviewed available literature and found that probiotics (in general) reduce the risk of respiratory tract infections (RR, 0.89; 95% CI, 0.82 to 0.96) [35]. Unfortunately, this meta-analysis included all age groups, was not strain specific and was not stratified based on the type of facility where probiotics were used. However, it was reported that although there was no effect on the duration of illness, absenteeism from the kindergarten was decreased [35].
Table 1. Probiotics in Prevention of Respiratory and Gastrointestinal Infections in Children Attending Day Care Centers.
LGG: Lactobacillus rhamnosus GG, CFU: colony-forming units, NS: not significant.
Based on the presented results in Table 1, it can be concluded that probiotics could have a place in the prevention of upper respiratory tract infections. However, questions that remain are what strain to use, in which dose and when. Based on well-designed RCTs in children (Table 1), LGG was examined in 3 studies [24,26,27] involving all together 1,375 children and all studies reported positive effect on the lowering the incidence of respiratory tract infections. Other strain Bifidobacterium animalis subsp. lactis was evaluated in 4 RCTs [25,30,32,34] from which all found negative results.
The question is whether to recommend probiotic use routinely in all children who are at increased risk for respiratory infection. Based on currently available evidence, it seems prudent to use strains with proven efficacy in more than 2 RCT (which is LGG). However, there are no cost-effective analyses. Regression analysis determined that children who would benefit the most from the LGG use were children of younger age and with recurrent respiratory infections during winter months [26].
Majority of studies which investigated probiotic use in the prevention of respiratory tract infections also investigated the risk of acquiring GI infection (Table 1). Results from those studies are weak. There is no meta-analysis which assessed overall effect, however, based on literature search there are no 2 RCTs which investigated same probiotic strain and yielding positive results. Of note is that both studies investigating LGG found no effect [24,26], similarly is for B. animalis subsp. lactis investigated by other two studies [30,32].
All these results, however, should be interpreted with caution because most of them were performed in the winter period when the incidence of GI infections is much lower, and therefore someone can argue that the sample size was not powered enough to assess GI risk.
Nosocomial infections
Nosocomial, hospital-acquired or healthcare-associated infections, develop during a hospital stay and they are not present or incubating at the admission; infections that occur more than 48 hours after the admission are usually considered as nosocomial [36]. The incidence of nosocomial infections on pediatric wards even in developed countries is still high, ranging from 5% to 10% and GI and respiratory tract infections account for the majority of them [37]. Nosocomial infections have several negative impacts; they worsen the treatment outcome, could prolong the hospitalization, and significantly increase hospital costs [38]. Standard preventive measures, mainly hand hygiene, isolation of sick children and reduction in the number of hospitalized patients decreases infection spreading, but cannot successfully prevent them [39,40]. Therefore, there is a place for new strategies, one of which is the use of probiotics.
Recently, ESPGHAN WG on pre- and probiotics performed systematic review on the role of different probiotic strains in the prevention of nosocomial diarrhea [41]. This meta-analysis identified 8 RCTs out of which 3 investigated LGG. The administration of LGG reduced the risk of nosocomial diarrhea from 13.9% to 5.2% (2 RCTs, n=1823; RR, 0.35; 95% CI, 0.19 to 0.65) [41]. On contrary, L. reuteri DSM 17938 was investigated by two studies (same probiotic strain but different doses: 108 CFU/day [42] and 109 CFU/day [43]) and had negative results (RR, 1.11; 95% CI, 0.68 to 1.81) [41]. Based on the evidence ESPGHAN WG concludes that if probiotics for preventing nosocomial diarrhea in children are considered, LGG (at least 109 CFU/day, for the duration of hospital stay) should be used (quality of evidence: moderate, recommendation: strong) [41].
Due to lack of cost effectiveness, currently there is a need for identifying children in risk for acquiring nosocomial diarrhea. Based on regression analysis published in one of the RCTs [38] children who stay longer in hospital are especially prone to nosocomial infection, therefore this group of children would benefit the most.
On contrary to role of probiotics in the prevention of nosocomial diarrhea, we have only limited evidence of the role of probiotics in the prevention of nosocomial respiratory tract infection outside of intensive care unit. There are only two (although big) RCTs. One RCT investigated LGG (n=742) at the dose of 109 CFU and found reduction in risk of upper respiratory tract infection [41]. Other study, performed at the same center used different probiotic strain, B. animalis subsp. lactis (Chr Hansen, Denmark) at the same dose, was not able to prove positive effect [44]. Authors also identified that children who stayed longer in the hospital and who were younger had higher chance of acquiring upper respiratory tract infections [41]. Although there is an evidence that some probiotic strain could have effect in the prevention of infection, still there is no enough evidence to recommend probiotics for the prevention of nosocomial respiratory tract infections.
