Abstract
Background
HIV and TB infections are both associated with elevated oxidative stress parameters. Anti-oxidant supplementation may offer beneficial effects in positively modulating oxidative stress parameters in HIV and HIV-TB infected patients. We investigated the effects of vitamin A and C supplementation on oxidative stress in HIV infected and HIV-TB co-infected subjects.
Methods
40 HIV/TB co-infected and 50 HIV mono-infected patients were divided into 2 equal groups. Participants provided demographic information and blood was collected to determine oxidative stress parameters before and after vitamin A (5000 IU) and C (2600 mg) supplementation for 1 month.
Results
There was a significantly (p < 0.05) higher level of Malondialdehyde (MDA) at baseline for HIV infected subjects compared with HIV-TB co-infected subjects. There was a significantly (p < 0.05) lower level of MDA and higher level of Catalase (CAT) in subjects administered supplementation compared to subjects without supplementation for the HIV infected group. There was a significantly lower level of Reduced Glutathione (GSH), Superoxide Dismutase (SOD) and higher level of MDA after one month of supplementation compared with baseline levels for HIV/TB co infected subjects. A similar result was also obtained for the HIV mono-infected groups which had a significantly lower level of SOD, MDA and CAT compared to the baseline. There was a significantly lower level of GSH and SOD, and higher level of MDA after supplementation compared with the baseline for HIV/TB co-infected subjects.
Comparing the indices at baseline and post no-supplementation in HIV/TB co-infection showed no significant differences in the oxidative stress parameters
Conclusion
HIV/TB co-infection and HIV mono-infection seems to diminish the capacity of the anti-oxidant system to control oxidative stress, however exogenous anti-oxidant supplementation appears not to have beneficial roles in positively modulating the associated oxidative stress.
Keywords: Oxidative stress, HIV, TB, anti-oxidants, vitamin A, vitamin C
Introduction
Acquired Immune Deficiency Syndrome is a fatal illness caused by a retrovirus known as the human immunodeficiency virus (HIV) that breaks down the body's immune system, infects CD4+ cells initially, progressively and ultimately leads to a chronic depletion of the immune system.1,2 This syndrome is often associated with rare opportunistic infections which includes Mycobacterium tuberculosis, pneumonia and others.3 Among the opportunistic pathogens associated with AIDS, Mycobacterium tuberculosis possesses a large number of virulence factors and has a higher potential for person-to-person transmission.4
Persons infected with HIV are particularly susceptible to tuberculosis, both from the reactivation of latent infection and from new infection with rapid progression to active disease.5,6 In 2008, more than one-third of HIV-infected individuals were also infected with tuberculosis (TB).7 Studies from 2007 show that of the 9.3 million new TB cases, 1.4 million were also living with HIV and 500,000 HIV positive TB patients died that same year. In many parts of the world TB is a leading cause of death in individuals infected with HIV.7,8
It is believed that oxidative stress played a major role in the progression of HIV infection.8,9,10 Oxidative stress is also associated with the pathophysiology of other disease like diabetes, and Alzheimer's disease.11,12 Substantial amount of evidence revealed roles of Oxidative Stress (OS) as a contributory factor in many infections and drug related toxicities.13
Experiments in animals have established the involvement of oxidative stress in drug induced toxic reaction.14 A dysfunction of anti-oxidant system have also been observed in patients on anti-retroviral therapy, suggesting the roles of oxidative stress in antiretroviral toxicities, this is because Highly Active Antiretroviral Therapy (HAART) seems to elevate reactive oxidative species in circulation, by producing more oxidized species due to the reactions between ROS and cellular molecular components.15,16 HIV alone or and in combination with anti-retroviral therapies contributes to the development of oxidative stress in humans.
