Abstract
Background
Camptothecin (CPT) is a potent drug against cancers, originally from plants. The endophytic fungi could produce the secondary metabolite same as the host and is used as medicine.
Objectives
The aim of this paper was to investigate an endophytic fungal CPT with anti-neoplastic activity.
Methods
Endophytic fungi were isolated from Camptotheca acuminata in China. CPT from strain S-019 was characterized by TLC, HPLC and EI-MS analysis. Anti-tumor activity of fungal CPT was detected by MTT and fluorescent dye methods using Vero and PC-3 cells.
Results
A total of 94 endophytic fungi strains were isolated from tissues of C. acuminata and 16 fungi strains displayed cytotoxic activity on Vero or PC3 cells. Of which, the fungal strain S-019, classified as Fusarium solani, displayed impressive cytotoxic activity on cancer cells and was found to produce CPT by analysis of TLC, HPLC and EI-MS methods. Bioassay studies confirmed that the fungi CPT had potent cytotoxicity on Vero cells and induced apoptosis of Vero cells.
Conclusion
The endophytic fungi from camptotheca trees are a reliable source for natural anticancer compounds. The endophytic fungi could produce CPT same as plant. The fungal CPT exhibited effective activity at inhibiting cell growth and inducing apoptosis on Vero cells.
Keywords: Endophytic fungi, camptothecin, anti-tumor, Camptotheca acuminate
Introduction
Camptotheca acuminata is a species of medium-sized deciduous trees indigenous to the Southern China1. It is usually classified in the tupelo family Nyssaceae, which includes two species: Camptotheca acuminata Decne and Camptotheca lowreyana. Camptotheca acuminata has been used as traditionally Chinese medicine for centuries, all parts of this fast growing tree contain the secondary metabolite, camptothecin (CPT, an indole alkaloid), which is an effective drug against several types of cancer2–4. It is reported that CPT can induce apoptosis or death in various cancer cells by interfering with the topoisomerase I, an essential enzyme in the DNA replication process5–8. Although CPT is present in many plants, but two of the plants used for the commercial isolation of CPT are Camptotheca acuminate and Nothapodytes foetida9. Because the over exploitation has resulted in these plant sources to be endangered in China10. It is indispensable to look for alternate sources for this class of natural products.
Endophytic fungi have attracted attention for their potentials for producing the secondary metabolite same as the host plant, or novel metabolites11. Endophytic fungi are a large group of micro-organisms colonizing in the tissue of plants without causing overt symptoms or apparent injury to the host. The plant is thought to provide nutrients to the endophytic fungi, while the microbe may produce factors that protect the host plant from attack by animals, insects or other microbes12. It has been proven that endophytic fungi are a source of providing secondary metabolites diverse in structure and biologic activity13,14. Thus, the endophytic fungi in C. acuminata plant are expected to be a potential source for CPT or new natural bioactive agents. This paper described the screening of endophytic fungi with antitumor activity isolated from C. acuminata collected from Guizhou province, SouthWest of China, and demonstrated that an endophytic Fusarium solani was capable of producing camptothecin which possessed of inhibitory activity to cancer cells.
Materials and methods
Acquisition of C. acuminata
Samples of C. acuminata were collected in April from the Botanical Garden, South of Guiyang, China. Small terminal, limbs (1×25 cm) from one to two years old were harvested and stored in a sealed plastic bag at room temperature and processed within 2 days.
Isolation of strains
The endophytic fungi were isolated from different tissues of C. acuminata according to a protocol described previously15. Briefly, stem fragments were treated by surface disinfection with 70% (v/v) ethanol for 1 minute to kill epiphytic micro-organisms. Epidermis layers of tissues were removed with a sharp blade and the layers of phloem-cambium and xylem tissues were cut into pieces and placed in Petri plates. Pieces of rhizome were thoroughly washed using distilled water and followed by 70% (v/v) ethanol for 1 minute and 5% sodium hypochlorite for 5 minutes to accomplish adequate surface sterilization. They were subsequently rinsed in sterile demineralized water thrice for 1 minute. Small pieces (1 cm) of inner tissue of rhizome were placed on aqueous agar in petri plates and incubated at 28 ± 1 °C until the fungal growth was initiated. After several days, individual hyphal tips were removed and placed on potato dextrose agar (PDA). They were incubated for 10 days, and periodically checked for purity. Again, each culture was transferred, by hyphal tipping, to a water agar plate containing gamma-irradiated carnation leaves which usually permit the development of fruiting structures.
