1. Introduction
β- Galactosidase (commonly known as β-lactase; EC 3.2.1.23) is a multifunctional enzyme that can catalyze the hydrolysis of terminal non-reducing β-d-galactose residues in β-d-galactosides or transfer the galactosyl residue to saccharide acceptors to yield galacto-oligosaccharides (GOS). β-Galactosidase has a variety of applications in food and medical industries such as hydrolysis of lactose in milk, manufacture of galactooligosaccharides (GOS) and treatment of lactose malabsorption [1]. Although β-galactosidase is an ubiquitous enzyme existing in plants, animals and microorganisms, only a few β-galactosidases from Kluveromyces lactis, Aspergillus niger and Aspergillus oryzae are regarded as safe for food related industry applications.
To achieve commercial scale production of β-galactosidase, heterologous expression systems were applied including Saccharomyces cerevisiae and Pichia pastoris [1]. P. pastoris is a methylotrophic yeast with great protein expression potential, and has been used as host for expression of many proteins both experimentally and industrially. P. pastoris has also been used for the extracellular expression of β-galactosidase from Paecilomyces aerugineus [2], Lactobacillus crispatus [3] and strains belonging to Aspergillus spp [4].
Despite its advantage in expression of proteins, P. pastoris system usually needs to be optimized to achieve maximum possible production level for a given protein. In achieving this, potential expression bottlenecks are analyzed and alleviated through perturbing and engineering of P. pastoris at different levels. And this process is often performed in a protein-specific manner, depending on the inherent nature and applications of target protein as well as its interaction with P. pastoris host. Take β-galactosidase for example, while some previous works have reported its successful expression in P. pastoris with reasonably high level, there are still several concerns needing to be addressed before further optimization, for instance: 1) Which kind of promoters are suitable to express β-galactosidase, the inducible or constitutive promoters? The strong AOX1 (alcohol oxidase I) promoter has been the most frequently used one. Nevertheless, the adoption of constitutive promoters has been appreciated in recent years because it does not need methanol to induce the expression, and therefore is safer (especially for food-grade β-galactosidase production) and eases the process control during the fermentation. 2) Unexpected N-glycosylation of foreign proteins are very commonly observed in P. pastoris system and its effects on the activity of expressed proteins remain unpredictable. In some cases, glycosylation is essential for maintaining the activities of expressed enzymes [5], [6], [7], while in other cases, glycosylation can negatively affect the enzyme activity [8], [9], [10]. Although β-galactosidase possesses multiple potential N-glycosylation sites, the effects of N-glycosylation on β-galactosidase activity were rarely investigated. 3) Of thousands of proteins that have been expressed using P. pastoris, the protein expression levels can range from tens of milligrams to tens of grams per liter. β-galactosidase can easily reach several grams per liter of production, which is obviously near the high end of this range. This raises the concern that the high enzyme yield may saturate the protein secretion ability of P. pastoris and thus limit the further improvement of its production level.
In order to address the above concerns and systematically assess P. pastoris system for optimized β-galactosidase, we expressed β-galactosidase from K. lactis and A. oryzae in P. pastoris, evaluated different constitutive promoters in addition to AOX1 promoter and examined co-expression of different chaperones in hope of enhancing the secretion of β-galactosidase in P. pastoris. We equally assess the effect of glycosylation on β-galactosidase activity using OCH1 disrupted strain. Combining these strategies, the production level of β-galactosidase from A. Oryzae reached 1434.75 U/mL in 1 L fermentor, which therefore provided a basis for further optimization and industrial scale production of β-galactosidase in future works.
2. Materials and methods
2.1. Strains and plasmids
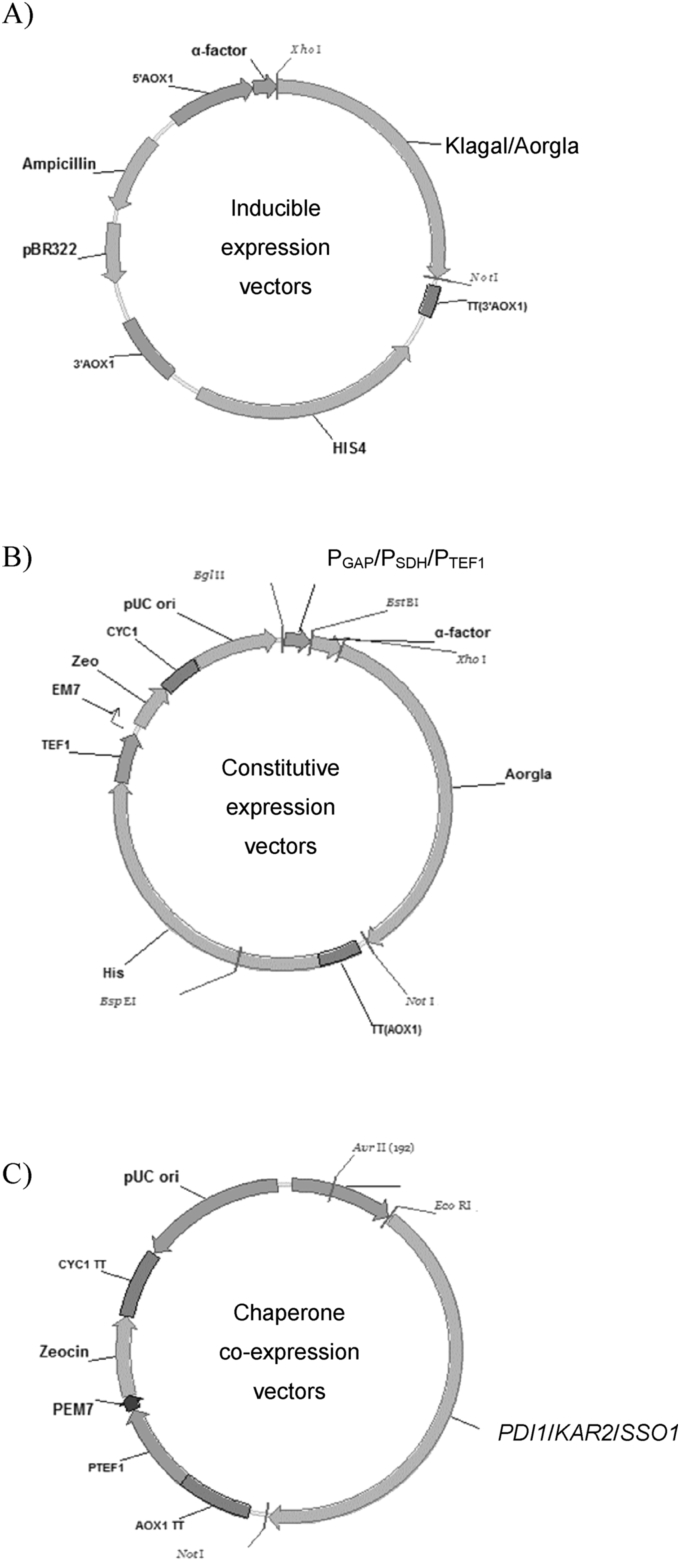
P. pastoris GS115, Escherichia coli DH5αand GS-OCH1 were stored in our lab, K. lactis and A. Oryzae were all purchased from China General Microbiological Culture Collection Center (CGMCC, Beijing, China). Plasmids pPICZαA, pGAPZα and pGAPZB were purchased from Invitrogen (Carlsbad, CA, USA). Information on the strains and plasmids used in this study were reported in Table 1, all primers synthesized by Invitrogen (Beijing, China) were also listed in Table 2 and the construction of recombinant plasmids were detailed in Fig. 1.
