Abstract
The mechanisms involved in sequential immunoglobulin G (IgG) class switching are still largely unknown. Sequential IG class switching is linked to higher levels of somatic hypermutation (SHM) in vivo, but it remains unclear if these are generated temporally during an immune response or upon activation in a secondary response. We here aimed to uncouple these processes and to distinguish memory B cells from primary and secondary immune responses. SHM levels and IgG subclasses were studied with 454 pyrosequencing on blood mononuclear cells from young children and adults as models for primary and secondary immunological memory. Additional sequencing and detailed immunophenotyping with IgG subclass-specific antibodies was performed on purified IgG+ memory B-cell subsets. In both children and adults, SHM levels were higher in transcripts involving more downstream-located IGHG genes (esp. IGHG2 and IGHG4). In adults, SHM levels were significantly higher than in children, and downstream IGHG genes were more frequently utilized. This was associated with increased frequencies of CD27+IgG+ memory B cells, which contained higher levels of SHM, more IGHG2 usage, and higher expression levels of activation markers than CD27−IgG+ memory B cells. We conclude that secondary immunological memory accumulates with age and these memory B cells express CD27, high levels of activation markers, and carry high SHM levels and frequent usage of IGHG2. These new insights contribute to our understanding of sequential IgG subclass switching and show a potential relevance of using serum IgG2 levels or numbers of IgG2-expressing B cells as markers for efficient generation of memory responses.
Immunoglobulin G (IgG) is the most abundant antibody class found in blood and tissue. In humans, there are four IgG subclasses, numbered based on their abundance in serum.1 Despite their high percentage of amino acid sequence identity, these four subclasses differ greatly in function, especially with regards to complement activation and cellular Fc receptor (FcγR) binding. IgG1 and IgG3 function quite similarly, because both bind to all six members of the three FcγR classes: FcγRI, FcγRII and FcγRIII. Furthermore, IgG1 and IgG3 bind to complement C1q.2 In contrast, IgG2 can only bind FcγRIIa and FcγRIIIa with low affinity, and IgG4 binds only FcγRI quite strongly.3 Furthermore, IgG2 and IgG4 are not potent activators of complement.4 Combined, these findings indicate that IgG1 and IgG3 are potent immune-activating subclasses, whereas IgG2 and IgG4 have a more regulatory function.
IgG production by B cells is only induced after these have encountered antigen. Following their generation in bone marrow, all naive B cells express IgM and IgD isotypes. These isotypes are replaced by one of the IgA or IgG subclasses or IgE through genomic recombination of the switch regions upstream of the immunoglobulin heavy chain (IGH) constant genes. This process of IG class switch recombination (CSR) is mediated by the enzyme activation-induced cytidine deaminase (AICDA), which also generates somatic hypermutations (SHM) in the Ig variable domains that form the basis of affinity maturation. AICDA can mutate residues in repetitive regions (switch regions) that are found upstream of each IGH constant gene. IG CSR is guided by cytokine signaling that induces germline transcription over the switch regions and thereby making this available for mutagenesis by AICDA.5
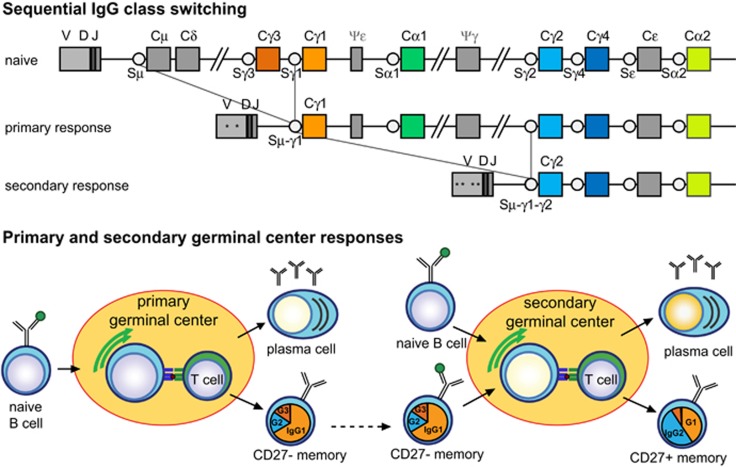
IG CSR is irreversible, because the intervening DNA is removed from the genome. Still, the genetic organization of the IGH constant genes allows for secondary switching to more downstream constant genes. Evidence for sequential in vivo IG CSR has been obtained through sequence analysis of hybrid switch regions that contained a fragment of another switch region, e.g., Sμ-Sγ1-Sγ2.6, 7, 8, 9 Importantly, the hybrid switch regions of the more downstream-located IGHG2 contained more frequently remnants of indirect class switching (24%) than those of IGHG1 (9%).6 Furthermore, IGHG2 transcripts contain higher levels of SHM in Ig variable domains than IGHG1 and IGHG3.10 This can be explained by the fact that both processes are mediated by AICDA and that prolonged exposure results in increased SHM and CSR.10 It remains unclear what would be the cause of longer exposure. One explanation would be that during the course of an immune response B cells that exit the germinal center early will carry lower numbers of mutations, and would be mostly switched to IGHG3 and IGHG1. The cells that remain longer in the germinal center would accumulate more mutations and undergo sequential switching, resulting in more usage of IGHG2 and IGHG4 (Figure 1b). In addition to this temporal model,10, 11 a reentry model can be envisioned where after a primary immune response, the antigen is cleared and resting IG-switched memory B cells will circulate. These will be activated upon secondary encounter by the same antigen and upregulate AICDA, resulting in the accumulation of more SHM and the possibility for sequential IG CSR (Figure 1c).12