Oxidative stress also has been shown to be associated with TB infection through activation of phagocytes by mycobacteria which may further contribute to immunosuppression. 17,18 High level of oxidative stress has been reported in patients with tuberculosis as a result of tissue inflammation, poor nutrition and poor immunity and this stress becomes more severe in those with HIV-TB co-infection. Furthermore, a report has shown high levels of oxidative stress in HIV-TB co-infected patients.19
Patients infected with HIV are in oxidative imbalance early in the disease; serum and tissue anti-oxidants levels are low and peroxidation products elevated.20 High plasma levels of malondialdehyde (MDA), reduced plasma glutathione (GSH), and decreased superoxide dismutase (SOD) activities are normally found. HIV and TB infection also result in considerably reduced vitamin C concentrations. 21
Anti-oxidant supplementation may help protect against oxidative stress associated with the infection and drug therapy. This study was therefore conducted to identify the modulatory roles of vitamin A and C supplementation on oxidative stress associated with HIV mono-infection and HIV-TB co-infection
Methods
Study site
The study was conducted at the AIDS Prevention Initiative of Nigeria (APIN) Clinic, Lagos University Teaching Hospital, Lagos, Nigeria; which is one of the treatment centers and a research center for the HIV relief program in Nigeria. The clinic runs from 8:00 a.m. to 4:00 p.m. (Monday to Friday) and has over 10,000 registered patients with an average of 200 patients attended to per day.
Subjects
A total of 90 consenting patients consisting of male and female adults with HIV infection (17 males, 33 females) and HIV-TB co-infection (10 males, 30 females) attending the AIDS Prevention Initiative of Nigeria (APIN) Clinic of the Lagos University Teaching Hospital (LUTH).
This was a prospective randomized study to determine the effect of vitamin A 5000 IU and vitamin C 2600 mg on oxidative stress markers in HIV infected and HIV-TB co-infected patients.
Ethical approval and patients' consent
Ethical approval was obtained from the Research and Ethics Committee of Lagos University Teaching Hospital, (LUTH), Lagos, Nigeria.
Inclusion criteria
Subjects with HIV infection and HIV-TB co-infection ≥ 18 years of age who gave informed consent were used.
Exclusion criteria
Patients with:
Co-morbidities, such as renal, endocrine, hepatic diseases were excluded from the study so that they would not act as confounders to the study.
Pregnant or lactating women were also excluded from the study.
CD4 count > 350 cells/micro-liter were also excluded from the study
Sample population and study procedure
Fifty (50) HIV infected and forty (40) HIV-TB co-infected subjects who met the inclusion criteria were recruited from 15th May 2013 to 30th January 2014. Information collected after obtaining consent includes bio-data, CD4+ count, viral load, hematological parameters and clinical chemistry parameters.
HIV treatment naive subjects (50 subjects) were recruited as they presented to the clinic and 5mls of blood samples in heparinized vacutainer was collected for baseline oxidative stress parameters. Blood samples were collected at subjects' first clinic visit for estimation of full blood count, chemistry, CD4+ count and viral load 2 weeks prior to when samples for estimation of baseline oxidative stress parameters were collected.
The 50 HIV infected subjects were systematically randomized into 2 groups as follows:
25 HIV infected subjects on supplementation (group A)
25 HIV infected subjects without supplementation (group B)
5mls of blood was collected from both groups; pre-supplementation and at 1month post-supplementation with vitamin A and vitamin C.
The 40 HIV-TB co-infected subjects were systematically randomized into:
20 HIV-TB co-infected subjects on supplementation (group C)
20 HIV-TB infected subjects without supplementation (group D)
5mls of blood was collected from both groups; pre-supplementation and at 1month post-supplementation with vitamin A and vitamin C.
Modalities of providing supplementation
Group A and C were given supplements (Vitamin A 5000 IU and Vitamin C 2600 mg) contained in EMVITE multivitamin tablets. EMVITE multi-vitamin tablets produced by EMZOR Pharmaceutical Industries Ltd, with batch number 3 19698 and 04, 2013 as date of manufacture and 04, 2016 as expiry date were used. Each tablet contained vitamin A acetate 2500 IU, vitamin B1 600 mcg, vitamin D 250 IU, vitamin C 13000 mcg. It was ensured that subjects adhered to the required number of tablets to be taken daily.
These doses can be tolerated in humans according to Pappolla et al, Schipper et al and Ayinde et al , hence were chosen for the study.22, 23,24
Sample and statistical analysis
Plasma samples were separated from whole blood by centrifuging in a Whisperfuge centrifuge Model 684 (GEA Westfalia, Northvale, NJ, USA) at 2500 rpm for 10 minutes and was stored in Nuaire - 80°C ultralow temperature freezer, model number NU-9668E, serial number 08040061, until ready for analysis.
CD4+ count, clinical chemistry and hematological parameters were determined using Automated Cy-flow counter; Hitachi 90 and Mindray BC 3200 respectively. Viral load was determined using Automated Ampliprep Cobas Taqman 48 analyzer.