Table 1.
Strains and plasmids used in this study.
| Plasmids or strains | Short descriptions | Reference or source |
|---|---|---|
| Plasmids | ||
| pPICZαA | Vector for extracellular expression | Invitrogen |
| pAO815 | Vector for extracellular expression | Invitrogen |
| pAOαMH | Vector for extracellular expression; derived from pPICZαA and pAO815, containing 6 × His tag | This study |
| pAOαMH-Aor | pAOαMH based vector, carryingβ-galactosidase gene from A. oryzae; HIS4, Ampr | This study |
| pAOαMH-Kla | pAOαMH based vector, carryingβ-galactosidase gene from K. lactis; HIS4, Ampr | This study |
| pGAPZα | Vector for extracellular expression; Zeor | Invitrogen |
| pSDHZα | Vector for extracellular expression; Zeor | This study |
| pTef1Zα | Vector for extracellular expression; Zeor | This study |
| pSDHZαH | Vector for extracellular expression; HIS4, Zeor | This study |
| pTef1ZαH | Vector for extracellular expression; HIS4, Zeor | This study |
| pGAPZα-Aor | pGAPZα based vector, carryingβ-galactosidase gene from A. oryzae; Zeor | This study |
| pSDHZαH-Aor | pSDHZαH based vector, carryingβ-galactosidase gene from A. oryzae; HIS4, Zeor | This study |
| pTef1ZαH-Aor | pTef1ZαH based vector, carryingβ-galactosidase gene from A. oryzae; HIS4, Zeor | This study |
| pGAPZB | Vector for intracelluar expression; Zeor | Invitrogen |
| pGAPZ-PDI | pGAPZ based vector, carrying PDI1gene; Zeor | This study |
| pGAPZ-KAR | pGAPZ based vector, carrying KAR2gene; Zeor | This study |
| pGAPZ-SSO | pGAPZ based vector, carrying SSO1gene; Zeor | This study |
| Strains | ||
| E. coli DH 5α | Commercial transformation host for cloning | Takara |
| A. oryzae | CGMCCNumber 3.05232 | CGMCC |
| K. lactis | CGMCCNumber 2.1494 | CGMCC |
| P. pastoris GS115 | Commercial transformation host for Cloning; his4-, Mut+ | Invitrogen |
| GS-OCH1 | GS115 with its OCH1 gene disrupted | Our lab |
| GSG-Aor | GS115integratedwith linearizedpGAPZα-Aor | This study |
| GSS-Aor | GS115integratedwith linearized pSDHZαH-Aor | This study |
| GST-Aor | GS115integratedwith linearizedpTef1ZαH-Aor | This study |
| GSA-Aor | GS115 integrated with inducible β-galactosidase from A. oryzae borne vector | This study |
| GSA-Kla | GS115integratedwith linearizedpAOαMH-KLA | This study |
| GSA-Aor-PDI | GSA-Aor integrated with linearizedpGAPZ-PDI | This study |
| GSA-Aor-KAR | GSA-Aor integrated with linearized pGAPZ-KAR | This study |
| GSA-Aor-SSO | GSA-Aor integrated with linearizedpGAPZ-SSO | This study |
| GSA-Aor-OCH1 | OCH1 disrupted strain integrated with inducible vector β-galactosidase gene from A. oryzae borne vector | This study |
Table 2.
All primers used in this study.
| Name | Sequence (5’→3′) |
|---|---|
| 5-GAP 5-AOX |
gtccctatttcaatcaattgaac gactggttccaattgacaagc |
| 3-AOX | gcaaatggcattctgacatcc |
| SDH-F | gtacagatctaagttgtatattattaatggcgggg |
| SDH-R | gtacttcgaagttggataatagtgagtgtaatga |
| Tef1-F | gtacagatctataactgtcgcctcttttatc |
| Tef1-R | gtacttcgaagttggcgaataactaaaatgta |
| His-F | gatcagatctatgacatttcccttgctacc |
| His-R | gatcggatccttaaataagtcccagtttctc |
| Aor-F | ctcgagaaaagatccatcaagcatcgtctcaatg |
| Aor-R | gcggccgcttagtatgctcccttccgctgc |
| Kla-F | Cttgctcgagaaaagatcttgccttattcctgagaatt |
| Kla-R | cttggcggccgcttattcaaaagcgagatcaaac |
| PDI-F | ggtcgaattcatgcaattcaactgggatat |
| PDI-R | attggcggccgcttaaagctcgtcgtgagcgt |
| KAR-F | ggtcgaattcatgaaagtgacattatctgt |
| KAR-R | attggcggccgcattactctatccctaggggtt |
| SSO-F | ggtcgaattcatgagtaaccagtataatccg |
| SSO-R | attggcggccgcaggtgacagaaatgaaaacgg |
Note: the italic fonts indicate restriction enzyme sites.
Fig. 1.
Schematic representation of the construction of the expression vectors in this work. (A) The inducible expression vectors, pAOαMH-Kla and pAOαMH-Aor, carrying β-galactosidase gene from Aspergillus oryzae and Kluyveromyces lactis, respectively. (B) The constitutive expression vectors, pGAPα-Aor, pSDHZαH-Aor and pTef1ZαH-Aor with PGAP, PSDH, and PTEF1 as promoters. pGAPα-Aor contains no HIS4 gene. (C) The chaperone co-expression vectors, pGAPZ-PDI, pGAPZ-KAR and pGAPZ-SSO carrying PD1, KAR2 and SSO1, respectively.