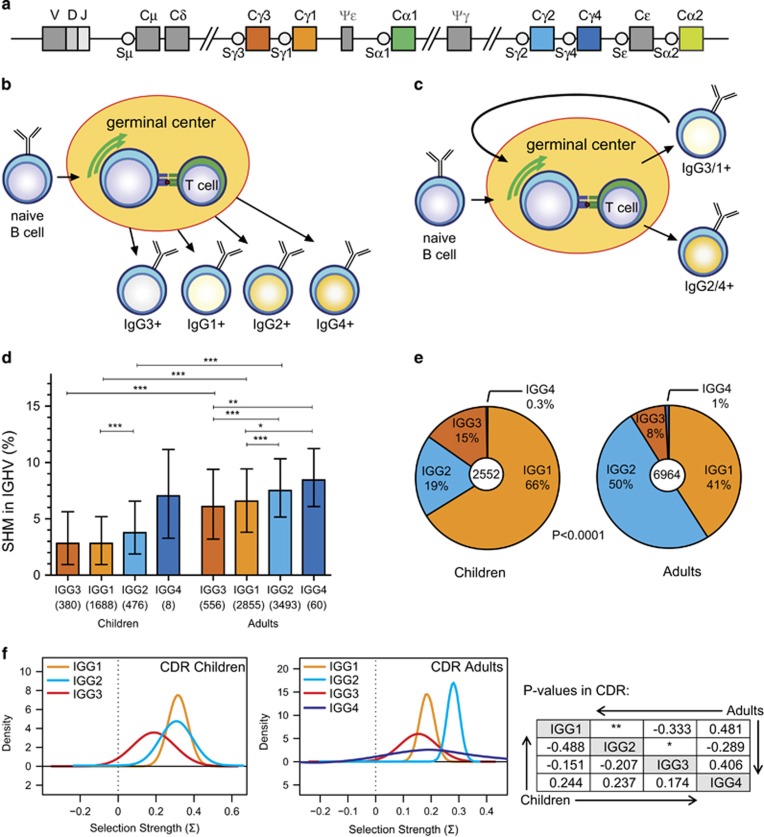
Figure 1.
Somatic hypermutation (SHM) levels and IGHG subclass usage in adults and children. (a) Schematic representation of the human IGH constant gene regions. (b) The temporal model of IgG class switching, where over the course of an immune response sequential switching towards IGHG3>IGHG1>IGHG2>IGHG4 occurs with increasing levels of SHM.10, 11 (c) Model for sequential IgG switching in secondary germinal center responses, where primary IgG memory B cells re-enter a germinal center in a secondary response, undergo additional SHMs and switch to a more downstream IgG subclass.6, 12 (d) SHM levels in IGHG transcripts determined with next generation sequencing (NGS) from nine children (aged 1–10 years) and 14 adults (aged 20–50 years). Data are shown as median bars with interquartile ranges. Statistical analysis of SHM levels between IGHG subclasses, and between children and adults, was done with the Kruskal–Wallis test and Dunn’s multiple comparison test: *P<0.05; **P<0.01; ***P<0.001. (e) IGHG subclass distribution in children and adults based on transcript analysis after next generation sequencing. Statistical analysis of CSR with the χ2 test. (f) Selection for replacement mutations in CDR regions of IGHG transcripts as calculated with the BASELINe program.32, 33 P values for CDR comparisons are shown in tabular format; *P<0.05; **P<0.01.
Studies on dissecting the contribution of both processes to sequential IGHG class switching would require a model system to separate primary from secondary responses. Unfortunately, the mouse IG constant gene domain differs greatly from that in humans and SHM levels are generally low, making it difficult to study sequential IGHG class switching in a specific-pathogen-free animal model. Therefore, we here studied SHM levels and IGHG subclass usages in young children as a model system enriched for primary immune responses, and we analyzed CD27+ and CD27− memory B cells in adults that differ in IGHG subclass usage.6 Together these studies provide new insights into when sequential IgG class switching takes place; does this mainly occur during the primary response, or also after repeated exposure?
Results
Molecular properties of IGHG transcripts in young children and adults
In healthy adults, transcripts involving the IGHM-distal IGHG2 and IGHG4 subclasses have been shown to contain more SHM than IGHG3 and IGHG1.10, 11 As adults have seen many antigens several times, it remains unclear if this phenomenon is the result of sequential switching during one response, or if this occurs in activated memory B cells in the secondary responses. To dissect these two processes, we performed 454 pyrosequencing of variable regions of IGH transcripts from peripheral blood mononuclear cells (PBMCs) of young children (aged 1–10 years) who will have generated fewer memory responses and compared these to adults (20–55 years). After filtering,13, 14 we obtained 2552 unique sequences from nine children (range per child, 65–984) and 6964 sequences from 14 adults (range per adult; 84–1469). From each sequence, the frequency of SHM in IGH variable genes (IGHV) was determined, and the median mutation frequencies were analyzed per IGHG subclass.
We observed a similar increase in SHM levels according to increasing genomic distance from IGHM; IGHG3, IGHG1, IGHG2, IGHG4 in adults (Figures 1a and d). This pattern was less obvious for young children (Figure 1d). Still, IGHG2 transcripts in these children carried significantly more SHM than IGHG1. In general, IGHG transcripts of children and adults differed in SHM frequencies in their variable genes. This was irrespective of the IGHG subclass as children had significantly lower SHM levels in IGHG3, IGHG1 and IGHG2 transcripts (Figure 1d). The numbers of IGHG4 transcripts were too low to properly assess.
All IGHG sequences were generated with a reverse primer that recognized all four IGHG subclasses, making it possible to determine the relative frequencies of all four subclasses in children and in adults. In adults, IGHG2 transcripts were most frequent, followed by IGHG1, IGHG3 and IGHG4 (Figure 1e). The frequencies of IGHG1 and IGHG3 in children were higher than in adults, mostly to the expense of IGHG2. This resulted in distinct patterns of subclass distribution for children and adults, observed for all donors (Supplementary Figure S1).