Identification of the endophytes
The endophytic fungal strains were identified by the morphological method and reinforced by 18S rDNA sequencing. The endophytes from C. acuminata were examined under light microscope stained with lactophenol cotton blue16. The total genomic fungal DNA was then extracted by CTAB method17. The endophytic fungus was identified by analysis of 18S ribosomal genes with primers 5′-TCCGTAGGTGAACCTGCGG-3′ and 5′-TCCTCCGCTTATTGATATGC-3′. The amplified products were purified utilizing Gel Extraction Kit (Omega, USA), and sequenced using ABI 3730 DNA Analyzer (ABI, USA) as per the manufacturer's instructions. The DNA sequences were submitted to Genbank for homology analysis by BLASTN program. The ribosomal gene database (http//ncbi.nim.nih.gov/) was accessed and sequence alignment was used as an underlying basis to identify the fungus. The sequence data of strain S-019 had been submitted to the GenBank databases under accession number of EF062312.
Fungal cultivation and extraction
The fungi were statically cultured for 10 days at 28°C in petri dishes. They were incubated into 1000 mL flasks containing 500 mL of PDA broth. After 7 days of incubation at 28 ± 1°C on rotary shaker at 150 rotations per minute (rpm), mycelia and broth were separated by filtration. Mycelia were thoroughly washed with sterile distilled water and homogenized in a cell disintegrator. Both cell homogenate and cell-free broths were extracted four times with equal volume of chloroform: methanol (4:1 v/v). After stripping off the solvent, a small quantity of the residue was applied on silica gel thin layer chromatography (TLC) plates. The chloroform: methanol (9:1 v/v) solvent system was used for TLC. TLC analysis exhibited spots which were superimposable with the authentic CPT. The spots were visualized under ultra violet (UV) light. The rest of the residue was used for quantification of CPT by HPLC.
High performance liquid chromatographic analysis
HPLC separation was performed on a LC-8 (A) HPLC of authentic CPT and system (Shimadzu, Japan). The mobile phase was set as methanol: water, 60:40 (pH ≤ 5) and separation was carried out at a flow rate of 1 mL min−1 over a period of 30 min. The compounds were detected using a UV detector at λmax of 360 nm. The electron ionization mass spectrometry (EI-MS) DATA were collected on an HP 5973 mass spectrometer (Hewlett-Packard, America).
Cell lines and normal culture conditions
The cell lines used in this paper were two cancer cell lines: Vero cell and human prostate cancer cell line pc-3 (PC3 cell). The cells were grown in 96-well plates (6000 cells per well) in Dulbecco's modified Eagle medium containing 10% fetal calf serum supplemented with 1000 units mL-1 penicillin and 100 µg mL−1 streptomycin sulfates, in a humidified atmosphere in 5% CO2 at 37°C. The exponentially growing cells (1×103) were harvested for next tests.
Analysis of cytotoxic activity
The cytotoxic effects of fermentation broth and mycelia extraction were tested by 3–4, 5-dimethylthiazol-2, 5-diphenyl tetrazolium bromide (MTT) assay and conventional ethidium bromide-acridine orange (EB/AO) staining. The MTT assay protocol was similar to the previous with a little modification18. The cell lines were inoculated into three 96-well plates when cells were in the denary logarithmic growth condition. The cells were divided into the control group and the experimental group, then the extracts from strain S-019 (0, 2.5, 5, 10, 20, 30 and 50 µg mL−1) were added. Three replicates were prepared for each treatment and the cells were cultured for 24 hours. MTT and dimethyl sulfoxide (DMSO) were added to the cell cultures, and the OD-value at 570 nm wavelength was measured for each treatment group using the MTT assay. Lastly, in order to calculate the survival ratio of the cells, the following formula was used: Survival ratio of cells = (OD treated well) / (OD control well) ×100%.