Antioxidant enzymes assay
Measurements of anti-oxidant enzyme activity and lipid peroxidation were performed according to standard procedures. Catalase, superoxide dismutase (SOD), 25 malondialdehyde (MDA),26 reduced glutathione (GSH) and were determined using the methods of Sedlak and Lindsay. 27
Result
Bio-demographics
Age range of the recruited HIV subjects
The results showed that the recruited HIV infected patients were in the age range 23 years to 58 years. Most were in the productive age group.
Sex distribution of recruited HIV subjects
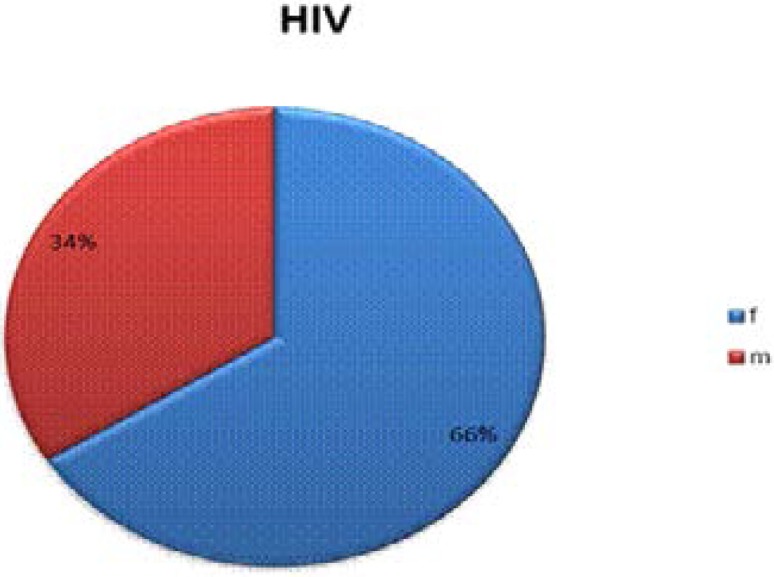
The sex distribution of the subjects showed that the female: male ratio was in proportion of 2:1. A total of 17 male and 33 female HIV infected subjects who gave informed consent were recruited. (Fig. 1)
Fig 1.
Sex distribution of recruited HIV subjects.
Age range of the recruited HIV-TB co-infected study subjects
The results showed that the recruited HIV-TB infected patients were in the age range 28 years to 58 years. Most were in their productive age group.
Sex distribution of the recruited HIV-TB co-infected study subjects
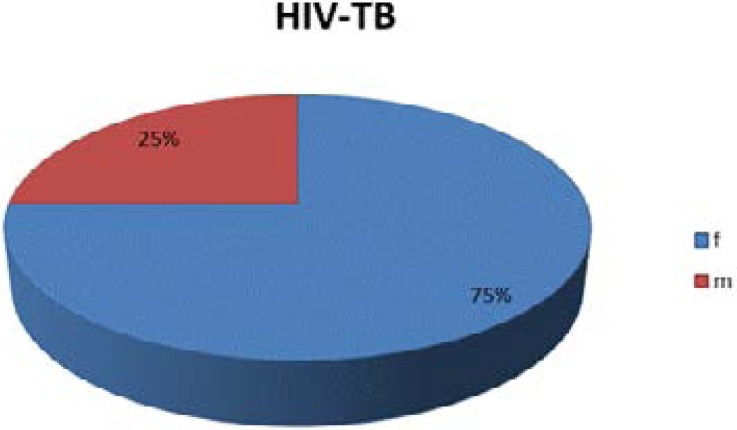
The sex distribution of the subjects showed that the female: male ratio was in proportion of 3:1. A total of 10 males and 30 females HIV-TB co-infected subjects who gave informed consent were recruited. (Fig. 2)
Fig 2.
Sex distribution of the recruited HIV-TB co-infected study subjects
Baseline CD+ count and viral load in HIV infected subjects
The mean baseline CD4+ count and viral load were 164.61 cells/µL and 540,284.44 copies/mL. The low CD4+ count and viral load showed both immune-depression and immuno-suppression.