2.2. Construction of recombinant plasmids
The β-galactosidase gene of K. lactis was amplified by PCR from genomic DNA of K. lactis (Klagal) CGMCC 2.1494 using Kla-F/Kla-R primers. The β-galactosidase gene from A. oryzae (Aorgal) were cloned from the cDNA of its native strain. Total RNA was extracted using RNA pure prep Kit (Tiangen Biotech, Beijing, China) and subjected to reverse transcription to get the single-strand cDNA, followed by PCR amplification using Aor-F/Aor-R primer pairs. The PCR products were inserted into XhoI and NotI site of inducible vector pAOαMH [11] to produce pAOαMH-Kla and pAOαMH-Aor respectively. The constitutive vector pGAPZα was double digested by XhoI and NotI for subsequent integration of DNA fragment containing Aorgal gene to produce pGAPZα-Aor. Promoter SDH AND TEF1 were cloned from genomic DNA of P. pastoris GS115 using SDH-F/SDH-R and Tef1-F/Tef1-R respectively and then double digested by BglII and BstBI prior to insertion into the same sites of pGAPZα to replace Gap promoter to obtain pSDHZα and pTef1Zα. A His fragment was double digested by BglII and BamHI and inserted into BamHI site of pSDHZα or pTef1Zα for integration into GS115 to produce pSDHZαH or pTef1ZαH. Aorgal gene was subsequently inserted into XhoI and NotI sites of pSDHZαH and pTef1ZαH to generate pSDHZαH-Aor and pTef1ZαH-Aor. The chaperone genes of PDI1, KAR2 and SSO1 were cloned from genomic DNA of P. pastoris GS115 using PDI-F/PDI-R, KAR-F/KAR-R and SSO-F/SSO-R respectively and double digested by EcoRI and NotI for subsequent insertion into intracellular expression vector pGAPZB thus generating the recombinant plasmids pGAPZ-PDI, pGAPZ-KAR and pGAPZ-SSO.
2.3. The generation of recombinant P. pastoris
All transformation with P. pastoris GS115 was performed by electroporation according to Invitrogen protocol. The recombinant vectors pAOαMH-Aor, pSDHZαH-Aor and pTef1ZαH-Aor were linearized by BspEI while pAOαMH-Kla by StuI. Transformants were screened on minimal plates (MD per liter: YNB 13.4 g, biotin 0.4 mg, glucose·H2O 20 g, and agar 20 g) and designated as GSA-Aor, GSS- Aor, GST- Aor and GSA-Kla respectively. The glycosylated P. pastoris GS-OCH1 [11] was transformed with BspEI linearized pAOαMH-Aor and transformants were designated as GSA-Aor-OCH1 upon screening on MD plates. For constitutive recombinant plasmid, GS115 was transformed with pGAPZα-Aor linearized with AvrII and for co-expression of chaperone genes, P. pastoris GSA-Aor was transformed with AvrII linearized pGAPZ-PDI, pGAPZ-KAR and pGAPZ-SSO and transformants were selected on YPD (per liter: yeast extract 10 g, peptone 20 g, glucose·H2O 20 g, agar 20 g and Zeocin 40 mg) and designated as GSG-Aor GSA-Aor-PDI, GSA-Aor-KAR and GSA-Aor-SSO respectively.
2.4. Shake-flask fermentation
For assessing β-galactosidase production, the constructed P. pastoris strains were pre-incubated on YPD at 30 °C until a stationary phase is reached. 1 mL of constitutive expression strains were re-inoculated into 25 mL BMGY (per liter: mono-potassium phosphate 8.7 g, YNB 13.4 g, biotin 0.4 mg, peptone 20 g, yeast extract 10 g, glucose·H2O 20 g; pH 6.0) while inducible expression strains were inoculated into BMMY (same as BMGY without glucose·H2O) in 250 mL shake flask and cultured at 30 °C, 200 r/m. The induction phase was initiated by adding 200 μL absolute methanol to each flask following subsequent methanol feeding at 12 h interval for 96 h.
2.5. High density fermentation
A 1-L stirred tank reactor (Infors, Switzerland) was used in the fermentation of GSA-Aor-KAR with 0.8 L of medium contained (per liter): 23.7 mL H3PO4, 0.6 g CaSO4·2H2O, 9.5 g K2SO4, 7.8 g MgSO4·7H2O, 2.6 g KOH, 40 g glycerol supplemented with 4.2 g histidine and 4.4 mL of trace salts. The following culture conditions were applied: 30 °C, pH 6.0 controlled by NH3 (25%) and dissolved oxygen (DO) controlled between 10 and 30% by stirrer (500–1000 rpm) with air flow rate at 2 L/min. A conventional P. pastoris fermentation protocol containing four phases was adopted: starting with a batch growth phase (phase I) lasting between 18 and 22 h followed by a glycerol (85% w/v) fed-batch phase (phase II) until OD600 reached 200. A transition phase (phase III) preceded by 30–60 min of starvation, began with induction of (0.2%) methanol until cells adapted to methanol metabolism followed by methanol feeding phase (phase IV) for 96 h. Cell growth was determined at OD600 and samples stored at −20 °C at 12 h interval.
2.6. Enzyme assays and other analyses
β-galactosidase activity was determined as described by Katrolia et al. [2]. 25 μL of fermented supernatant (diluted with 0.1 mol/L sodium acetate buffer, pH 5.2) was added to 100 μL of reaction mixture consisting of 0.25% (w/v) oNPG in 0.1 mol/L sodium acetate buffer (pH 5.2) and incubated at 60 °C for 10 min. The reaction was quenched by adding 125 μL of 1 M Na2CO3 and o-nitrophenol (oNP) was measured at 420 nm. One unit of β-galactosidase was defined as 1 μmol of oNP released per minute. For enzymatic glycosylation (EM) of N-linked glycans, 10 μg of β-galactosidase was denatured with 1 × glycoprotein denaturing buffer (0.5% SDS, 40 mmol/L DTT) at 100 °C for 10 min prior to the addition of 1 × glycoprotein reaction buffer. Two-fold dilutions of Endoglycosidase H (Endo H, New England Biolabs Beijing, China) were added and the reaction mix were incubated for 1 h at 37 °C. The extracellular proteins and the separation of reaction products of EM were analyzed on 10% SDS-PAGE stained with Coomassie blue.