Conventional EB/AO staining procedures were followed as described previously19. Briefly, after various treatments with fermentation broth and mycelia extraction at various concentrations (2.5 to 50 µg mL-1) and at different periods (0 to 60 h), suspended cells and trypsinized adherent cells were harvested at previous determined time periods, washed in phosphate-buffered saline (PBS) and centrifuged at 1000 rpm for 10 min to obtain a pellet, and then resuspended in 0.5 mL of 1% PBS. EB/AO in PBS (5 µL of a 100 µg mL-1 solution) was added to a 0.1 mL cell suspension, and stained cells were observed by fluorescence microscopy (Leica, wetzlar, Germany). A total of 300 cells were counted in multiple randomly selected fields, and the percentage of apoptotic and dead cells was then calculated.
Results
Screening of endophytic fungi having cytotoxic activity by the MTT assay
A total of 94 isolates of endophytic fungi were isolated from C. acuminata in the present survey. Among them, there were 24 strains from leaves, 56 from barks and 14 from roots. Fermentation broths and mycelia of 94 endophytic fungi were tested for cytotoxicity. Table 1 shows the number and percentage of the broths with endophytes at a dilution at 1:50, displaying activity of cells with growth inhibition rate at 50%. There were 16% (15/94) and 13.8% (13/94) of endophytic fungi cultures displayed cytotoxic activity on Vero cells and PC3 cells. A total of 17% (16/94) strains from the plant showed anticancer activity. Most active strains isolated from C. acuminata belong to several genera, such as Alternaria sp., Cephalosporium sp., Pestalotiopsis sp. and Fusarium sp. Furthermore, the cytotoxic activity in strains was much divergent even though two strains were classified into the same genus.
Table 1.
Screening of fermentation broths with cytotoxic activity (ID50 dilution ≤ 1:50) from endophytic fungi by MMT assay
| Parts of C. acuminata leave |
Number of strains 24 | Number of strains with cytotoxic activity (%) | Total strains (%) |
|
| Vero cell | PC-3 cell | |||
| leave | 24 | 4 (16.7) | 3 (12.5) | 5 (20.8) |
| bark | 56 | 10 (17.9) | 9 (16.1) | 10 (17.9) |
| root | 14 | 1 (7.1) | 1 (7.1) | 1 (7.1) |
| Total | 94 | 15 (16.0) | 13(13.8) | 16(17.0) |
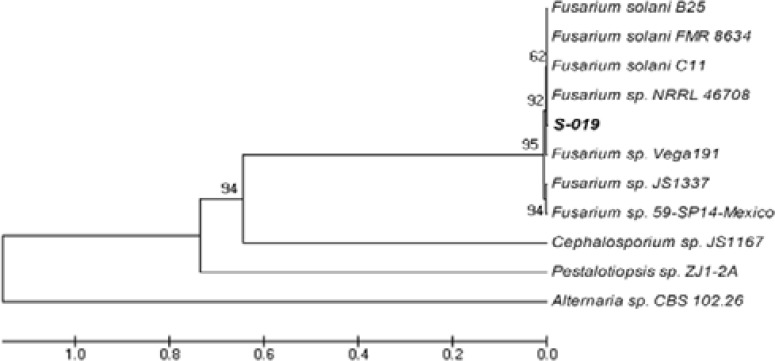
One of the most interesting fungal endophytes, strain S-019, displayed impressive cytotoxic activity on cancer cells and was found to produce CPT by TLC analysis. This fungus was identified as a member of Fusarium genera according the morphological characters described previously20,21 (Fig. 1), which was reinforced by the sequence of its 18S rDNA that gave a 99% sequence similarity to those genes of Fusarium solani deposited at the GenBank of NCBI (Fig. 2).
Fig. 1.
The mycelial colonies and spores of the S-019 isolates grown on PDA medium for 10 days.
Fig. 2.
Phylogenetic analysis based on the rDNA ITS sequence data showing the relationships of the strain S-019 with reference taxa. Sequences from ITS1, 5.8S rRNA to ITS2 were aligned among fungi (GenBank accession number): S-019 (EF062312), Fusarium solani strain B25 (EF488413), Fusarium solani strain FMR 8643 (AM412600), Fusarium solani strain C11 (EF488412), Fusarium sp. NRRL 46708 (EU329717), Fusarium sp. Vega191 (EF694669), Fusarium sp. JS1337 (AM176729), Fusarium sp. 59-SP14-Mexico (AY755617), Cephalosporium sp. (AM176753), Pestalotiopsis sp. (FJ037738), Alternaria sp. (FJ266485).