Baseline clinical chemistry parameters in HIV-infected subjects
The mean baseline clinical chemistry parameters in the recruited HIV infected subjects showed that levels of urea and creatinine were 12.27 mol/L and 48.98 µmol/L respectively. These suggest that there was renal impairment. The mean values of hepatic enzymatic biomarkers; AST, ALT, ALP was also increased suggesting evidence of liver injury.
Oxidative stress parameters in HIV infected subjects one month post-supplementation
There was significant (p < 0.05) differences in CAT and MDA levels in HIV subjects one month post-supplementation. (Table 1)
Table 1.
Oxidative stress parameters in HIV infected subjects one month post-supplementation
| Oxidative parameters (U/mg) |
Supplementation Vitamin A & C combination |
No supplementation | p-value |
| Mean ± SEM | Mean ± SEM | ||
| GSH | 0.67 ± 0.54 | 0.56 ± 0.55 | 0.603 |
| SOD | 1.97 ± 1.16 | 2.29 ± 0.89 | 0.307 |
| CAT | 14.21 ± 4.90 | 17.42 ± 3.41 | 0.026* |
| MDA | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.008* |
GSH, Reduced Glutathione; SOD, Superoxide Dismutase; CAT, Catalase; MDA, Malondialdehyde. Result are expressed as mean±SEM
p < 0.05 Supplementation vs No supplementation
Discussion
Oxidative stress is thought to play an important role in the progression of HIV infection. There is clear evidence that oxidative stress contributes to several aspects of HIV disease, including viral replication, inflammatory response and decreased immune cell proliferation.28 There is a profound interplay of oxidative stress in tuberculosis. In pulmonary tuberculosis, there is increase in several circulating markers of free radical activity, indicating ongoing oxidative stress and decrease in anti-oxidant activity which may contribute to development of lung function abnormalities.29 This study was conducted to investigate the modulatory effects of exogenous anti-oxidant supplementation on disease progression in HIV mono — infected and HIV-TB co-infected subjects.
Ninety subjects were recruited for this study; fifty HIV infected, and forty HIV/TB co-infected. Sex distribution of the recruited subjects showed that the ratio of male to female for the HIV mono-infected subjects was 2:1 while HIV/TB co-infected subjects had a ratio of 3:1. Therefore recruited subjects had similar sex distribution with a higher proportion of females in both groups.
The hematological parameters at baseline in the HIV infected group showed lower hemoglobin and red blood cell count compared with the general reference value hence suggesting anemia. A major feature in HIV patients is the presence of low level of hemoglobin and red blood cell.30,31 Apart from HIV induced anemia, zidovudine is also associated with anemia especially in women.31 The level of clinical chemistry parameters in HIV infected subjects at baseline shows elevated mean total protein, AST and ALT levels and a lower level of creatinine compared to the normal reference range. This shows a possibility of hepatotoxicity that is characterized by an elevation of hepatic enzymatic biomarkers. One of the adverse effects of anti-retroviral therapy is hepatotoxicity and is frequently reported in patients taking nevirapine containing highly active antiretroviral therapy. Case reports, clinical trials and other studies have linked nevirapine with hepatotoxicity in HIV patients taking nevirapine containing ART.32
In this study, there was a significantly higher level of CAT in HIV mono-infected subjects who had no supplementation when compared with subjects administered vitamin A and vitamin C. Also, the oxidative stress indices were significantly lower in the post-supplementation group compared to baseline. HIV infected subjects have been shown to have decreased anti-oxidant concentrations, disruption in glutathione metabolism and enhanced spontaneous generation of reactive oxygen species.33 Friis-Moller et al.20 have shown that HIV-infected patients are in oxidative imbalance early in the disease; serum and tissue anti-oxidants levels are low and lipid peroxidation products elevated. These supplements were unable to correct this oxidative state or induce an increase/stimulation of the anti-oxidant system. The first stage of metabolism of vitamin C converts it into a free radical34 which could exacerbate oxidative stress in conditions characterized by excessive oxidation. This is further corroborated by the significantly lower levels of MDA in subjects who had no supplementation when compared with the subjects who had supplementation. According to Mehta and Fawzi,35 advanced HIV disease may suppress release of vitamin A from the liver and would result in low levels of vitamin A in the plasma despite the body having enough vitamin A liver stores. These authors35 also reported that the HIV genome has a retinoic acid receptor element hence vitamin A may increase HIV replication via interacting with this receptor. Vitamin A is also known to increase lymphoid cell differentiation, which leads to an increase in CCR5 receptors which are essential for attachment of HIV to the lymphocytes and therefore, an increase in their number is likely to increase HIV replication. This may be a probable mechanism for the non-efficacious effect of this vitamin seen in this study.