3. Results
3.1. Expression of different sources of β-galactosidase genes in P. pastoris GS115
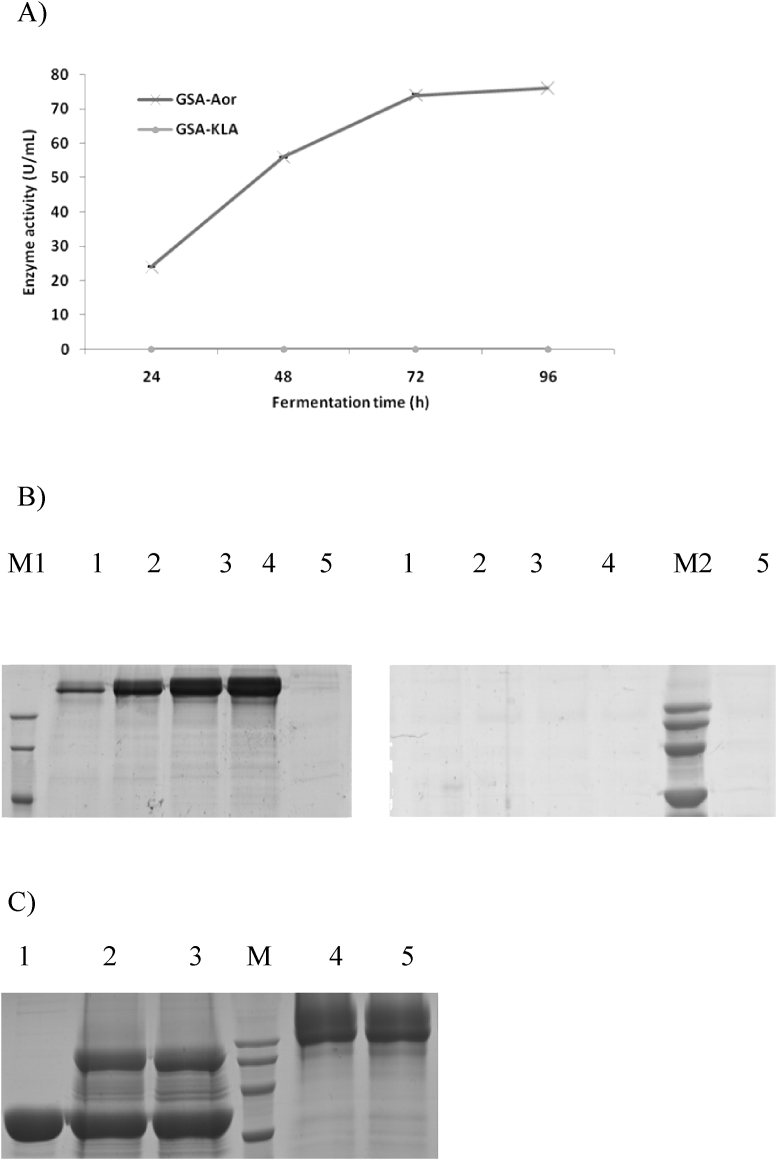
β-galactosidase genes were amplified from K. lactis and A. oryzae using genomic DNA and cDNA respectively. Both genes were placed under AOX1 promoter by inserting them into the secretory expression vector pAOαMH and then transformed into P. pastoris GS115. The generated recombinant strains GSA-Kla and GSA-Aor were then evaluated for their enzyme expression in shake flasks. After 96 h of induction, a final activity of 76.06 U/mL was achieved for Aorgal (Fig. 2A) and the time course accumulation of proteins was also observed during the induction process (Fig. 2B). In contrast, neither protein expression nor β-galactosidase activity could be detected for GSA-Kla revealing unsuccessful expression of Klagal in P. pastoris. Therefore, only β-galactosidase derived from A. oryzae was used for the rest of the studies.
Fig. 2.
Expression of β-galactosidase gene from Aspergillusoryzae and Kluyveromyces lactis in Pichia pastoris GS115 with inducible AOX1 promoter. (A) Comparison of the β-galactosidase expression levels of GSA-Aor and GSA-Kla in shake-flask cultures. Three parallel flasks were tested for each strain. (B) SDS-PAGE of β-galactosidase from GSA-Aor and GSA-Kla cultures. Samples were subjected to 10% SDS-PAGE and stained with Coomassie blue. Lane M1, the protein molecular weight standards (94 kDa, 66 kDa, 45 kDa); Lane M2, the protein molecular weight standards (120 kDa, 100 kDa, 80 kDa, 60 kDa); Lane 1–4, supernatant from cultures of GSA-Aor (left) and GSA-KLA (right) sampled at 24 h, 48 h, 72 h and 96 h. Lane 5, supernatant from cultures of P. pastoris GS115 at 96 h (C) SDS-PAGE of β-galactosidase from GSA-Aor at 96 h and treated with Endo H. Lane 1, Endoglycosidase H (Endo H); Lane 2–3, supernatants from culture of GSA-Aor treated with Endo H; Lane 4–5, supernatants from culture of GSA-Aor; Lane M, the protein molecular weight standards (120 kDa, 100 kDa, 80 kDa, 60 kDa).
Despite observed remarkable expression, the Aorgal showed extensive diffusion and smearing on the SDS-PAGE and the average molecular mass of the enzyme was larger than the calculated value (108.1 kDa) thereby suggesting a high degree of glycosylation of the recombinant enzyme. Further Endo H treatment of the samples showed that the band heterogeneity was considerably reduced and the average molecular mass of the β-galactosidase was closer to the theoretical size confirming the glycosylation of β-galactosidase in P. pastoris (Fig. 2C).
