Abstract
Our acceptance of exposure to radiation is somewhat schizophrenic. We accept that the use of high doses of radiation is still one of the most valuable weapons in our fight against cancer, and believe that bathing in radioactive spas is beneficial. On the other hand, as a species, we are fearful of exposure to man-made radiation as a result of accidents related to power generation, even though we understand that the doses are orders of magnitude lower than those we use everyday in medicine. The 70th anniversary of the detonation of the atomic bombs in Hiroshima and Nagasaki was marked in 2015. The 30th anniversary of the Chernobyl nuclear power plant accident will be marked in April 2016. March 2016 also sees the fifth anniversary of the accident at the Fukushima nuclear power plant. Perhaps now is an opportune time to assess whether we are right to be fearful of the effects of low doses of radiation, or whether actions taken because of our fear of radiation actually cause a greater detriment to health than the direct effect of radiation exposure.
Keywords: Environmental, health effects, nuclear accident, radiation
Statement of Search Strategies Used and Sources of Information
This paper reflects expert opinion and current literature accessed by the authors; no formal search strategy has been defined.
Health Effects of Low-dose Radiation in our Environment: What do we Know?
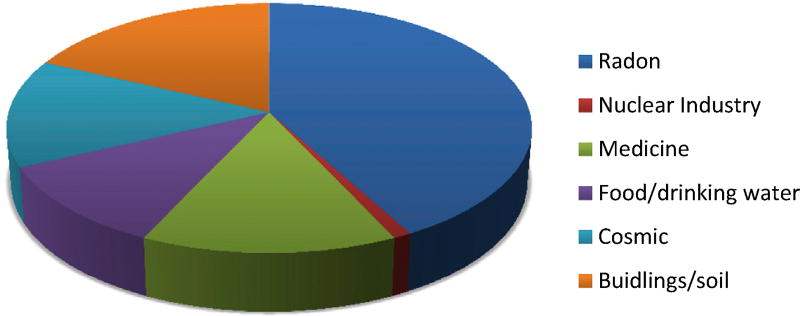
We are all exposed to a certain level of background radiation. Most background radiation comes from radon, which is generated fromthe rocks that comprise the crust of our planet. A smaller amount (16%) comes from artificial sources, mainly medical exposures, with a very small amount (1%) coming from the nuclear industry as a whole, including atmospheric testing of atomicweapons (Figure 1). The average dose received by all of us from background radiation is around 2.4 mSv/year, which can vary depending on the geology and altitude where people live – ranging between 1 and 10 mSv/year, but can be more than 50 mSv/year. The highest known level of background radiation affecting a substantial population is in Kerala and Madras states in India, where some 140 000 people receive doses that average over 15 mSv/year from gamma radiation, in addition to a similar dose from radon. Comparable levels occur in Brazil and Sudan, with average exposures up to about 40 mSv/year to many people. Taking the individual average dose of 2 mSv/year, someone who lived to the age of 80 years would have accumulated 160 mSv of radiation from natural sources during their lifetime.
Fig 1.
Sources of background radiation. Eighty-five per cent of an individual’s annual dose of radiation comes from natural sources (radon, a gas that is emitted from the rocks that form the crust of the planet; food/drinking water; cosmic radiation; exposure from buildings and soil). Fifteen per cent is from man-made sources, largely from exposures for medical reasons (14%). The remaining 1% comes from the nuclear industry. Fallout from atomic weapons testing or use and nuclear accidents accounts for around 0.3% of an individual’s annual radiation dose. Figure redrawn from data available at http://www.world-nuclear.org/info/Safety-and-Security/Radiation-and-Health/Nuclear-Radiation-and-Health-Effects.
The health effects of radiation can be divided into two, and show subtly different relationships between dose and effect. Early, deterministic or tissue effects are seen at high doses (>1 Sv), associated with cell killing in the tissues exposed, and show a direct correlation with dose. We are used to seeing these effects in cancer patients treated with radiation – vomiting and diarrhoea, loss of hair, etc. The longer-term effects or stochastic effects are seen at lower doses, where the dose is correlated with the probability of the effect, rather than directly with dose. The stochastic effect of most public concern is that of cancer.