Despite differences in mutation levels and subclass distribution, IGHG1 and IGHG2 transcripts of children did not differ significantly with regards to selection for replacement mutations in complementarity determining regions (CDR; Figure 1f). In contrast, IGHG2 transcripts of adults did show significantly increased selection for replacement mutations in CDR than IGHG1 transcripts.
Thus, in children who have undergone less memory responses than adults, SHM levels are lower, and downstream IGHG genes were less used. Accumulation of SHM in adults was associated with more usage of downstream IGHG genes and increased selection in CDR in these downstream IGHG subclasses.
Confirmation of SHM and CSR patterns by Sanger sequencing
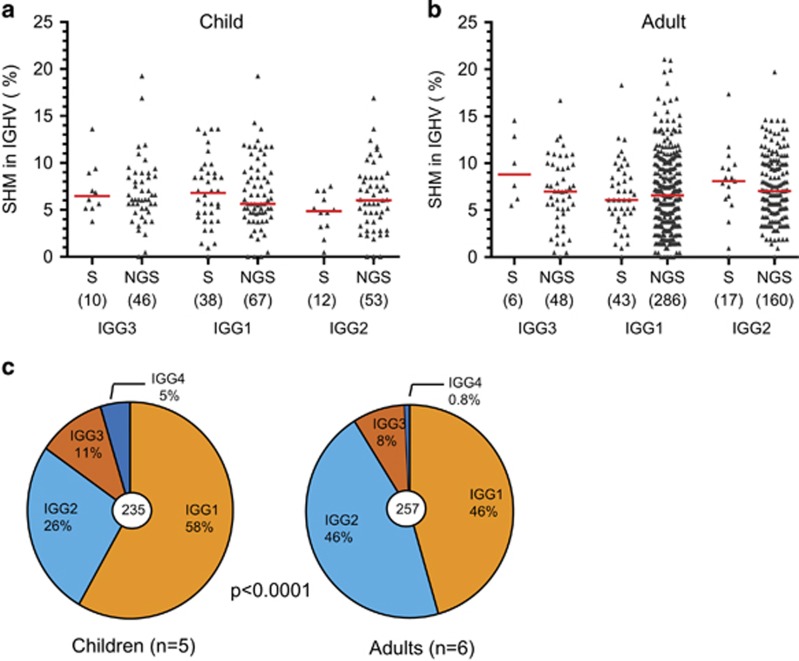
Next generation sequencing techniques are very efficient in generating large amount of data. Still, Sanger sequencing is less sensitive to technical artefacts of the sequencing method.15 To ensure that the observed differences in SHM levels between IGHG subclasses and between adults and children were not affected by technical artefacts, we generated additional data using Sanger sequencing. Mutation levels generated with NGS and Sanger sequencing from the same individuals revealed similar data spread and median values (Figures 2a and b). Furthermore, in combined data sets generated by Sanger sequencing from five children and six adults, we observed similar differences in IGHG gene usage: IGHG3 and IGHG1 subclasses were significantly more frequent in children than in adults (Figure 2c). Thus, in our hands, sequence analysis of IGHG transcripts was not affected by the nature of the sequencing procedure.
Figure 2.
Comparison of SHM levels in IGHG transcripts obtained by Sanger (S) and next generation sequencing (NGS). (a) Results from one child (C9). (b) Results from one adult (A12). Statistical analyses did not reveal any significant differences (Kruskal–Wallis; P>0.05). (c) IGHG subclass distributions in children (n=5) and adults (n=6) based on transcript analysis after Sanger sequencing. Statistics: χ2 test.
SHM and CSR in memory B-cell subsets
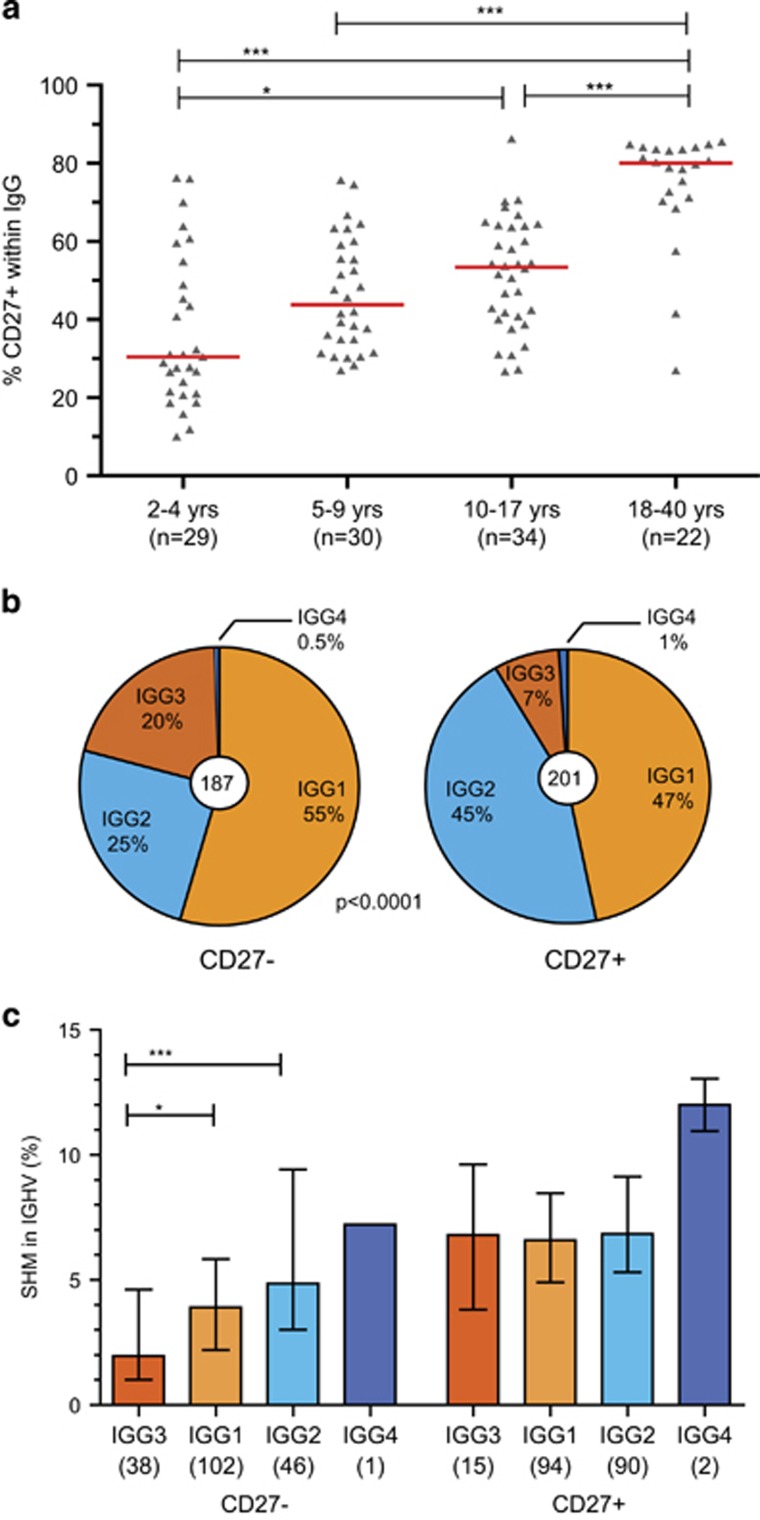