3.2. Comparison of constitutive promoters for expression of β-galactosidase
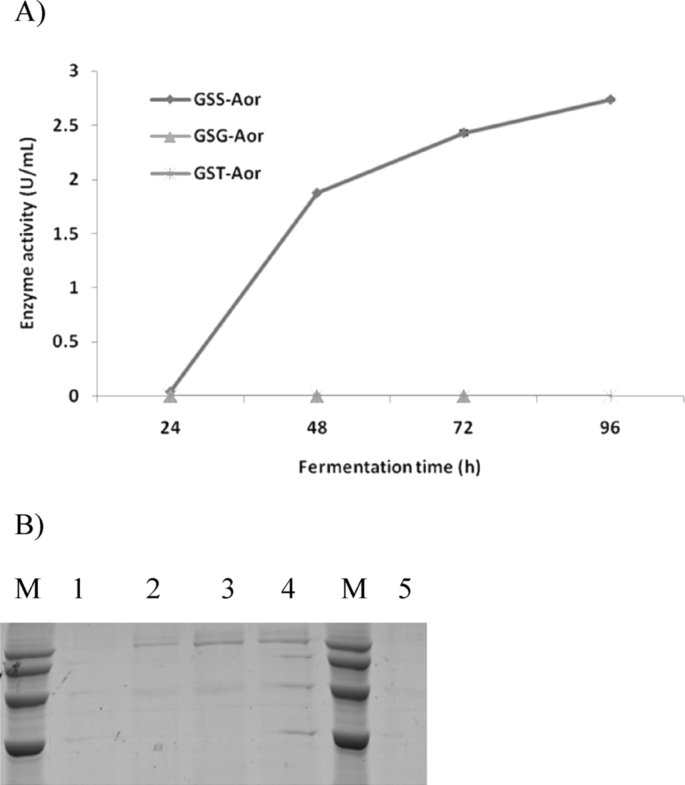
The effect of constitutive promoter was also assessed by placing Aorgal under the control of some of the strongest constitutive promoters reported to date which include GAP (glyceraldehyde 3-phosphate dehydrogenase), SDH (sorbitol dehydrogenase) and TEF1 (elongation factor 1-alpha) promoters. The generated recombinant expression vectors pGAPα-Aor, pSDHZαH-Aor and pTef1ZαH-Aor were then transformed into P. pastoris GS115, resulting in the positive recombinant strains GSG-Aor, GSS-Aor and GST-Aor respectively. A 96 h cultural system in BMGY medium revealed that neither GSG-Aor nor GST-Aor exhibited detectable enzyme activity in the fermentation broth and only 2.74 U/mL of β-glactosidase was detected for GSS-Aor, which was significantly lower than that obtained using AOX1 promoter (Fig. 3A). SDS-PAGE results indicated that the protein expression levels of GSS-Aor were also very low compared with GSA-Aor (Fig. 3B).
Fig. 3.
Expression of β-galactosidase gene from Aspergillus oryzae with different constitutive promoters. (A) The enzyme activities of β-galactosidase in GSS-Aor, GSG-Aor and GST-Aor in shake-flask cultures. β-Galactosidase activities were assayed over the time course for 96 h. Three parallel flasks were tested for each strain. (B) SDS-PAGE of β-galactosidase from GSS-Aor culture. Samples were subjected to 10% SDS-PAGE and stained with Coomassie blue. Lane M, the protein molecular weight standards (120 kDa, 100 kDa, 80 kDa, 60 kDa).; Lane 1–4, supernatant from cultures of GSS-Aor sampled at 24 h, 48 h, 72 h and 96 h. Lane 5, supernatant from cultures of P. pastoris GS115 at 96 h.
3.3. The effects of OCH1 knock-out strain on the β-galactosidase expression
In vitro experiment using Endo H treatment confirmed that β-galactosidase was expressed as a glycoprotein in P. pastoris. To investigate the effects of glycosylation on β-galactosidase expression, we attempted to produce the enzyme in a P. pastoris strain with disrupted OCH1 gene (denoted as GS-OCH1) which encodes α-1,6-mannosyltransferases that initiates the first step of out-chain elongation of high mannose type N-glycan in P. pastoris since previous reports showed that disruption of OCH1 could significantly reduce the hyper-glycosylation and increase the homogeneity of expressed proteins in P. pastoris.
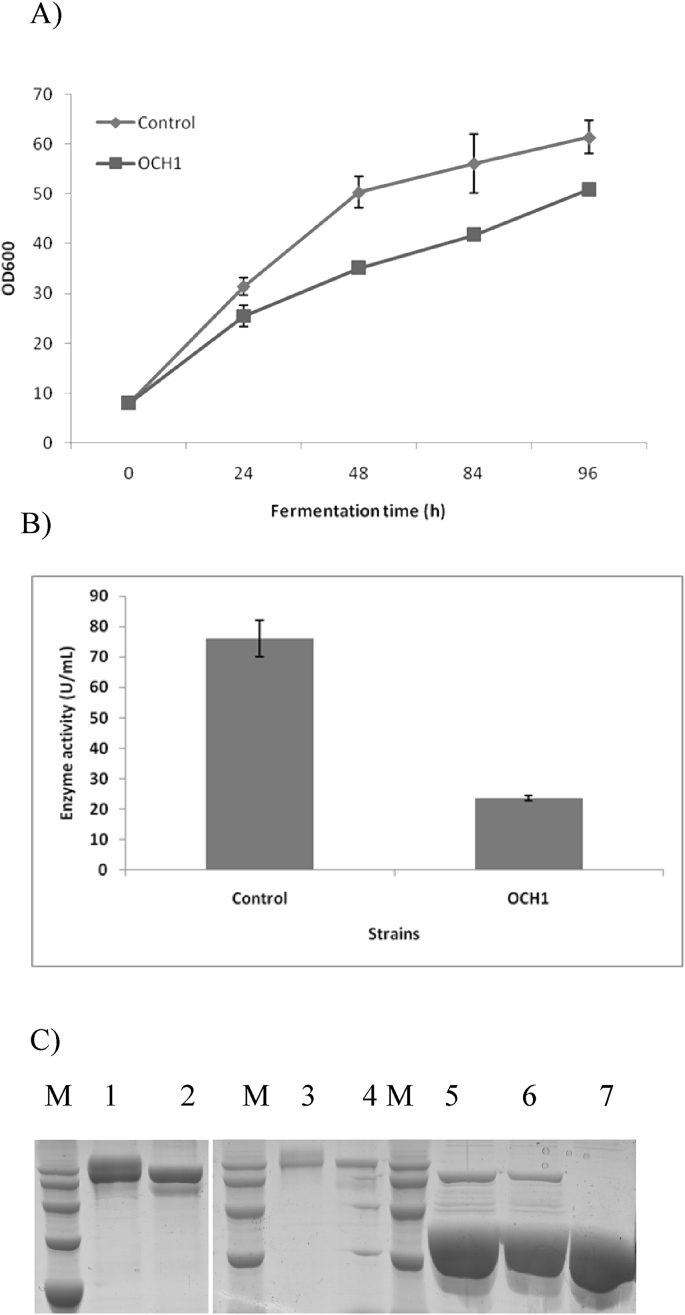
The expression plasmid pAOαMH-Aor was transformed into the GS-OCH1 and generated the recombinant strain GSA-Aor-OCH1 which was then compared with GSA-Aor for β-galactosidase expression. As expected, SDS-PAGE analysis showed that the heterogeneity of glycoproteins was remarkably reduced in GSA-Aor-OCH1 as only one single band could be observed. However, GSA-Aor-OCH1 only reached a production level of 23.58 U/mL, a 31% value of the control strain (76.06 U/mL) (Fig. 4B, Table 3). The protein level of GSA-Aor-OCH1 (94 mg/L) was also 44% lower than that of GSA-Aor (168 mg/L) which could probably be attributed to its relatively slow growth (Fig. 4A and C). The specific enzyme activity of GSA-Aor-OCH1 was estimated to be 250.85 U/mg, 44.6% lower than the value of 452.76 U/mg achieved for GSA-Aor, showing that deglycosylated protein was less active than the glycosylated counterparts.