Isolation, identification and quantification of secondary metabolite
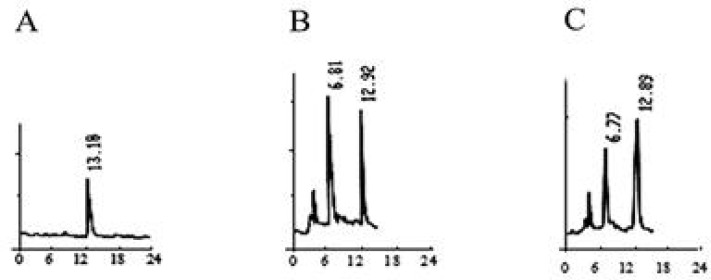
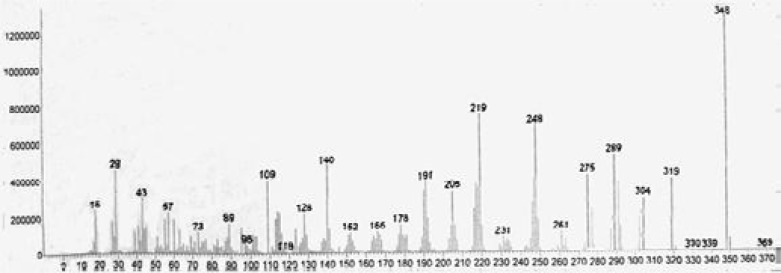
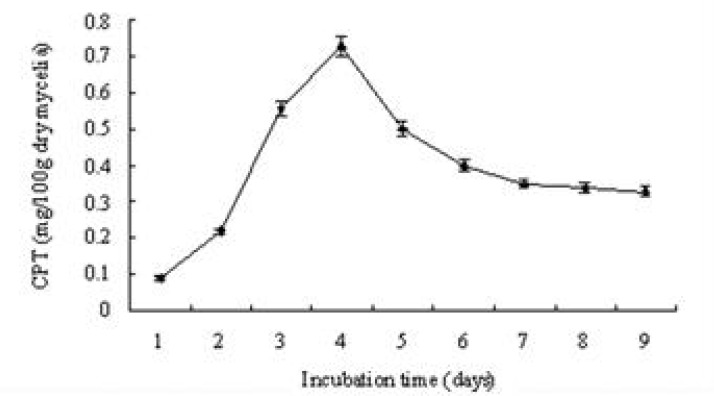
In order to determine the camptothecin produced by the endophytic fungi, the S-019 strain was incubated into PDA broth. The mycelia were collected and washed described above. Cell homogenates of mycelia were sonicated. The cell lysate and the broth were extracted using ethanol and methanol solvent systems. After extraction with methanol, thin layer chromatography (TLC) in solvent system chloroform: acetone (7:3 v/v) was used to separate the production from S-019. The plate was examined under short wave ultraviolet rays. A band had the same chromatographic mobility (Rf = 0.5) with that of authentic CPT. The band was removed by scraping and the compound was eluted with methanol. It displayed anti-Vero cell activity whereas none of the other bands were active (data not shown). Furthermore, HPLC analyses of fractions showed retention times of 12.92 (Fig. 3B) that corresponded to that of authentic CPT (Fig. 3A), which was further confirmed by co-spiking (Fig. 3C). Further convincing evidence for the identity of CPT were obtained by electron ionization mass spectrometry (EI/MS). Characteristically, authentic CPT yielded an m/z (M+, 100%) peak at 348. On the other hand, the fungal compound (M+) also exhibited a peak at 348 (Fig. 4). It was found to be identical to that of authentic plant-derived CPT. Based on those information, the compound from fungus S-019 was considered homogenous with plant-derived CPT. The camptothecin content of the organic extracts of mycelia, collected at every day, was determined to have an insight into the kinetics change of production (Fig. 5). The camptothecin initiated at 24 h of incubation. Maximum production of camptothecin was observed on day 4 (96 h) in terms of CPT (µg g-1 dry weight of mycelia). The camptothecin content gradually declined after 96 h of incubation. The mean maximum yield of camptothecin in S-019 (fraction collected after 96 h of incubation) was 40 ± 5 µg g-1 dry weight mycelia, and 150 ± 20 µg L−1 of broth (the results were mean of three experiments).