Since we observed that the IgG-expressing B cells in adults are more frequently switched to a downstream IGHG subclass and carry more SHM in their IG variable regions than children, we studied if this difference resulted from an altered cellular composition. We performed flowcytometric immunophenotyping of IgG+ B cells in children and adults between 2–40 years of age. We focused on the proportion of CD27+ B cells as these are reported to carry more SHM and more IgG2 than CD27− memory B cells.6, 16, 17 Indeed, the minority of IgG+ memory B cells in most of the children below 10 years of age were CD27+, while in most adults 80% of the IgG+ B cells were CD27+ (Figure 3a).
Figure 3.
SHM levels and IGHG subclass distributions in memory B cell subsets. (a) The frequencies of IgG+ B cells that express CD27 increase with age. (b) IGHG subclass distributions of CD27+ (eight donors) and CD27− IgG+ memory B-cell subsets (six donors). (c) SHM frequencies in IGHV of IGHG transcripts from memory B-cell subsets. Numbers of transcripts are indicated in brackets. Statistical analysis of SHM was done with the Kruskal–Wallis test and Dunn’s multiple comparison test and of CSR with the χ2 test; *P<0.05; **P<0.01; ***P<0.001.
Further detailed IGH transcript analysis of IgG+ B cell subsets from adults revealed more frequent IGHG2 usage and higher SHM levels in the CD27+ than in the CD27− subset (Figures 3b and c). IGHG3 transcripts in the CD27− subset carried significantly fewer mutations than the more downstream IGHG subclasses. Interestingly, SHM levels in CD27+ cells were similar between IGHG3, IGHG1 and IGHG2 (Figure 3c).
In previous studies, we have shown that both CD27− and CD27+-expressing memory B cells show a pre-activated immunophenotype.6, 18 Detailed gene expression profiling of the CD27+IgG+ and CD27−IgG+ subsets confirmed and extended these observations (Supplementary Table S4). Transcripts of CD58, CD80, CD86, CD95, and TACI were upregulated in both subsets as compared to naive mature B cells. Furthermore, CD22 and CD72 were downregulated. Still, in addition to CD27, CD27+IgG+ memory B cells had significantly more transcripts of CD95 and TACI, and less of CD72, suggestive of a more activated state than CD27−IgG+ cells. In contrast, these CD27−IgG+ memory B cells expressed significantly more CXCR4, CCR7 and IL4R. Together, these results show that with age CD27+ memory B cells accumulate and that these appear to be in a more pre-activated state and less receptive cytokines and chemokines involved in germinal center responses than their CD27− counterparts.