Fig. 4.
Effect of OCH1 disruption on β-galactosidase expression level. (A) Comparison of the growth curves between GSA-Aor (Control) and GSA-Aor-OCH1 in shake-flask cultures. (B) Comparison of the β-galactosidase expression levels between GSA-Aor (Control) and GSA-Aor-OCH1 in shake-flask cultures. β-Galactosidase activities were assayed at the end of fermentation for 96 h. Three parallel flasks are tested for each strain. (C) SDS-PAGE of β-galactosidase between GSA-Aor and GSA-Aor-OCH1 in shake-flask cultures. Samples were subjected to 10% SDS-PAGE and stained with Coomassie blue. Lane M, the protein molecular weight standards (120 kDa, 100 kDa, 80 kDa, 60 kDa); Lane 1, supernatant from culture of GSA-Aor; Lane 2, supernatant of GSA-Aor-OCH1; Lane 3, supernatant of GSA-Aor (five-time dilution); Lane 4, supernatant of GSA-Aor-OCH1 (five-time dilution); Lane 5, supernatant of GSA-Aor (five-time dilution) treated with Endo H; Lane 6, supernatant of GSA-Aor-OCH1 (five-time dilution) treated with Endo H; Lane 7, Endo H.
Table 3.
β-galactosidase expression levels of GSA-Aor and GSA-Aor-OCH1 in shake-flask cultures.
| Strains | Enzyme production (U/mL) | Protein production (mg/mL) | Crude specific activity (U/mg) |
|---|---|---|---|
| GSA-Aor | 76.06 | 0.168 | 452.76 |
| GSA-Aor-OCH1 | 23.58 | 0.094 | 250.85 |
3.4. Effect of co-expression of chaperone genes on the β-galactosidase expression level
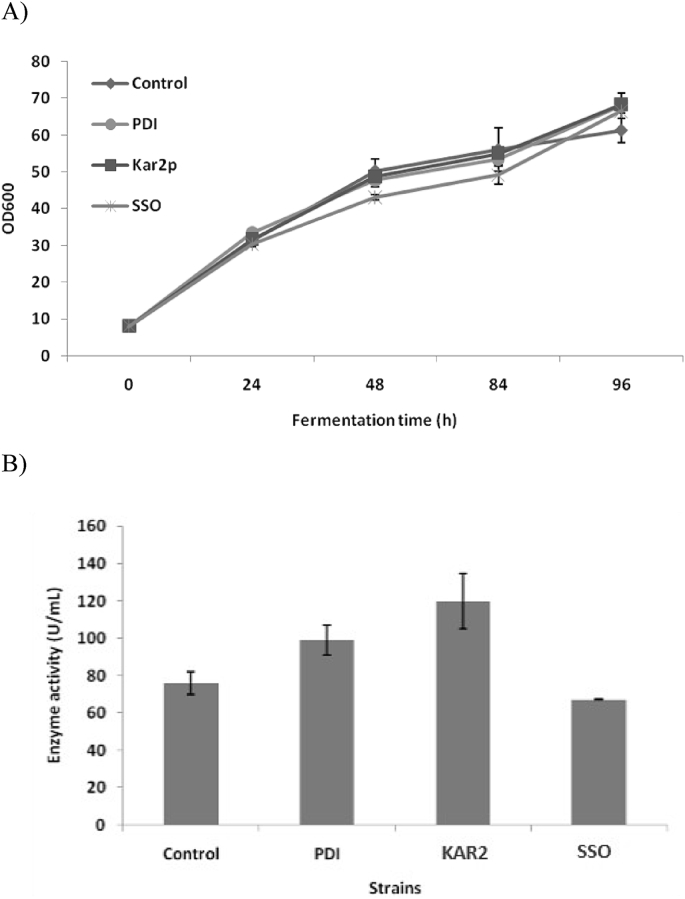
Optimization of β-galactosidase expression by co-expressing chaperones PDI1, KAR2 and SSO1 from pastoris GS115 genome to generate GSA-Aor-PDI, GSA-Aor-KAR and GSA-Aor-SSO revealed no significant difference in cell growth between the three strains and the control GSA-Aor (Fig. 5A) while the β-galactosidase expression of GSA-Aor-SSO remained almost the same as that of control (Fig. 5B). The enzyme expression levels of GSA-Aor-PDI and GSA-Aor-KAR reached 98.88 and 119.81 U/mL, 30% and 57.51% higher than that of GSA-Aor (76.06 U/mL) respectively, while the SDS-PAGE results also confirmed that co-expression of chaperone genes of KAR2 or PDI1 could improve the secretion of β-galactosidase (Fig. 4C).
Fig. 5.
Effects of co-expression of chaperone genes on β-galactosidase expression. The chaperone genes PDI1, KAR2 and SSO1 were all driven by GAP promoter, and the GSA-Aor was used as a control. (A) Growth curves of co-expression strains. (B) β-galactosidase expression levels of co-expression strains. β-Galactosidase activities were assayed at the end of fermentation for 96 h. Three parallel flasks are tested for each strain.
3.5. High level expression of β-galactosidase by high density fermentation
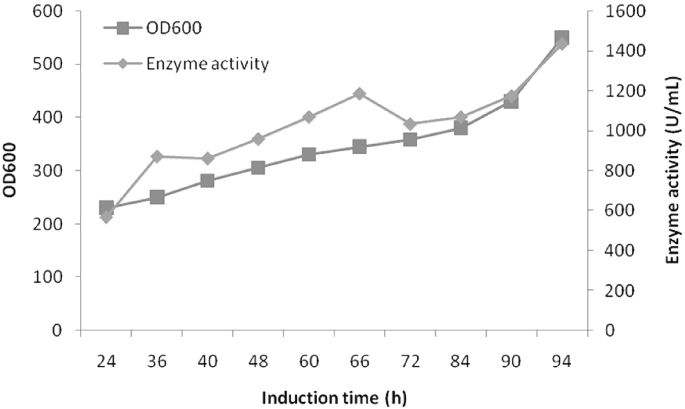
The β-galactosidase producing potential of GSA-Aor-KAR was further investigated by high density fermentation in a 1-L fermentor. The cell growth of GSA-Aor-KAR increased steadily during the entire 94 h fermentations and a final OD600 value of 550 (estimated to be 138 gDCW/L) was obtained (Fig. 6). The β-galactosidase activity kept increasing after induction, which was also confirmed by building up of secreted proteins on SDS-PAGE (data not shown). The maximum volumetric β-galactosidase productivity reached 1434.75 U/mL at 94 h of induction, which was 17.9-fold of that in shake-flask cultivation (Fig. 6).