Many of the health effects that we attribute to radiation are not produced exclusively by radiation and not all types of cancer have been shown to be elevated in populations exposed to ionising radiation. Cancer can be caused by a variety of chemical carcinogens, exposure to sunlight, obesity and a great many other factors. There are no validated biomarkers that enable us reliably to identify a cancer as being caused by radiation. Radiation increases the number of cancers within a given exposed population, rather than changing the biology of the cancers induced. This makes it impossible to separate the number of cancers that have been caused by radiation from those that are due to other causes. Because the same health effects can be caused by factors other than radiation, we define the contribution that a given dose of radiation makes to a health outcome (e.g. cancer) as the excess relative risk. This is defined as the rate of disease in the exposed population divided by the rate of disease in an unexposed population minus 1. The risk is usually defined as being a given percentage per Sievert, which enables the risk to be defined regardless of the type of radiation to which the population is exposed.
Most of the information on the health risks of radiation in healthy populations comes from the life span studies, which were established after the detonation of the atomic bombs in Japan in 1945. Assembled in 1950 these cohorts have now been followed for 65 years. Of the 120 000 original subjects, 54 000 were within 2.5 km of the epicentre of the detonations and 45 000 were located 2.5–10 km away. Forty per cent are still alive. The control population (26 000 individuals) were not present at the detonations, but lived in Hiroshima or Nagasaki between 1951 and 1953. Individual dose estimates are available for 92% of the population, with some survivors receiving over 2 Gy and the mean dose 200 mSv. The results of these studies have been recently reviewed by Kamiya et al. [1].
In brief, survivors of the atomic bombs in Hiroshima and Nagasaki have a dose–response relationship that is linear for solid cancer, but the precise shape of the curve is still unclear at low doses. Survivors who were children when exposed have a higher risk of cancer than those exposed at older ages; the risk of cardiovascular diseases and some other non-cancer diseases is increased at higher doses. In children exposed to high doses of atomic bomb radiation in the womb, development of the central nervous system and stature were affected, and the risk of cancer increased with maternal dose. Risks of hereditary malformations, cancer, or other diseases in children of atomic bomb survivors did not increase detectably with paternal or maternal dose, based on follow-up to date; atomic bomb survivors exposed to high doses of radiation tend to show deterioration of the immune system similar to that observed with ageing, and many survivors exposed to high doses of radiation have minor inflammatory reactions. Cancer risk increases after exposure to moderate and high doses of radiation (more than 0.1–0.2 Gy); however, whether cancer risk is increased by acute low doses (0.1 Gy or lower) or low dose rates is unclear.
There are a number of other large cohort studies involving both acute and protracted radiation exposures that confirm the data from the lower dose range of the life span study. These include the National Registry of Radiation Workers (NRRW), a study of UK nuclear workers [2]; the Techa River residents who were exposed to discharges of radioactive waste into the river near which they lived [3]; the cohorts of workers who cleaned up after the Chernobyl accident [4]. There are also data from Yangjiang, an area of high natural background radiation in China [5] and from the workers at British Nuclear Fuels Limited (BNFL) [6]. It is to be noted that most of the estimates of excess relative risk lie close to 0, particularly in the dose range between 0 and 0.1 Sv, and in most cases the 95% confidence intervals (where given) span 0. This indicates that there is no statistical evidence that an effect of radiation at these levels is proven scientifically, but rather could be a chance association.
Because of their ‘all or nothing’ nature and the difficulty in separating out low level, but prolonged, radiation exposure from background, it is difficult to estimate risks or a threshold of effect due to occupational radiation exposure when stochastic effects are considered. In practice, risk is extrapolated from high levels of exposure and a linear, no threshold (LNT) model assumed, i.e. there is no exposure level below which the risk is zero. When the individual radiation dose, from sources other than background radiation, falls below 100 mSv, it is generally accepted that it is difficult to show statistically that any cancers in the population under study are caused by radiation, as it is much more likely that those cancers are caused by all the other factors that we know also cause them. At the present time it is not clear whether there is a difference between a single dose exposure of 100mSv or a protracted low dose exposure that totals 100 mSv over time. However, it is generally accepted that the effect is the same, although there is considerable debate about the effects of confounders in the various studies [7–10].