Fig. 3.
Identification of camptothecin using HPLC. (A) HPLC of authentic CPT and (B) HPLC of the S-019 extracts, (C) HPLC of the mixture of authentic CPT and the S-019 extracts.
Fig. 4.
EI-MS spectrum of fungalcamptothecin.
Fig. 5.
The production kinetics of the camptothecin in the organic extracts of S-019 mycelia
Cytotoxic analysis in vitro
Vero cell line was used as a model system to evaluate the cytotoxicity of CPT from the broth and mycelium extracts of S-019. The cells were exposed to escalating concentrations (0–50 µg mL−1) of the extracts for 24 h, and the cell viability was determined by MTT assay. The extracts exhibited cytotoxic effects on the viability of Vero cells in a dose-dependent manner (P<0.05) (Fig. 6). It showed significant decrease in surviving (%) at a dose from 2.5 to 50 µg mL-1 and potent cytotoxicity was exhibited at 50 µg mL-1 (three times higher cytotoxicity).
Fig. 6.
Comparison of cell surviving fraction of cell-line Vero cells treated with different concentrations of the CPT from S-019. Untreated cells served as the negative control and were considered equivalent as 100% survival.
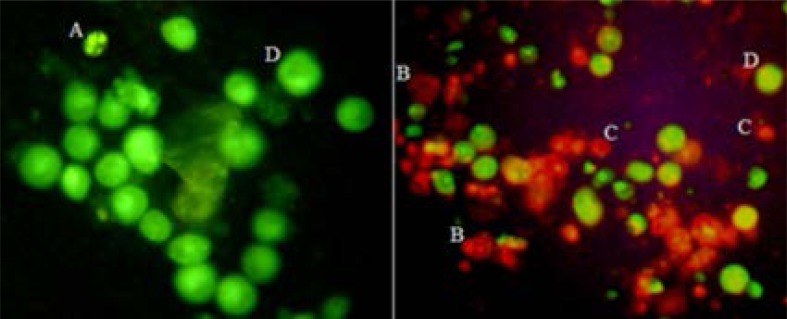
Apoptosis was further assessed by the fluorescent dye method using AO/EB. Apoptotic cells treated with the fungal CPT were observed by fluorescence microscopy (Fig. 7).
Fig. 7.
Fungi CPT-induced apoptosis, as assessed by AO/EB. (A) Early apoptotic Vero cell, showing the presence of green patches of fragmented and condensed chromatin. (B) Late apoptotic cells fluoresce orange. As the plasma membrane lost its integrity, EB entered the cell and intercalates fragmented DNA, staining the cell red. (C) Necrotic cell that had lost its selective permeability, allowing EB to intercalate DNA and produce a uniform red color. (D) Viable cells were uniformly green. Original approximate magnifications, ×400.