Fig. 6.
High-density culture of GSA-Aor-KAR in 1 L scale fermentor. Data shown are mean values from experiments performed in triplicate. Closed square, cell growth; closed diamond, β-galactosidase activity.
4. Discussion
In this study, P. pastoris was used as host to express β-galactosidase from K. lactis and A. oryzae both of which are regarded safe for food related industrial applications. The K. lactis β-galactosidase is produced industrially by intracellular expression in its native host [1]. Due to the high cost associated with its extraction and following downstream process, secretory expression of K. lactis β-galactosidase was explored in K. lactis and S. cerevisiae [13], [14], but only trace amount of enzyme activities could be detected in these works, suggesting that Klagal may not be suitable for extracellular expression due to its inherent nature. On the contrary, the Aorgal is a native extracellular protein and have shown successful expression in P. pastoris in this work as well as previous reports [4].
Despite the significant expression level of β-galactosidase on inducible strong AOX1 promoter, different reports has raised concern on the use of AOX1 promoter ranging from drawbacks during process scale up, sophisticated operation and longer fermentation period to safety issues raised as a result of large amount of methanol used during the process [15], [16]. Constitutive promoters were therefore applied as alternatives to overcome these problems [17]. While the GAP promoter was the most commonly used constitutive promoter in P. pastoris system [18], other promoters like TEF1 [19] and SDH [20] were also reported to have promoting strength comparable to GAP. These three promoters were thereby evaluated and compared. Unfortunately, GAP and TEF1 promoters exhibited no expression while only trace expression was noticed on SDH promoter. The reason for this is still not clear yet. β-galactosidase contains multiple potential glycosylation sites and are expressed in gram-per-liter level which might cause a severe folding stress upon P. pastoris and subsequently impaired cell growth and even decreased stability of Aorgal gene [21]. The dissociation of cell growth with induction phase as applied in inducible expression under AOX1 promoter would help to minimize this adverse effects and thus remarkably improve the protein expression level.
N-glycosylation is ubiquitous in eukaryotic systems, where the asparagine residues within the N-X-S/T (X is any amino acid except proline) sequence is glycosylated by glycotransferases. Although not as hyper-mannosylated as S. cerevisiae (adding up to 50 mannoses), the N-glycans of P. pastoris are also of the high mannose type (8–14 mannoses) [22], [23]. The effect of glycosylation on expressed proteins in P. pastoris are unpredictable and vary on a case-by-case basis. For β-galactosidase, a previous work showed that removal of glycans would decrease the specific activity of the β-galactosidase from P. aerugineus by treating the enzyme with Endo H in vitro [2]. In this work, we investigated the effect of glycosylation on β-galactosidase with an in vivo strategy through the use of an OCH1 disrupted strain. OCH1 encoding the α-1, 6-mannosyltransferase is responsible for triggering the afterwards outer-chain elongation of N-glycans [24], [25] and disruption of OCH1 would thus eliminate hyper-mannosylation of glycoproteins. As shown in this work, knockout of OCH1 successfully generate a more unified β-galactosidase protein band. Unfortunately, the prevention of hyper-glycosylation of β-galactosidase seems to decrease the specific activity of β-galactosidase, which is in accordance with previous report [24], [25], thereby suggesting certain degree of glycosylation was necessary for maintaining the activity of this enzyme.
Previous work showed β-galactosidase can easily reach gram/liter production. This large protein synthesis flux was assumed to cause the overloading on the secretion capacity of P. pastoris. We therefore confirm this hypothesis and overcome the limitation by co-expressing chaperone genes. Three chaperone genes relating to protein folding, disulfide bond formation and protein transporting which includes KAR2 (or BiP, a ER-resident chaperone of the HSP70 class that mediate protein folding in the ER), PDI1 (a chaperone that is responsible for the correct formation of disulfide bonds during oxidative folding) and SSO1 (involved in fusion of secretory vesicles at the plasma membrane) were chosen because they are closely related to the folding and formation of disulfide bond. Previous literature has reported that the three chaperones could significantly improve the secretion of heterologous proteins in some cases [24], [25]. Overexpression of PDI1 and KAR2 resulted in 30% and 57.51% increase in the β-galactosidase production due to the increase in protein expression level (data not shown). The positive effects of PDI1 and KAR2 confirmed the secretion bottleneck hypothesis and provided solutions for further improvement of β-galactosidase which has not been reported before.
Finally, the evaluation of the optimized strain on a 1-L fermentor with final production level of 1434.75 U/mL (approximately 2.5 g/L of protein) in 96 h of induction. P. pastoris has been shown to be the ideal host for β-galactosidase expression, and very high enzyme titers were achieved in some cases, e.g. 3.5 g/L of protein for Aspergillus niger β-galactosidase [4], an enzyme level of 22 g/L or 9500 U/ml for P. aerugineus β-galactosidase [2]. This work systematically examined some of the major concerns regarding to high expression of β-galactosidase in P. pastoris and successfully identified enzyme secretion as a potential limiting factor, which will help to guide further improvement of β-galactosidase in P. pastoris system.
Acknowledgments
This work was supported by Key International Cooperation Project from Chinese Academy of Sciences (155112KYSB20160010), Beijing Municipal Natural Science Foundation (5132024) and National Natural Science Foundation of China (31000026).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Taicheng Zhu, Email: zhutc@im.ac.cn.
Yin Li, Email: yli@im.ac.cn.