In HIV-TB co infected subjects in this study, there were significantly higher levels of GSH and SOD in subjects who had no supplementation compared with the Vitamin A and C administered subjects. Comparing post-supplementation levels of oxidative stress indices with baseline showed significantly lower levels of SOD and CAT with a corresponding significantly higher level of MDA at post-supplementation. Loss of appetite, poor intestinal absorption, increased urinary loss of vitamin A or acute phase reaction in tuberculosis36 may contribute to these findings and portends that these supplements were unable to confer protective roles as anti-oxidants against oxidative stress at the experimental doses used and may provide no benefit also in HIV-TB coinfection.
A higher level of MDA in these subjects points to increased lipid peroxidation further indicating the inability of vitamin C and A to shield the patients from medication or infection induced oxidation and hence disease progression.
Conclusion
This study shows that there are no positive modulatory roles and benefits of vitamin C and A supplementation on oxidative stress indices in HIV mono-infected and HIV-TB co-infected patients.
Conflict of interest
There are no conflicts of interest with respect to the study.
References
- 1.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003;5:172–177. PubMed. [PubMed] [Google Scholar]
- 2.Scott MC, Walusimbi M, Johnson DF. Tuberculosis Treatment in HIV Infected Ugandans with CD4 Counts > 350 Cells/mm3 reduces Immune Activation with No Effect on HIV Load or CD4 Count. PLoS One. 2010;5:e9138. doi: 10.1371/journal.pone.0009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quagliarello V. The Acquired Immunodeficiency Syndrome: current status. Yale J Biol Med. 1982;55:443–452. [PMC free article] [PubMed] [Google Scholar]
- 4.Awodele O, Olayemi S O, Nwite J A, Adeyemo T A. “Investigation of the Levels of Oxidative Stress Parameters in HIV and HIV-TB Co-Infected Patients,”. Journal of Infection in Developing Countries. 2012;6(1):79–85. doi: 10.3855/jidc.1906. [DOI] [PubMed] [Google Scholar]
- 5.Daley CL, Small PM, Schecter GF. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Murcia-Aranguren MI, Gómez-Marin JE, Alvarado Frequency of tuberculous and non-tuberculous mycobacteria in HIV infected patients from Bogota, Colombia. BMC Infect Dis. 2001;1:21. doi: 10.1186/1471-2334-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization, author. Tuberculosis Facts. 2009. www.who.int/tb/publications/2009/tbfactsheet2009onepage.pdf.
- 8.Chin J. The AIDS Pandemic: the collision of epidemiology with political correctness. Oxford: Radcliffe Publishing; 2007. 248 p. [Google Scholar]
- 9.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. The EMBO Journal. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carole L, Sterrit C. Anti-oxidants, oxidative stress and N acetyl cysteine. Gay Men's Health Crises: Treatment Issues. 1994;7:1–4. PubMed. [Google Scholar]
- 11.Abrescia P, Golino P. Free radicals and anti-oxidants in cardiovascular diseases. Expert Rev Cardiovasc Ther. 2005;3:159–171. doi: 10.1586/14779072.3.1.159. [DOI] [PubMed] [Google Scholar]
- 12.Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med Sci Sports Exerc. 2006;38:411–417. doi: 10.1249/01.mss.0000191347.73848.80. [DOI] [PubMed] [Google Scholar]
- 13.Kashou AH, Agarwal A. Oxidants and antioxidants in the pathogenesis of HIV/AIDS. Open Reproductive Sci J. 2011;3:154–161. doi: 10.2174/1874255601103010154. [DOI] [Google Scholar]
- 14.Adaramoye OA, Adesanoye OA, Adewumi OM, Akanni O. Studies on toxicological effects of nevirapine, an antiretroviral drug, on liver, kidney and testis of male wister rats. Hum Exp Tox. 2012;13:676–885. doi: 10.1177/0960327111424303. PubMed. [DOI] [PubMed] [Google Scholar]
- 15.Martín JA, Sastre J, De la Asunción J, Pallardó FV, Vinña J. Hepatic γ-cystathionase deficiency in patients with AIDS. JAMA. 2001;285:1444–1445. doi: 10.1001/jama.285.11.1444. [DOI] [PubMed] [Google Scholar]