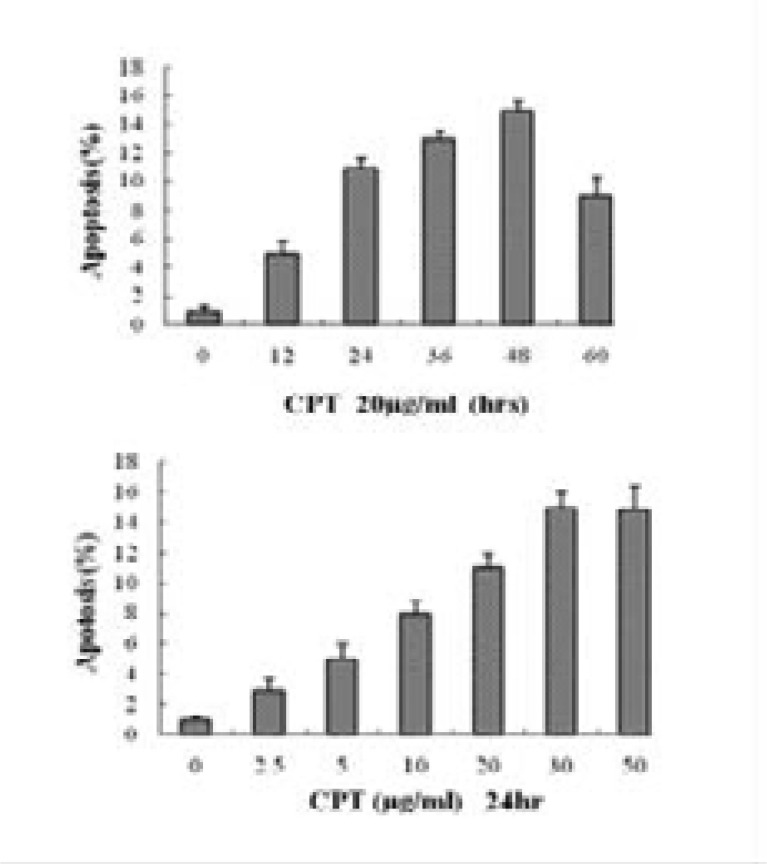
The fungi CPT at a concentration of 20 µg mL−1 induced apoptosis of Vero cells in a time-dependent manner (Fig. 8, down), and maximal induction of apoptosis occurred after 48 h of incubation (15.3% ± 0.8%; P<0.005). Furthermore, Vero cells also exhibited apoptosis in a dose-dependent manner when exposed to escalating concentrations (0–50 µg mL-1) of the fungi CPT for 24 h (Fig. 8, Down), and maximal induction of apoptosis occurred at a concentration of 30 µg mL-1 (15.3% ± 1.8%; P<0.001). The nucleus shapes of necrotic Vero cells were round and yellow in color because of the uptake of EB (Fig. 7). There was a high level of necrosis induced by the fungal CPT treatment (40 to 70%) that was also observed by fluorescent dye staining.
Fig. 8.
Fungi CPT induced apoptosis in Vero cells in a time- and dose- dependent manner. (Up) Apoptosis was quantified by fluorescent dye staining of Vero cells treated with fungi CPT (20 µg mL-1) for various times (n=3 for each time point; P<0.005). (Down) Vero cells treated with various concentrations of fungi CPT for 24 h. Apoptosis was quantified by fluorescent dye staining. Untreated cells (bar 0) served as the negative control (n = 3; P<0.001).
Discussion
In this study, we described the isolation of endophytic fungi from C. acuminata and investigated the inhibition activity on tumor cells. A total of 94 endophytic fungi were isolated from the various parts of C. acuminata plants and grouped into several genera based on partial ITS sequencing. We found that 16 endophytic fungi showed cytotoxic activity on Vero cells and/or PC3 cells with different level of inhibition. One of them (S-019, Fusarium solani) displayed higher inhibition activity than other isolates. The results demonstrated that endophytic fungi are a reliable source for natural anti-cancer active compounds. Because of the diversity of the endophytic fungi and its cytotoxicity, the potential of using endophytes as an effective alternative or novel source for therapeutic compounds has been recognized11,22.
The endophytic fungi are known to produce the secondary metabolite same as the host plant23. For example, taxol is the most famous metabolite, which was originally produced by the yew tree and can be produced by endophytic fungi of yew trees24. Endophytic fungal strains of Fusarium solani from Apodytes dimidiata and Nothapodytes nimmoniana produce camptothecin25,26. In order to ascertain whether any of those isolates obtained in this study produced camptothecin same as the host plant, we selected the strain S-019 to determine CPT from endophytic fungi. TLC bioautography revealed that the extracts of S-019 from the mycelia and the fermentation broth showed an inhibition band against Vero cells, which had the same chromatographic mobility with that of authentic CPT. This compound from fungus S-019 was further analyzed by HPLC and EI-MS. Organic solvent extracts of mycelia and broth of surface culture were found to contain CPT after 24 h of incubation. The results indicated that endophytic fungus Fusarium solani from C. acuminata could produce camptothecin.
Camptothecin and its derivatives are well-known topoisomerase I inhibitors, which inhibit cell growth and induce apoptotic pathways through decelerating the dissociation of topoisomerase I-DNA to trap topoisomerase I-DNA cleavage complexes27. In this study, biological activity of fungal CPT was assayed in vitro against Vero cells. It was found that fungal CPT displayed remarkable inhibitory effects at inhibiting cell growth and inducing apoptosis on Vero cells in a dose- and time-dependent manner. The results suggested that fungal CPT, which was similar to plant CPT, was responsible for the death of Vero cells.