References
- 1.Oliveira C., Guimaraes P.M., Domingues L. Recombinant microbial systems for improved β-galactosidase production and biotechnological applications. Biotechnol Adv. 2011;29:600–609. doi: 10.1016/j.biotechadv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Katrolia P., Yan Q., Jia H., Li Y., Jiang Z., Song C. Molecular cloning and high-level expression of a β-galactosidase gene from Paecilomyces aerugineus in Pichia pastoris. J Mol Catal B-Enzym. 2011;69:112–119. [Google Scholar]
- 3.Nie C., Liu B., Zhang Y., Zhao G., Fan X., Ning X. Production and secretion of Lactobacillus crispatus β-galactosidase in Pichia pastoris. Protein Expr Purif. 2013;92:88–93. doi: 10.1016/j.pep.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Dragosits M., Pflugl S., Kurz S., Razzazi-Fazeli E., Wilson I.B., Rendic D. Recombinant Aspergillus β-galactosidases as a robust glycomic and biotechnological tool. Appl Microbiol Biotechnol. 2014;98:3553–3567. doi: 10.1007/s00253-013-5192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez D.L.S.A., Cayetano-Cruz M., Gutierrez-Anton M., Santiago-Hernandez A., Plascencia-Espinosa M., Farres A. Improvement of catalytical properties of two invertases highly tolerant to sucrose after expression in Pichia pastoris. Effect of glycosylation on enzyme properties. Enzyme Microb Technol. 2016;83:48–56. doi: 10.1016/j.enzmictec.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Capone S., Corajevic L., Bonifert G., Murth P., Maresch D., Altmann F. Engineering for the production of glyco-engineered horseradish peroxidase C1A in Pichia pastoris. Int J Mol Sci. 2015;16:23127–23142. doi: 10.3390/ijms161023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maestre-Reyna M., Liu W.C., Jeng W.Y., Lee C.C., Hsu C.A., Wen T.N. Structural and functional roles of glycosylation in fungal laccase from Lentinus sp. PLoS One. 2015;10:e120601. doi: 10.1371/journal.pone.0120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranaei S.S., Mollasalehi HHeydarzadeh N. Substrate affinity and catalytic efficiency are improved by decreasing glycosylation sites in Trichoderma reesei cellobiohydrolase I expressed in Pichia pastoris. Biotechnol Lett. 2016;38:483–488. doi: 10.1007/s10529-015-1997-8. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y.L., Chang S.H., Gong X., Wu J., Liu B. Expression, purification and characterization of low-glycosylation influenza neuraminidase in α-1,6-mannosyltransferase defective Pichia pastoris. Mol Biol Rep. 2012;39:857–864. doi: 10.1007/s11033-011-0809-z. [DOI] [PubMed] [Google Scholar]
- 10.Yurimoto H., Yamane M., Kikuchi Y., Matsui H., Kato N., Sakai Y. The pro-peptide of Streptomyces mobaraensis transglutaminase functions in cis and in trans to mediate efficient secretion of active enzyme from methylotrophic yeasts. Biosci Biotechnol Biochem. 2004;68:2058–2069. doi: 10.1271/bbb.68.2058. [DOI] [PubMed] [Google Scholar]
- 11.Zhu T., You L., Gong F., Xie M., Xue Y., Li Y. Combinatorial strategy of sorbitol feeding and low-temperature induction leads to high-level production of alkaline β-mannanase in Pichia pastoris. Enzyme Microb Technol. 2011;49:407–412. doi: 10.1016/j.enzmictec.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Becerra M., Prado S.D., Siso M.I., Cerdan M.E. New secretory strategies for Kluyveromyces lactis β-galactosidase. Protein Eng. 2001;14:379–386. doi: 10.1093/protein/14.5.379. [DOI] [PubMed] [Google Scholar]
- 14.Becerra M., Díaz Prado S., Cerdán E., González Siso M.I. Heterologous Kluyveromyces lactis β-galactosidase secretion by Saccharomyces cerevisiae super-secreting mutants. Biotechnol Lett. 2001;23:33–40. [Google Scholar]
- 15.Zhu T., Sun H., Li P., Xue Y., Li Y., Ma Y. Constitutive expression of alkaline β-mannanase in recombinant Pichia pastoris. Process Biochem. 2014;49:2025–2029. [Google Scholar]
- 16.Cos O., Ramon R., Montesinos J.L., Valero F. Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: a review. Microb Cell Fact. 2006;5:17. doi: 10.1186/1475-2859-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogl TGlieder A. Regulation of Pichia pastoris promoters and its consequences for protein production. N Biotechnol. 2013;30:385–404. doi: 10.1016/j.nbt.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhang A.L., Luo J.X., Zhang T.Y., Pan Y.W., Tan Y.H., Fu C.Y. Recent advances on the GAP promoter derived expression system of Pichia pastoris. Mol Biol Rep. 2009;36:1611–1619. doi: 10.1007/s11033-008-9359-4. [DOI] [PubMed] [Google Scholar]
- 19.Ahn J., Hong J., Lee H., Park M., Lee E., Kim C. Translation elongation factor 1-α gene from Pichia pastoris: molecular cloning, sequence, and use of its promoter. Appl Microbiol Biotechnol. 2007;74:601–608. doi: 10.1007/s00253-006-0698-6. [DOI] [PubMed] [Google Scholar]
- 20.Periyasamy S., Govindappa N., Sreenivas S., Sastry K. Isolation, characterization and evaluation of the Pichia pastoris sorbitol dehydrogenase promoter for expression of heterologous proteins. Protein Expr Purif. 2013;92:128–133. doi: 10.1016/j.pep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Curvers S., Linnemann J., Klauser T., Wandrey C., Takors R. Recombinant protein production with Pichia pastoris in continuous fermentation – kinetic analysis of growth and product formation. Eng Life Sci. 2002;2:229–235. [Google Scholar]
- 22.Krainer F.W., Gmeiner C., Neutsch L., Windwarder M., Pletzenauer R., Herwig C. Knockout of an endogenous mannosyltransferase increases the homogeneity of glycoproteins produced in Pichia pastoris. Sci Rep. 2013;3:3279. doi: 10.1038/srep03279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinna LSTschopp J.F. Size distribution and general structural features of N-linked oligosaccharides from the methylotrophic yeast, Pichia pastoris. Yeast. 1989;5:107–115. doi: 10.1002/yea.320050206. [DOI] [PubMed] [Google Scholar]
- 24.De Pourcq K., De Schutter KCallewaert N. Engineering of glycosylation in yeast and other fungi: current state and perspectives. Appl Microbiol Biotechnol. 2010;87:1617–1631. doi: 10.1007/s00253-010-2721-1. [DOI] [PubMed] [Google Scholar]
- 25.Bobrowicz P., Davidson R.C., Li H., Potgieter T.I., Nett J.H., Hamilton S.R. Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: production of complex humanized glycoproteins with terminal galactose. Glycobiology. 2004;14:757–766. doi: 10.1093/glycob/cwh104. [DOI] [PubMed] [Google Scholar]