How can we best put the health risks from radiation exposure into context? There are large databases drawn from cancer registries, notably the Surveillance, Epidemiology and End Results (SEER) database in the USA, which enable reliable statistics to be generated on the incidence of cancer in a given population. SEER is one of the largest of these databases, pooling information from 14 different cancer registries. Cancer incidence varies in different countries with respect to lifestyle factors, such as smoking, drinking, diet, etc. Radiation increases the frequency of cancers in the population, and the degree by which it does this has been defined in the Life Span cohort studies after the atomic bombs in Hiroshima and Nagasaki. On average, assuming a gender and age distribution similar to that of the entire US population, the BEIR VII lifetime risk model predicts that about one individual in 100 persons would be expected to develop cancer (solid cancer or leukaemia) from a dose of 100 mSv, whereas about 42 of the 100 individuals would be expected to develop solid cancer or leukaemia from other causes. Lower doses would produce proportionally lower risks, if we assume that the LNT hypothesis is correct. For example, it is predicted that about one individual in 1000 would develop cancer from an exposure to 10 mSv [11].
Environmental Exposure from Nature and Man versus Exposure from Nuclear Power Plant Accidents
The above studies focus largely on radiation doses from gamma radiation, rather than exposure to radioactive particles in fallout. The latter exposure is more relevant to the general public exposed to nuclear power plant accidents, whereas the former is more relevant in determining risk to workers involved in the clean-up of damaged nuclear power plants. Accidents at nuclear power plants are graded on the International Nuclear Radiological Event Scale [12]. There have been only two events that have been classed as major accidents where there have been widespread health and environmental effects as a result of external release of a significant fraction of reactor core inventory. The first was the Chernobyl power plant accident in 1986; the second was the Fukushima accident in 2011. Clinical Oncology produced a special issue to mark the 25th anniversary of the Chernobyl accident [13]. It was published 1 month after the Fukushima accident in 2011. The findings of 25 years of careful scientific investigation into the effects of the Chernobyl accident are summarised below.
The only proven radiobiological effect of the Chernobyl accident on the general population has been an increase in thyroid cancer in those who were young at the time of the accident. The increase was rapid, and is still apparent today, although the level of thyroid cancer is back to that prior to the accident for those who were born from 1987 onwards, after the radioactive iodine disappeared from the environment. There seems to be little difference in the type or the clinical outcome of radiation-induced thyroid cancer when compared with age-matched controls. Thyroid cancer is very amenable to treatment and although 30% of patients may suffer a relapse, only 1% may eventually die of their disease [14]. Of around 6000 diagnosed cases since 1986, only 15 have so far proved fatal [15]. Many of these cases would have been prevented if better measures to limit exposure to radioiodine had been put in place. There is, thus far, no evidence for increases in other diseases in the exposed population at large. Now that the human population has been reduced as a result of the establishment of an exclusion zone around the nuclear power plant, the thriving natural environment around the reactor accident suggests that the presence of higher than background levels of caesium-137 in the environment poses little risk to human or animal health [16]. Life span studies, similar to those carried out in Japan, would be needed in order to identify any further minor health effects [17]. However, these will be expensive to carry out, and may well be expected to give results that will not satisfy the concerns of those who have already made up their minds.
So, what did we learn from Chernobyl? Cancer risk is determined by the age at exposure and the concentration of radioisotopes in particular tissues. Low dose exposure to caesium-137, even over a long period of time, is perhaps not as deleterious to health as we would have predicted. The one thing we did not learn was how to deliver information about radiation risk to an exposed population. There have been considerable psychological consequences, unrelated to the direct radiation risks for human health, from the Chernobyl accident, which have been inadequately quantified [18].
What do we Already Know about Fukushima and What Should we Learn?
This special issue focuses on the evidence that we already have concerning radiation doses and the immediate consequences of the actions taken to minimise radiological risk. The accident at Fukushima happened as a direct consequence of two natural disasters – the Great Tohoku Earthquake and the resultant unprecedented tsunami. The nuclear incident that followed made these events of international concern, particularly given the important political debates around greenhouse gas emissions, power generation and climate change and gave the whole scenario a political dimension that was not present to the same degree after Chernobyl.