- 16.Hulgan T, Morrow J, D'Aquila RT, Raffanti S, Morgan M, Rebeiro P, Haas DW. “Oxidant stress is increased during treatment of human immunodeficiency virus infection,”. Clin Infect Dis. 2003;37(12):1711–1717. doi: 10.1086/379776. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Jack CI, Jackson MJ, Hind CR. Circulating markers of free radical activity in patients with pulmonary tuberculosis. Tuber Lung Dis. 1994;75:132–137. doi: 10.1016/0962-8479(94)90042-6. PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Grimble RF. Malnutrition and the immune response. Impact of nutrients on cytokine biology in infection. Trans R Soc Trop Med Hyg. 1994;88:615–619. doi: 10.1016/0035-9203(94)90195-3. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Macallan DC. Malnutrition in tuberculosis. Diag Microbiol Infect Dis. 1999;34:153–157. doi: 10.1016/s0732-8893(99)00007-3. PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Friis-Moller N, Reiss P, Sabin CA. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Day BJ, Lewis W. Oxidative stress in NRTI-induced toxicity: evidence from clinical experience and experiments in vitro and in vivo. Cardiovasc Toxicol. 2004;4:207–216. doi: 10.1385/ct:4:3:207. PubMed. [DOI] [PubMed] [Google Scholar]
- 22.Pappolla MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol. 1992;140(3):621–628. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 23.Schipper HM, Cissé S, Stopa EG. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol. 1995;37(6):758–768. doi: 10.1002/ana.410370609. PubMed. [DOI] [PubMed] [Google Scholar]
- 24.Ayinde OC, Ogunnowo S, Ogedegbe RA. Influence of vitamin C and vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol Toxicol. 2012;14(13):17. doi: 10.1186/2050-6511-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun M, Zigma S. An improved spectrophotometric assay of superoxide dismutase based on ephinephrine antioxidation. Anal Biochem. 1978;90:81–89. doi: 10.1016/0003-2697(78)90010-6. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Buege J A, Aust S D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. PubMed. [DOI] [PubMed] [Google Scholar]
- 27.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:1192–1205. doi: 10.1016/0003-2697(68)90092-4. PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Aquaro S, Scopelliti F, Pollicita M, Perno CF. Oxidative Stress and HIV Infection: Target Pathways for Novel Therapies? Future HIV Therapy. 2008;2(4):327–338. PubMed. [Google Scholar]
- 29.Ragunath RR, Madhavi SP. Plasma oxidant antioxidant status in different respiratory disorder. Indian J of Clinical Biochemistry. 2006;21(2):161–164. doi: 10.1007/BF02912934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannessen A, Naman E, Gundersen SG, Bruun JN. Antiretroviral treatment reverses HIV-associated anemia in rural Tanzania. BMC Infectious Diseases. 2011;11:190. doi: 10.1186/1471-2334-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meidani M, Rezaei F, Maracy MR, Avijgan M, Tayeri K. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17(2):138–142. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 32.Adikwu E, Brambaifa N, Oputiri D, Geoffrey OP. Antiretroviral Toxicity and Oxidative Stress. American Journal of Pharmacology and Toxicology. 2013;8(4):187–196. [Google Scholar]
- 33.Delmas-Beauvieux MC, Peuchant E, Couchouron A, Constans J, Sergeant C, Simonoff M, Pellegrin JL, Leng B, Conri C, Clerc M. The enzymatic antioxidant system in blood and glutathione status in human immunodeficiency virus (HIV)-infected patients: effects of supplementation with selenium or b-carotene. Am J Clin Nutr. 1996;64(1):101–117. doi: 10.1093/ajcn/64.1.101. PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100(11):2842–2848. doi: 10.1172/JCI119832. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta S, Fawzi W. Effects of vitamins, including vitamin A, on HIV/AIDS patients. Vitam Horm. 2007;75:355–383. doi: 10.1016/S0083-6729(06)75013-0. PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Mathur ML. Role of vitamin A supplementation in the treatment of tuberculosis. Natl Med J India. 2007;20(1):16–21. PubMed. [PubMed] [Google Scholar]