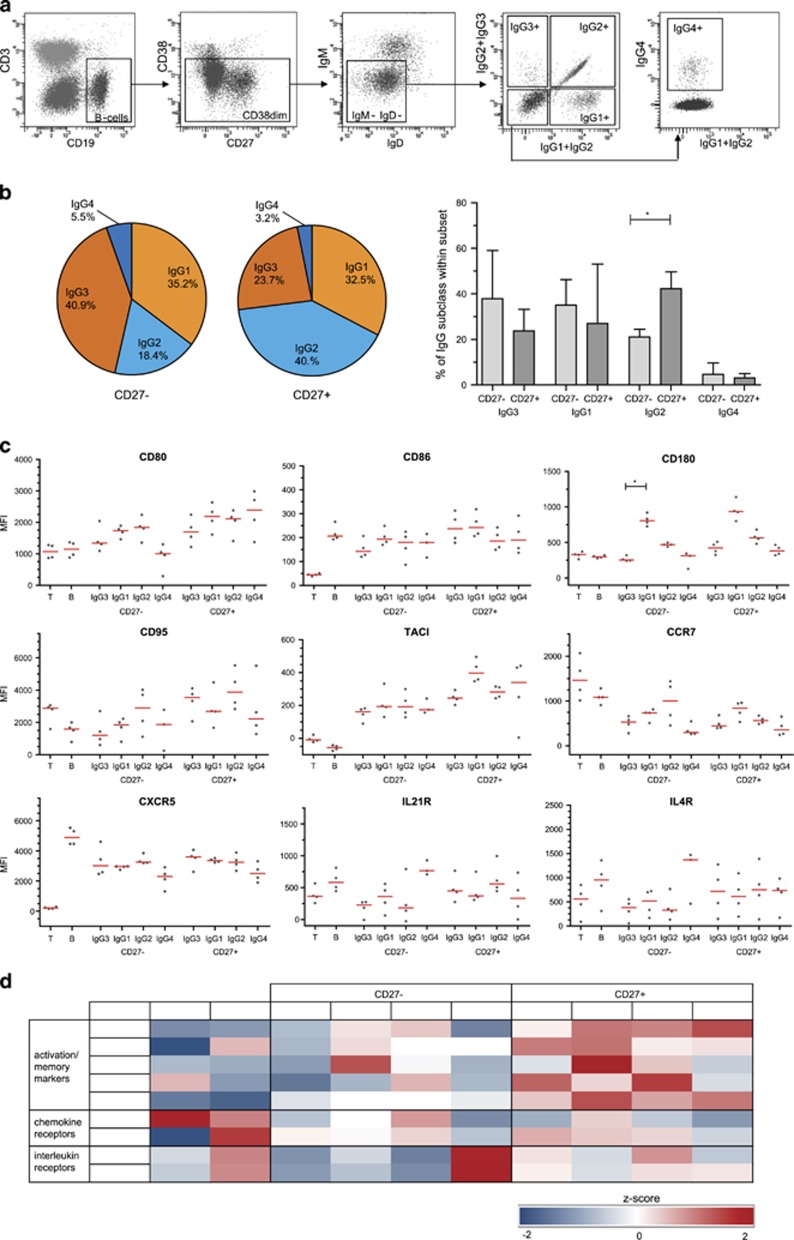
Phenotyping of IgG-subclass expressing memory B-cell subsets
The differences in gene expression profiles of the CD27+ and CD27− subsets of IgG+ memory B cells could be related to their distinct IgG subclass usage. To study this, we performed extensive flowcytometric immunophenotyping using antibodies that are specific for each of the four human IgG subclasses (Figure 4a), and analyzed these separately within the CD27+ and CD27− memory B-cells compartments of healthy adults (Figure 4b). In line with our molecular analysis (Figure 3b), the IgG3 and IgG1 subclasses were more frequently used in CD27− cells, whereas IgG2 usage was significantly higher in CD27+ memory B cells.
Figure 4.
Immunophenotypes of IgG subclass expressing memory B cells. (a) Gating strategy for the detection of IgG subclass expressing memory B cells. (b) IgG subclass distribution based on phenotyping for both CD27− and CD27+ B-cell subsets. Depicted as the average of four healthy adult donors in pie charts, or as median with interquartile range of the four healthy adult donors. (c) Expression of different markers in CD27− and CD27+ IgG-subclass expressing B cells. (d) Heatmap based on z-scores of MFI values of four healthy adult donors. Z-scores were maximized to −2 and 2. CD27+ T cells and naive mature B cells were used as controls.
Subsequently, we analyzed the expression levels of activation markers and typical chemokine and cytokine receptors on the memory B cells expressing the different IgG-subclasses (Figures 4c and d). In general, the expression levels of activation markers were higher on CD27+ than on CD27− memory B cells (Figure 4d). For most markers, except for CD95, this pattern was seen across the subclasses and did not correlate with more downstream positioning in the IGH locus (IgG2 and IgG4). Strikingly, CD180 was highly expressed on both CD27+ and CD27− IgG1-expressing cells. The lymph node homing receptor CCR7 and B-cell follicle homing receptor CXCR5 were expressed on all memory B cells, with only consistently lower levels on IgG4-expressing subsets. In contrast to the microarray data, most CD27+ subsets had higher expression of cytokine receptors IL4 and IL21. Only the CD27−IgG4+ subset had very high levels of both, potentially causing the observed high levels of transcripts observed in the unsorted subsets (Supplementary Table S4). Thus, we here confirm previous observations that CD27+IgG+ memory B cells have a more activated phenotype than CD27−IgG+ B cells.
Discussion
We here provide experimental evidence that sequential IgG subclass switching does not only occur over the course of a single immune response,10, 11 but that usage of Cμ-distal IGHG2 and IGHG4 subclasses with high SHM frequencies increases with age. IGHG2 and IGHG4 transcripts are predominantly present in CD27+ memory B cells, which carry higher expression levels of activation molecules than their CD27− counterparts. Together with our previous findings that CD27+ memory B cells in adults have a more extensive replication history and molecular signs of indirect IgG subclass switching to IgG2, these new results support a model for secondary IgG subclass switching in consecutive germinal center responses (Figure 5).
Figure 5.
Summarizing model for sequential IgG switching during consecutive germinal center responses. In a primary response, naive B cells are activated and can undergo IgG subclass switching prior to differentiation into memory B cells. These cells are predominantly CD27− and utilize IGHG3 or IGHG3. Upon secondary encounter, both naïve and pre-existing CD27− memory IgG1+ B cells enter the germinal center, where the latter can undergo secondary switching resulting in the CD27+ memory B cells which are enriched in IGHG2 transcripts with high levels of SHM.
It is challenging to dissect ex vivo if sequential IGHG class switching in humans has occurred during a primary response or from re-entry of memory B cells into secondary responses.10, 11, 12 Therefore, we here compared SHM levels and IGHG subclasses from young children with adults. SHM is thought to increase with age, although this has not been studied in early childhood in great detail.13, 19, 20 Indeed, SHM levels in adults were significantly higher than in young children, fitting with accumulation during memory responses. Importantly, this accumulation with age was associated with more frequent usage of IGHG2 at the expense of IGHG3 and IGHG1. Naturally, not all memory B cells in children will be derived from primary responses only. Repeated vaccinations and repeated exposure to pathogens will have resulted in memory responses. However, chance of repeated exposures will be much higher in adults, and the resulting composition of the memory B-cell compartment will be much more enriched for those generated from memory responses.