Conclusion
This work presented the isolation of endophytic fungi from C. acuminata and investigated the inhibition activity on tumor cells. The results showed that the endophytic fungi of camptotheca trees are a reliable source for natural anti-cancer active compounds. The endophytic fungi could produce CPT same as camptotheca trees. The fungal CPT exhibited effective activity at inhibiting cell growth and inducing apoptosis on Vero cells.
Acknowledgement
This study were supported by the 863 Program [2013AA102503], the Guizhou Province “Hundred” Innovative Talents Project QKHRC[2016]-4012, the Guizhou Province Collaborative Innovation Center for Mountain Ecology & Agro-Bioengineering (qjhxtcxz[ 2014]-01), and the Governor Special Project of Guizhou Province, China (qz[2005]-108).
References
- 1.Monacelli B, Valletta A, Rascio N, Moro I, Pasqua G. Laticifers in Camptotheca acuminata Decne: distribution and structure. Protoplasma. 2005;226:155–161. doi: 10.1007/s00709-005-0118-2. [DOI] [PubMed] [Google Scholar]
- 2.Jain PT, Fornari FA, Randolph JK, Orr MS, Gewirtz DA. Induction of DNA damage, inhibition of DNA synthesis, and suppression of c-myc expression by the topoisomerase I inhibitor, camptothecin, in MCF-7 human breast tumor cells. Biochemical Pharmacology. 1998;55:1263–1269. doi: 10.1016/s0006-2952(97)00618-7. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Carpenter SB, Bourgeois WJ, Yu Y, Constantin RJ, Falcon MJ, et al. Variations in the secondary metabolite camptothecin in relation to tissue age and season in Camptotheca acuminata. Tree Physiology. 1998;18:265–270. doi: 10.1093/treephys/18.4.265. [DOI] [PubMed] [Google Scholar]
- 4.Sriram D, Yogeeswari P, Thirumurugan R, Bal TR. Camptothecin and its analogues: a review on their chemotherapeutic potential. Natural Product Research. 2005;19:393–412. doi: 10.1080/14786410412331299005. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay K, Li P, Gjerset RA. CK2-mediated hyperphosphorylation of topoisomerase I targets serine 506, enhances topoisomerase I-DNA binding, and increases cellular camptothecin sensitivity. PLoS One. 2012;7:e50427. doi: 10.1371/journal.pone.0050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mei Y, Xie C, Xie W, Tian X, Li M, Wu M. Noxa/Mcl-1 balance regulates susceptibility of cells to camptothecin-induced apoptosis. Neoplasia. 2007;9:871–881. doi: 10.1593/neo.07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parent N, Winstall E, Beauchemin M, Paquet C, Poirier GG, Bertrand R. Proteomic analysis of enriched lysosomes at early phase of camptothecin-induced apoptosis in human U-937 cells. Journal of Proteomics. 2009;72:960–973. doi: 10.1016/j.jprot.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soukasene S, Toft DJ, Moyer TJ, Lu H, Lee HK, Standley SM, et al. Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS Nano. 2011;5:9113–9121. doi: 10.1021/nn203343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash L, Middha SK, Mohanty SK, Swamy MK. Micropropagation and validation of genetic and biochemical fidelity among regenerants of Nothapodytes nimmoniana (Graham) Mabb. employing ISSR markers and HPLC. 3 Biotech. 2016;6:171. doi: 10.1007/s13205-016-0490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amna T, Khajuria RK, Puri SC, Verma V, Qazi GN. Determination and quantification of camptothecin in an endophytic fungus by liquid chromatography - positive mode electrospray ionization tandem mass spectrometry. Current Science. 2006;91:208–212. [Google Scholar]
- 11.Wang J, Wang G, Zhang Y, Zheng B, Zhang C, Wang L. Isolation and identification of an endophytic fungus Pezicula sp. in Forsythia viridissima and its secondary metabolites. World Journal of Microbiology & Biotechnology. 2014;30:2639–2644. doi: 10.1007/s11274-014-1686-0. [DOI] [PubMed] [Google Scholar]