CONCLUSION
Above mentioned evidence further demonstrates that not all probiotics have the same efficacy for every specific clinical indication. Based on currently available evidence certain probiotic strains (LGG and S. boulardii) have proven effect in the treatment of acute gastroenteritis and prevention of AAD. Furthermore, LGG was proven to be effective in prevention of nosocomial diarrhea and respiratory tract infection in day care centers.
Field of probiotics increases tremendously, thus it is hard for clinicians to follow the literature. Therefore, it is of utmost importance to recognize scientific authorities and to follow up their guidelines.
ACKNOWLEDGEMENTS
Iva Hojsak has participated as a clinical investigator for Biogaia and Chr Hansen and speaker for Biogaia and Medis Adria.
References
- 1.Food and Agricultural Organization of the United Nations, World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córboda: FAO, WHO; 2001. [Google Scholar]
- 2.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 3.Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132–152. doi: 10.1097/MPG.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 4.Enserink R, Mughini-Gras L, Duizer E, Kortbeek T, Van Pelt W. Risk factors for gastroenteritis in child day care. Epidemiol Infect. 2015;143:2707–2720. doi: 10.1017/S0950268814003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon M, Akobeng A. Racecadotril for acute diarrhoea in children: systematic review and meta-analyses. Arch Dis Child. 2016;101:234–240. doi: 10.1136/archdischild-2015-309676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szajewska H, Guarino A, Hojsak I, Indrio F, Kolacek S, Shamir R, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531–539. doi: 10.1097/MPG.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 7.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048. doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children--updated analysis of randomised controlled trials. Aliment Pharmacol Ther. 2013;38:467–476. doi: 10.1111/apt.12403. [DOI] [PubMed] [Google Scholar]
- 9.Dinleyici EC, Eren M, Ozen M, Yargic ZA, Vandenplas Y. Effectiveness and safety of Saccharomyces boulardii for acute infectious diarrhea. Expert Opin Biol Ther. 2012;12:395–410. doi: 10.1517/14712598.2012.664129. [DOI] [PubMed] [Google Scholar]
- 10.Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol. 2008;74:6032–6040. doi: 10.1128/AEM.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szajewska H, Urbańska M, Chmielewska A, Weizman Z, Shamir R. Meta-analysis: Lactobacillus reuteri strain DSM 17938 (and the original strain ATCC 55730) for treating acute gastroenteritis in children. Benef Microbes. 2014;5:285–293. doi: 10.3920/BM2013.0056. [DOI] [PubMed] [Google Scholar]
- 12.Dinleyici EC, Dalgic N, Guven S, Metin O, Yasa O, Kurugol Z, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J) 2015;91:392–396. doi: 10.1016/j.jped.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Szajewska H, Canani RB, Guarino A, Hojsak I, Indrio F, Kolacek S, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495–506. doi: 10.1097/MPG.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 14.Missaghi B, Valenti AJ, Owens RC., Jr Clostridium difficile infection: a critical overview. Curr Infect Dis Rep. 2008;10:165–173. doi: 10.1007/s11908-008-0028-5. [DOI] [PubMed] [Google Scholar]
- 15.Slattery J, MacFabe DF, Frye RE. The significance of the enteric microbiome on the development of childhood disease: a review of prebiotic and probiotic therapies in disorders of childhood. Clin Med Insights Pediatr. 2016;10:91–107. doi: 10.4137/CMPed.S38338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arvola T, Laiho K, Torkkeli S, Mykkänen H, Salminen S, Maunula L, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 17.Issa I, Moucari R. Probiotics for antibiotic-associated diarrhea: do we have a verdict? World J Gastroenterol. 2014;20:17788–17795. doi: 10.3748/wjg.v20.i47.17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112(Suppl 6A):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 19.Dellepiane RM, Pavesi P, Patria MF, Laicini E, Di Landro G, Pietrogrande MC. Atopy in preschool Italian children with recurrent respiratory infections. Pediatr Med Chir. 2009;31:161–164. [PubMed] [Google Scholar]
- 20.Silverstein M, Sales AE, Koepsell TD. Health care utilization and expenditures associated with child care attendance: a nationally representative sample. Pediatrics. 2003;111:e371–e375. doi: 10.1542/peds.111.4.e371. [DOI] [PubMed] [Google Scholar]
- 21.Haskins R. Acute illness in day care: how much does it cost? Bull N Y Acad Med. 1989;65:319–343. [PMC free article] [PubMed] [Google Scholar]
- 22.Hojsak I, Kolaček S. Probiotics and prebiotics in the prevention of respiratory tract infections. In: Orel R, editor. Intestinal microbiota, probiotics and prebiotics. Ljubljana: Institute for Probiotics and Functional Foods, Ltd; 2014. pp. 117–128. [Google Scholar]