We here showed that SHM is significantly higher in adults compared to children, and that these groups also differ in their distribution of IGHG subclasses. Recently, it was shown that SHM in young children increases with age and stabilizes around 6 years of age.13 With increasing age, IGHG1 usage decreased and IGHG2 usage increased.13 In line with IJspeert et al.,13 we did not find an increase in selection for replacement mutations with age. We here did observe increased selection in transcripts utilizing the Cμ-distal IGHG subclasses, in line with what was found previously.10
Based on previous studies, we hypothesized that secondary IgG memory B cells would have a CD27+ phenotype.6, 16, 17 These cells are highly mutated, have undergone more cell divisions and more frequently utilize the IgG2 subclass than CD27− IgG+ memory B cells.6 Indeed, with age, the frequencies of IgG+ memory B cells expressing CD27 increased, and these contained more SHM, more frequently utilized IgG2 and showed increased expression of activation markers. Importantly, the differences between the subsets did not only result from a different composition of cells utilizing the four distinct IgG subclasses. SHM levels in CD27+ B cells were similarly high in IGHG3, IGHG1 and IGHG2 transcripts. Furthermore, the expression levels of activation markers were not higher in IgG2-expressing than IgG3- or IgG1-expressing cells. Thus, the relative abundance of IgG2 is potentially not the main factor underlying the strength of these memory responses. Rather, all CD27+IgG+ B cells show extensive molecular and cellular signs of immunological memory.
The high mutation frequencies in IGHG3 and IGHG1 transcripts from these memory responses might indicate that there is uncoupling between SHM and sequential class switching, and not all cells switch in consecutive responses. Alternatively, these IgG3- and IgG1-expressing B cells are generated from IgM-expressing memory B cells. This is the main mechanism for secondary responses in mice, where IgM-expressing memory B cells reenter the germinal center and give rise to higher affinity IgM+ and IgG+ memory B cells, whereas IgG+ memory B cells predominantly differentiate directly into IgG+ plasma cells.21 In mice, this could explain or compensate lack of sequential IgG class switching, despite the presence of four IGHG subclasses in the IGH locus.22
Genetic remnants of secondary recombination in humans have previously been detected in IG switch regions of IGHG1 and IGHG2.6 In addition, transcripts from clonally-related cells utilizing distinct IGHG subclasses have been identified in large-scale Ig repertoire studies.13, 23 Interestingly, these B cells did not acquire more SHM, which is again suggestive of uncoupling between SHM and CSR.
SHM could be viewed as a reflection of the history of antigenic exposure. This has been studied previously by comparing mutation levels in people from rural Papua New Guinea and from urban Australia,10, 24 hypothesizing that endemic parasitism would lead to higher SHM levels. Unexpectedly, lifelong exposure to parasites did not increase the IGHG SHM level. This could be explained by the fact that there is a contribution of naive B cells undergoing primary responses. These would yield IgG memory B cells with lower numbers of mutations, resulting in a similar SHM distribution as in individuals with less exposure to endemic parasites. In contrast, chronic inflammatory diseases, such as sarcoidosis and Crohn’s disease do result in increased IGHG2 usage and increased SHM levels.25, 26 Rather than self-limiting immune responses, these diseases are associated with chronic inflammation. Potentially, this prolonged pathogenic stimulation drives SHM in memory B cells. At present, it remains unclear if the increased usage of regulatory IgG2 and IgG4 isotypes affects immunity. One could envisage that these isotypes regulate immune responses to recurrent (harmless) antigens, thereby limiting inflammation.11
In conclusion, we here confirmed and extended previous observations that SHM levels are higher in Cμ-distal IGHG subclasses. On top of a potential temporal contribution to secondary class switching from the Cμ-proximal IGHG3 and IGHG1 to the Cμ-distal IGHG2 and IGHG4, we demonstrate accumulation of IGHG2 with age through reentry. These memory B cells generated from secondary immune responses express CD27 and higher levels of activation markers. These new insights contribute to our understanding of sequential IgG class switching and show a potential relevance of using serum IgG2 levels or numbers of IgG2-expressing B cells as markers for efficient generation of memory responses.
Methods
Blood samples
Blood samples were collected from healthy pediatric and adult donors after written informed consent was obtained according to the declaration of Helsinki. This study was approved by the Medical Ethics Committee of the Erasmus MC.
RNA isolation and cDNA synthesis
Peripheral blood samples were obtained from healthy donors (Supplementary Table S1). RNA from post-Ficoll mononuclear cells was isolated by using a mammalian RNA Miniprep kit (Sigma-Aldrich, St. Louis, MO, USA) and reverse transcribed with random hexamers.
Sanger Sequencing of IGH gene rearrangements from PBMC
Complete IGH V-D-J gene rearrangements were amplified as described before27 with L-VH forward primers28 or IGHV-FR1 forward primers29 in a one-step PCR with a IGHG consensus reverse primer.6, 18, 28 PCR products were cloned into the pGEM-T easy vector (Promega, Fitchburg, WI, USA) and single clones were prepared for sequencing on an ABI Prism 3130XL (Applied Biosystems, Foster City, CA, USA). Obtained sequences were analyzed using the IMGT database (http://www.imgt.org/IMGT_vquest/vquest) to assign the IGHV, IGHD and IGHJ genes and alleles, and to identify SHM.30, 31 Of each unique clone, the position and frequency of mutations were determined within the IGHV gene (CDR1-FR3). SHM was determined as variations on the best matched V-gene and represented as the percentage of mutations of the total sequenced V-gene nucleotides. The IgG receptor subclasses were determined using the IGH reference sequence (NG_001019). Selection for replacement mutations in framework regions (FR) and CDR was analyzed using Bayesian estimation of Antigen-driven SELectIoN (BASELINe; http://selection.med.yale.edu/baseline/).32, 33
Next generation sequencing of IGH gene rearrangements from PBMC
IGH transcripts were amplified in a multiplex PCR using six IGHV-subgroup consensus primers in FR1,29 and a universal IgG reverse primer.28 The PCR products were purified and sequenced using Roche 454 sequencing as previously described.13 In short, PCR products were purified by gel extraction (Qiagen, Valencia, CA, USA) and Agencourt AMPure XP beads (Beckman Coulter, Fullerton, CA, USA). Subsequently, the PCR concentration was measured using the Quant-it Picogreen dsDNA assay (Invitrogen, Carlsbad, CA, USA). The purified PCR products were sequenced on the 454 GS junior instrument according the manufacturer’s recommendations.