- 12.Carrell AA, Frank AC. Pinus flexilis and Picea engelmannii share a simple and consistent needle endophyte microbiota with a potential role in nitrogen fixation. Frontiers in Microbiology. 2014;5:333. doi: 10.3389/fmicb.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eo JK, Choi MS, Eom AH. Diversity of endophytic fungi isolated from Korean ginseng leaves. Mycobiology. 2014;42:147–151. doi: 10.5941/MYCO.2014.42.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain H, Kock I, Al-Harrasi A, Abbas G, Rehman NU, Shah A, et al. Coniothyren: a new phenoxyphenyl ether from the endophytic fungus, Coniothyrium sp. Journal of Asian Natural Products Research. 2014;16:1094–1098. doi: 10.1080/10286020.2014.931843. [DOI] [PubMed] [Google Scholar]
- 15.Puri SC, Nazir A, Chawla R, Arora R, Riyaz-Ul-Hasan S, Amna T, et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. Journal of Biotechnology. 2006;122:494–510. doi: 10.1016/j.jbiotec.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Parija SC, Prabhakar PK. Evaluation of lacto-phenol cotton blue for wet mount preparation of feces. Journal of Clinical Microbiology. 1995;33:1019–1021. doi: 10.1128/jcm.33.4.1019-1021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd Ed. New York: cold spring harbor laboratory press; 2001. [Google Scholar]
- 18.Segu VB, Li G, Metz SA. Use of a soluble tetrazolium compound to assay metabolic activation of intact beta cells. Metabolism. 1998;47:824–830. doi: 10.1016/s0026-0495(98)90120-2. [DOI] [PubMed] [Google Scholar]
- 19.Ching JC, Jones NL, Ceponis PJ, Karmali MA, Sherman PM. Escherichia coli Shiga-like toxins induce apoptosis and cleavage of poly (ADP-ribose) polymerase via in vitro activation of caspases. Infection and Immunity. 2002;70:4669–4677. doi: 10.1128/IAI.70.8.4669-4677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess LW, Summerell BA, Bullock S, Gott KP, Backhouse D. Laboratory manual for fusarium research. 3rd Ed. Sydney: Fusarium Research Laboratory University of Sydney and Royal Botanic Gardens; 1994. p. 133. [Google Scholar]
- 21.Booth C. Fusarium: laboratory guide to the identification of the major species. Kew, surrey, England: Commonwealth Mycological Institute; 1977. [Google Scholar]
- 22.Konig GM, Wright AD, Aust HJ, Draeger S, Schulz B. Geniculol, a new biologically active diterpene from the endophytic fungus Geniculosporium sp. 1. Journal of Natural Products. 1999;62:155–157. doi: 10.1021/np9802670. [DOI] [PubMed] [Google Scholar]
- 23.Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, et al. A Friendly relationship between endophytic fungi and medicinal plants: a systematic review. Frontiers in Microbiology. 2016;7:906. doi: 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivera-Orduña FN, Suarez-sanchez RA, Flores-Bustamante ZR, Gracida-Rodriguez JN, Flores-Cotera LB. Diversity of endophytic fungi of Taxus globose (Mexican yew) Fungal Diversity. 2011;47:65–74. [Google Scholar]
- 25.Bhalkar BN, Patil SM, Govindwar SP. Camptothecine production by mixed fermentation of two endophytic fungi from Nothapodytes nimmoniana. Fungal Biology. 2016;120:873–883. doi: 10.1016/j.funbio.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Venugopalan A, Srivastava S. Enhanced camptothecin production by ethanol addition in the suspension culture of the endophyte Fusarium solani. Bioresource Technology. 2015;188:251–257. doi: 10.1016/j.biortech.2014.12.106. [DOI] [PubMed] [Google Scholar]
- 27.Hsua JL, Hoa YF, Lib TK, Chenc CS, Hsua LC, Guha JH. Rottlerin potentiates camptothecin-induced cytotoxicity in human hormone refractory prostate cancers through increased formation and stabilization of topoisomerase I-DNA cleavage complexes in a PKCδ-independent pathway. Biochemical Pharmacology. 2012;84:59–67. doi: 10.1016/j.bcp.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]