The initial priority was the safety of those living in the immediate area. Japan is home to a large number of foreign residents, and became the immediate focus of the international press. The Japanese have great respect for advice from foreigners – therefore the advice given by foreign governments to their residents was watched with interest. Where this advice conflicted with that provided by the local government created considerable psychological pressure. The paper by Oppenheim and Franklin [19] provides a perspective from the staff based at the UK Embassy, and shows the importance of good scientific advice to government at all levels.
Three comprehensive reports have been written following the Fukushima accident [20–22]. The first of these comes under scrutiny in the paper by Yamashita [23] in this special issue and highlights the problems of using conservative estimations of radiation dose when trying to communicate potential health risks to the public. As discussed above, the dose of radiation received by individuals is of paramount importance when determining the risk of a health effect. Actual doses, in particular to the thyroid, were much lower than originally predicted, and this was due in part to the prompt action of the Japanese authorities in evacuating the local population and severing the food chain [24]. The evacuation was itself not without health risks, and the paper by Hasegawa et al. [25] details the harrowing consequences of the need to be seen to respond quickly in response to the public’s fears of radiation exposure. The Japanese public, in particular, given the country’s history of exposure to radiation from the atomic bombs, show heightened concern. This paper should make us question our response to a nuclear accident. Throughout our lives we learn to balance benefit with risk, but in the face of radiation we seem to lose the ability to do this based on science, and rely on instinct alone.
Perhaps it is helpful to view the radiation doses in and around Fukushima in the context of medical radiation exposure. Ninety-six per cent of the restoration workers at the Daiichi reactor site received doses less than 50 mSv [25]. Adults in the evacuation area were exposed to between 1.1 and 13 mSv [24]. A few decades ago, radiotherapy staff received similar doses as a matter of course. In 1981, the average dose received by a nurse at the Royal Beatson Memorial Hospital Glasgow was 19 mSv [26]. Some consultant oncologists received more than 50 mSv annually. Modern medical imaging can produce similar absorbed doses as those recorded in Fukushima. Albert [27] lists mean effective doses from a computed tomography scan of the head of 2 mSv and 31 mSv (range 6–90 mSv) for a multiphase abdominopelvic computed tomography scan. Indeed, the ‘worried well’ will pay for this degree of exposure during whole body screening computed tomography for reassurance they do not have an asymptomatic cancer. The lifetime individual doses for the vast majority of the population exposed after Fukushima (even assuming no remediation, e.g. soil skimming, etc) and some 6 million residents after Chernobyl is 9–10 mSv, i.e. similar to that of a whole body computed tomography scan, compared with 170 mSv lifetime dose from background radiation for a typical resident of Japan [15,22].
In response to the public concern about possible health consequences, a comprehensive medical surveillance study was established (reviewed in [23]). As the only radiobiological consequence of the previous nuclear power plant accident for the population at large had been shown to be thyroid cancer in the young, a comprehensive thyroid ultrasound examination programme in those aged under 19 years at exposure has been instigated [28]. The results of the initial round of screening were intended to be regarded as a baseline. In the interests of transparency, the Japanese authorities and Fukushima Medical University have made all their data publicly available [29]. However, as with all health-related matters, the data need careful interpretation, or will probably be misinterpreted by the media and others, for example as in the recent paper by Tsuda et al. [30]. The paper by Suzuki [28] outlines the results of the thyroid ultrasound examination so far, and puts these into context with data from a similar study carried out in areas of Japan not exposed to fallout from Fukushima.