Data analysis was performed as previously described by IJspeert et al 2016.13 In short, sequences were demultiplexed based on their multiplex identifier sequence and trimmed using the Antigen Receptor Galaxy (ARGalaxy) tool.34 Fasta files were uploaded in IMGT/High V-Quest,35 and subsequently, the IMGT output files were analyzed in the IGGalaxy tool. To exclude low-quality reads, we only included transcripts of which the exact CDR1-CDR3 nucleotide sequence occurred twice or more.14 Information on junction characteristics, CDR3 length, and composition were extracted from the output provided by IMGT/High V quest using ARGalaxy.34 Of each unique clone, the position and frequency of mutations were determined within the IGHV gene (CDR1-FR3). SHM was determined as variations on the best matched V-gene and represented as the percentage of mutations of the total sequenced V-gene nucleotides. The IgG receptor subclasses were determined using the IGH reference sequence (NG_001019). Selection for replacement mutations in FR and CDR was analyzed using Bayesian estimation of Antigen-driven SELectIoN (BASELINe; http://selection.med.yale.edu/baseline/).32, 33 The FASTQ files of the raw and filtered data are available from the European Nucleotide Archive (project number PRJEB15348, Supplementary Table S2).
Isolation and IG gene transcript analysis of B-cell subsets
B cells were isolated from buffy coat post-Ficoll mononuclear cells by magnetic separation with CD19 beads (Miltenyi Biotech, Carlsbad, Germany). From these, two memory B-cell populations were sorted which were defined as CD3/56/IgD negative and CD19/IgG positive, and either CD27+ or CD27−. Complete IGH gene rearrangements were amplified from cDNA of these populations as described above using L-VH forward primers28 and an IGHG reverse primer,18, 28 cloned into the pGEMT easy vector (Promega) and prepared for Sanger sequencing as described above.
Cell sorting and gene-expression profiling
Cell sorting and gene-expression profiling were performed on naive and memory B-cell subsets as previously described,18, 36 and all data were deposited in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession numbers E-MEXP-3767 and E-MTAB-3637. In short, expression profiles of naive mature and CD27+ and CD27− IgG memory B cell subsets from three healthy donors were compared based on the perfect-match probe-intensity levels. RMA background removal and quantile normalization were performed, followed by a per-probe set two-way ANOVA (with factors probe and cell type). This resulted in average expression levels for each probe set in each cell type, as well as P values for the significance of the difference between cell types. The P-values were adjusted for multiple testing using Šidák stepdown adjustment, and all differences with adjusted P-values <0.05 were considered significant.37
Flowcytometric immunophenotyping of IgG subclass expressing B cells
Detailed 10-color flowcytometric phenotyping was performed on blood lymphocytes from four healthy adults. Cell surface expression levels of selected activation and migration markers (Supplementary Table S3) were measured on a 4-laser LSRFortessa flowcytometer (BD Biosciences, San Jose, CA) with standardized instrument settings,38 and analyzed with FACS DIVA software version 8 (BD Biosciences).
Statistical analyses
Statistical analyses were performed with the Mann–Whitney U test, Kruskal–Wallis test, or χ2 test as indicated in Figure legends. P-values <0.05 were considered statistically significant.
Acknowledgments
The authors are indebted to Drs JJ Heeringa, D van den Heuvel, PA van Schouwenburg and Mr D van Zessen for sharing their data and for technical support. This work was performed in part in the Department of Immunology in the context of the Molecular Medicine Postgraduate School of the Erasmus MC. Part of this work is supported by a grant for BGL, JJMvD and MCvZ of the University of Amsterdam into the focal point Oral Infections and Inflammation. MCvZ is supported by an NHMRC Senior Research Fellowship (APP1117687) and MvdB is supported by ZonMW Vidi grant 91712323.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
The authors declare no conflict of interest.