- 23.Gutierrez-Castrellon P, Lopez-Velazquez G, Diaz-Garcia L, Jimenez-Gutierrez C, Mancilla-Ramirez J, Estevez-Jimenez J, et al. Diarrhea in preschool children and Lactobacillus reuteri: a randomized controlled trial. Pediatrics. 2014;133:e904–e909. doi: 10.1542/peds.2013-0652. [DOI] [PubMed] [Google Scholar]
- 24.Hatakka K, Savilahti E, Pönkä A, Meurman JH, Poussa T, Näse L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1327. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hojsak I, Močić Pavić A, Kos T, Dumančić J, Kolaček S. Bifidobacterium animalis subsp. lactis in prevention of common infections in healthy children attending day care centers-randomized, double blind, placebo-controlled study. Clin Nutr. 2016;35:587–591. doi: 10.1016/j.clnu.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Hojsak I, Snovak N, Abdović S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2010;29:312–316. doi: 10.1016/j.clnu.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Kumpu M, Kekkonen RA, Kautiainen H, Järvenpää S, Kristo A, Huovinen P, et al. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2012;66:1020–1023. doi: 10.1038/ejcn.2012.62. [DOI] [PubMed] [Google Scholar]
- 28.Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009;124:e172–e179. doi: 10.1542/peds.2008-2666. [DOI] [PubMed] [Google Scholar]
- 29.Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW, et al. Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized, controlled study. Vaccine. 2009;27:1073–1079. doi: 10.1016/j.vaccine.2008.11.114. [DOI] [PubMed] [Google Scholar]
- 30.Merenstein D, Gonzalez J, Young AG, Roberts RF, Sanders ME, Petterson S. Study to investigate the potential of probiotics in children attending school. Eur J Clin Nutr. 2011;65:447–453. doi: 10.1038/ejcn.2010.290. [DOI] [PubMed] [Google Scholar]
- 31.Merenstein D, Murphy M, Fokar A, Hernandez RK, Park H, Nsouli H, et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: the DRINK study. A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur J Clin Nutr. 2010;64:669–677. doi: 10.1038/ejcn.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merenstein DJ, Smith KH, Scriven M, Roberts RF, Sanders ME, Petterson S. The study to investigate the potential benefits of probiotics in yogurt, a patient-oriented, double-blind, cluster-randomised, placebo-controlled, clinical trial. Eur J Clin Nutr. 2010;64:685–691. doi: 10.1038/ejcn.2010.30. [DOI] [PubMed] [Google Scholar]
- 33.Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr. 2004;79:261–267. doi: 10.1093/ajcn/79.2.261. [DOI] [PubMed] [Google Scholar]
- 34.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y, et al. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e4509. doi: 10.1097/MD.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Prevention of hospital-acquired infections. 2nd ed. Geneva: World Health Organization; 2002. [Google Scholar]
- 37.World Health Organization. Report on the burden of endemic health care-associated infection worldwide. Geneva: World Health Organization; 2011. [Google Scholar]
- 38.Hojsak I, Abdović S, Szajewska H, Milosević M, Krznarić Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 2010;125:e1171–e1177. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- 39.Posfay-Barbe KM, Zerr DM, Pittet D. Infection control in paediatrics. Lancet Infect Dis. 2008;8:19–31. doi: 10.1016/S1473-3099(07)70310-9. [DOI] [PubMed] [Google Scholar]
- 40.Ejemot-Nwadiaro RI, Ehiri JE, Arikpo D, Meremikwu MM, Critchley JA. Hand washing promotion for preventing diarrhoea. Cochrane Database Syst Rev. 2015;(9):CD004265. doi: 10.1002/14651858.CD004265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hojsak I, Szajewska H, Canani RB, Guarino A, Indrio F, Kolacek S, et al. Probiotics for the prevention of nosocomial diarrhea in children. J Pediatr Gastroenterol Nutr. 2017 doi: 10.1097/MPG.0000000000001637. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Wanke M, Szajewska H. Lack of an effect of Lactobacillus reuteri DSM 17938 in preventing nosocomial diarrhea in children: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2012;161:40–43.e1. doi: 10.1016/j.jpeds.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 43.Urbańska M, Gieruszczak-Białek D, Szymański H, Szajewska H. Effectiveness of lactobacillus reuteri DSM 17938 for the prevention of nosocomial diarrhea in children: a randomized, double-blind, placebo-controlled trial. Pediatr Infect Dis J. 2016;35:142–145. doi: 10.1097/INF.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 44.Hojsak I, Tokić Pivac V, Močić Pavić A, Pasini AM, Kolaček S. Bifidobacterium animalis subsp. lactis fails to prevent common infections in hospitalized children: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2015;101:680–684. doi: 10.3945/ajcn.114.102004. [DOI] [PubMed] [Google Scholar]