A phase of recovery follows any societal upheaval, whether the cause of that upheaval was natural or manmade. The personal perspective offered by Mrs Ando [31] gives insight into how taking control of the situation for yourself can enhance resilience. Providing mechanisms for all sectors of society is beneficial and allows individuals to put risks into perspective for themselves. A recent study carried out by Japanese school children has been published in the Journal of Radiological Protection [32]. This study, designed by schoolchildren, used personal dosimeters worn by children and their teachers in four countries (Japan, France, Belarus and Poland). The aim of the study was to compare radiation doses in different areas in Japan and elsewhere. There are a number of monitoring stations across the Fukushima Prefecture that measure the ambient radiation dose in air. These stations were set up with the aim of reassuring the local population and visitors. However, unless context is given to the measurements, most of us would not be able to interpret the data they provide, as we are unaware of what is a ‘normal’ reading for airborne radiation. The study gave the children the ability to understand whether the dose in Fukushima Prefecture was different from elsewhere in the world – particularly important as the media frequently refers to levels of radiation in Fukushima Prefecture as ‘high’, with no context to this assertion. It also enabled them to explore what affected their doses, e.g. the material from which their houses/schools were built. It is disturbing to note that in some of the villages in Fukushima Prefecture some people still feel there is a likelihood of acute radiation syndrome 3 years after the accident [33], despite a considerable amount of effort in risk communication by local scientists. Perhaps initiatives like those undertaken by the schoolchildren could help to dispel some of these misconceptions?
Data are only useful if put into context. Information on radiation dose and the risk it poses to health is no exception to this. One recent article has suggested that exposure to radiation from the atomic bomb or as a clean-up worker after Chernobyl is less damaging to your health than obesity or passive smoking [34]. One question we need to ask ourselves is how we have allowed the scientific facts around the real risks of health effects of low-dose radiation to become occluded by science fiction? Perhaps the reason that we only now feel confident enough to try to set the record straight is that as scientists we prefer not to say anything until we are sure of our facts. We have 70 years of data from the atomic bomb cohorts and 30 years of data from Chernobyl. Surely we should now be able to interpret that data with enough confidence to say that the popular beliefs around radiation risk are incorrect – the scientific data tell a totally different story. What further data do we need? Do we really need to wait a further 30 or 40 years after Fukushima to start drawing the same conclusions as we already have the data to draw – and potentially make the same mistakes over again in the event of another power plant accident? All the public opinion polls [35] tell us that scientists and medical doctors are those most trusted by the public – surely our community should be making our voices heard?
We accept a level of risk in many other areas of life – many of which are statistically more likely to result in something that is deleterious to our health when compared with exposure to low doses of radiation. It is the context in which those risks are put that helps us make a personal decision on whether we wish to take that risk. The personal risk from radiation at low doses is very small, but the fact that scientists are honest and say that it cannot be quantified with any certainty below a certain level of dose, for all the reasons given above, make any exposure to radiation (above that from natural sources) unacceptable to many people. Interestingly, most of us, including those who find risks from nuclear power unacceptable, will not even think about the risk of the dose of radiation we will experience from long-haul flights as we take off for our holidays, or when consenting to a medical procedure involving radiation.
The papers in this special issue should give us pause for thought. Ethics teaches us that we should do no harm to others. If we do not learn the lessons from Fukushima regarding risk communication to the general public (and politicians!) and allow a knee jerk response to a nuclear power plant accident again, we will probably put more people at risk by our actions than the radiation will. There are some efforts being made in this direction. SHAMISEN, a project recently funded by the European Commission (http://www.crealradiation.com/index.php/en/shamisen-home) focuses on the real consequences of a nuclear accident, and one area will look at the ethics of research on those who have to live with these consequences. Does the research we ask them to participate in help them to recover from the accident – or does our thirst for knowledge make things worse?
As a global society we have some hard decisions to make with regard to power generation and global warming. If we are honest, nothing in life is risk free, including methods of generating power. The potential health effects of global warming are considerable, and will certainly result in many more deaths than either of the two nuclear power plant accidents that have resulted in radioactive releases to the general environment.
It is to be hoped that this time we will learn the lessons from Fukushima that our Japanese colleagues have shared so openly with us in this special issue and revise the advice given to government and emergency planners in dealing with nuclear emergencies. We have focused too much on the science of radiation and not enough on the communication of what that science really means for those around us – something we would all do well to consider in our scientific careers.