Supplementary Material
References
- Schauer U, Stemberg F, Rieger CHL, Borte M, Schubert S, Riedel F et al. IgG subclass concentrations in certified reference material 470 and reference values for children and adults determined with the binding site reagents. Clin Chem 2003; 49: 1924–1929. [DOI] [PubMed] [Google Scholar]
- Schroeder HW, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immun 2010; 125: S41–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth PM, Pietersz GA. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Discov 2012; 11: 311–331. [DOI] [PubMed] [Google Scholar]
- Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014; 5: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol 2008; 26: 261–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 2011; 118: 2150–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowska MA, Heeringa JJ, Hajdarbegovic E, van der Burg M, Thio HB, van Hagen PM et al. Human IgE(+) B cells are derived from T cell-dependent and T cell-independent pathways. J Allergy Clin Immunol 2014; 134: 688–697. [DOI] [PubMed] [Google Scholar]
- Zhang K, Mills FC, Saxon A. Switch circles from IL-4-directed epsilon class switching from human B lymphocytes. Evidence for direct, sequential, and multiple step sequential switch from mu to epsilon Ig heavy chain gene. J Immunol 1994; 152: 3427–3435. [PubMed] [Google Scholar]
- Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity 2008; 28: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Wang Y, Collins AM. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol Cell Biol 2014; 92: 729–733. [DOI] [PubMed] [Google Scholar]
- Collins AM, Jackson KJ. A temporal model of human IgE and IgG antibody function. Front Immunol 2013; 4: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zelm MC. B cells take their time: sequential IgG class switching over the course of an immune response? Immunol Cell Biol 2014; 92: 645–646. [DOI] [PubMed] [Google Scholar]
- Ijspeert H, van Schouwenburg PA, van Zessen D, Pico-Knijnenburg I, Driessen GJ, Stubbs AP et al. Evaluation of the antigen-experienced B-Cell receptor repertoire in healthy children and adults. Front Immunol 2016; 7: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaari G, Vander Heiden JA, Uduman M, Gadala-Maria D, Gupta N, Stern JN et al. Models of somatic hypermutation targeting and substitution based on synonymous mutations from high-throughput immunoglobulin sequencing data. Front Immunol 2013; 4: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou G, Ippolito GC, Beausang J, Busse CE, Wardemann H, Quake SR. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat Biotechnol 2014; 32: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol 2006; 177: 3728–3736. [DOI] [PubMed] [Google Scholar]
- Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 2007; 178: 6624–6633. [DOI] [PubMed] [Google Scholar]
- Berkowska MA, Schickel J-N, Grosserichter-Wagener C, de Ridder D, Ng YS, van Dongen JJM et al. Circulating human CD27−IgA+ memory B cells recognize bacteria with polyreactive Igs. J Immunol 2015; 195: 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatorje EJ, Driessen GJ, van Hout RW, van der Burg M, de Vries E. Levels of somatic hypermutations in B cell receptors increase during childhood. Clin Exp Immunol 2014; 178: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridings J, Nicholson IC, Goldsworthy W, Haslam R, Roberton DM, Zola H. Somatic hypermutation of immunoglobulin genes in human neonates. Clin Exp Immunol 1997; 108: 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S et al. Multiple layers of B cell memory with different effector functions. Nat Immunol 2009; 10: 1292–1299. [DOI] [PubMed] [Google Scholar]
- Collins AM. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol Cell Biol 2016; 94: 949–954. [DOI] [PubMed] [Google Scholar]
- Horns F, Vollmers C, Croote D, Mackey SF, Swan GE, Dekker CL et al. Lineage tracing of human B cells reveals the in vivo landscape of human antibody class switching. Elife 2016; 5: e16578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jackson KJ, Chen Z, Gaeta BA, Siba PM, Pomat W et al. IgE sequences in individuals living in an area of endemic parasitism show little mutational evidence of antigen selection. Scand J Immunol 2011; 73: 496–504. [DOI] [PubMed] [Google Scholar]
- Kamphuis LS, van Zelm MC, Lam KH, Rimmelzwaan GF, Baarsma GS, Dik WA et al. Perigranuloma localization and abnormal maturation of B cells: emerging key players in sarcoidosis? Am J Respir Crit Care Med 2013; 187: 406–416. [DOI] [PubMed] [Google Scholar]
- Timmermans WM, van Laar JA, van der Houwen TB, Kamphuis LS, Bartol SJ, Lam KH et al. B-Cell dysregulation in crohn's disease is partially restored with Infliximab therapy. PLoS ONE 2016; 11: e0160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zelm MC, Bartol SJ, Driessen GJ, Mascart F, Reisli I, Franco JL et al. Human CD19 and CD40L deficiencies impair antibody selection and differentially affect somatic hypermutation. J Allergy Clin Immunol 2014; 134: 135–144. [DOI] [PubMed] [Google Scholar]
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods 2008; 329: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003; 17: 2257–2317. [DOI] [PubMed] [Google Scholar]
- Lefranc MP. IMGT, the international ImMunoGeneTics information system: a standardized approach for immunogenetics and immunoinformatics. Immunome Res 2005; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res 2009; 37 (Database issue): D1006–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaari G, Uduman M, Kleinstein SH. Quantifying selection in high-throughput Immunoglobulin sequencing data sets. Nucleic Acids Res 2012; 40: e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uduman M, Yaari G, Hershberg U, Stern JA, Shlomchik MJ, Kleinstein SH. Detecting selection in immunoglobulin sequences. Nucleic Acids Res 2011; 39 (Web Server issue): W499–W504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJspeert H, van Schouwenburg PA, van Zessen D, Pico-Knijnenburg I, Stubbs AP, van der Burg M. Antigen receptor galaxy: a user-friendly, web-based tool for analysis and visualization of T and B cell receptor repertoire data. J Immunol 2017; 198: 4156–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamyar E, Duroux P, Lefranc MP, Giudicelli V. IMGT((R)) tools for the nucleotide analysis of immunoglobulin (IG) and T cell receptor (TR) V-(D)-J repertoires, polymorphisms, and IG mutations: IMGT/V-QUEST and IMGT/HighV-QUEST for NGS. Methods Mol Biol 2012; 882: 569–604. [DOI] [PubMed] [Google Scholar]
- Berkowska MA, Grosserichter-Wagener C, Adriaansen HJ, de Ridder D, Mirani-Oostdijk KP, Agteresch HJ et al. Persistent polyclonal B-cell lymphocytosis: extensively proliferated CD27+IgM+IgD+ memory B cells with a distinctive immunophenotype. Leukemia 2014; 28: 1560–1564. [DOI] [PubMed] [Google Scholar]
- van Zelm MC, van der Burg M, de Ridder D, Barendregt BH, de Haas EF, Reinders MJ et al. Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J Immunol 2005; 175: 5912–5922. [DOI] [PubMed] [Google Scholar]
- Kalina T, Flores-Montero J, van der Velden VH, Martin-Ayuso M, Bottcher S, Ritgen M et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012; 26: 1986–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.