References
- 1.Kamiya K, Ozasa K, Akiba S, et al. Long-term effects of radiation exposure on health. Lancet. 2015;386(9992):469–478. doi: 10.1016/S0140-6736(15)61167-9. [DOI] [PubMed] [Google Scholar]
- 2.Muirhead CR, O’Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100(1):206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schonfeld SJ, Krestinina LY, Epifanova S, Degteva MO, Akleyev AV, Preston DL. Solid cancer mortality in the Techa river cohort (1950–2007) Radiat Res. 2013;179(2):183–189. doi: 10.1667/RR2932.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashcheev VV, Chekin SY, Maksioutov MA, et al. Incidence and mortality of solid cancer among emergency workers of the Chernobyl accident: assessment of radiation risks for the follow-up period of 1992–2009. Radiat Environ Biophys. 2015;54(1):13–23. doi: 10.1007/s00411-014-0572-3. [DOI] [PubMed] [Google Scholar]
- 5.Tao Z, Akiba S, Zha Y, et al. Cancer and non-cancer mortality among inhabitants in the high background radiation area of Yangjiang, China (1979–1998) Health Phys. 2012;102(2):173–181. doi: 10.1097/HP.0b013e31822c7f1e. [DOI] [PubMed] [Google Scholar]
- 6.Gillies M, Haylock R. The cancer mortality and incidence experience of workers at British Nuclear Fuels plc, 1946–2005. J Radiol Prot. 2014;34(3):595–623. doi: 10.1088/0952-4746/34/3/595. [DOI] [PubMed] [Google Scholar]
- 7.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2:e276–e281. doi: 10.1016/S2352-3026(15)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardis E, Vrijheid M, Blettner N, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 9.Krestinina LY, Davis FG, Schonfeld S, et al. Leukaemia incidence in the Techa River Cohort: 1953–2007. Br J Cancer. 2013;109:2886–2893. doi: 10.1038/bjc.2013.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwasaki T, Murata M, Ohshima S, et al. Second analysis of mortality of nuclear industry workers in Japan, 1986–1998. Radiat Res. 2003;159:228–238. doi: 10.1667/0033-7587(2003)159[0228:saomon]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health risks from exposure to low levels of Ionizing Radiation: BEIR VII phase 2. Washington, DC: National Academy Press; 2006. [PubMed] [Google Scholar]
- 12.International Nuclear Radiological Events Scale. Available at: https://www.iaea.org/sites/default/files/ines.pdf.
- 13.http://www.journals.elsevierhealth.com/periodicals/chernobyl.
- 14.Tuttle RM, Vaisman F, Tronko MD. Clinical presentation and clinical outcomes in Chernobyl-related paediatric thyroid cancers: what do we know now? What can we expect in the future? Clin Oncol (R Coll Radiol) 2011;23(4):268–275. doi: 10.1016/j.clon.2011.01.178. [DOI] [PubMed] [Google Scholar]
- 15.UNSCEAR Report to the General Assembly of the United Nations. Annex D. Health effects due to radiation from the Chernobyl accident. 2008 Available at: http://www.unscear.org/docs/reports/2008/Advance_copy_Annex_D_Chernobyl_Report.pdf.
- 16.Deryabina TG, Kuchmel SV, Nagorskaya LL, et al. Long-term census data reveal abundant wildlife populations at Chernobyl. Curr Biol. 2015;25(19):R824–R826. doi: 10.1016/j.cub.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Final Report: Agenda for Research on Chernobyl Health (ARCH): To develop a strategic plan for research on the health consequences of radiation from the Chernobyl accident. Available at: http://cordis.europa.eu/publication/rcn/13497_en.html.
- 18.Bromet EJ, Havenaar JM, Guey LT. A 25 year retrospective review of the psychological consequences of the Chernobyl accident. Clin Oncol (R Coll Radiol) 2011;23(4):297–305. doi: 10.1016/j.clon.2011.01.501. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheim RJ, Franklin KC. The aftermath of the Fukushima Daiichi accident: a perspective from the British Embassy in Tokyo. Clin Oncol (R Coll Radiol) 2016;28:000–000. doi: 10.1016/j.clon.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 20.WHO report on health risk assessment from the nuclear accident after the 2011 Great East Japan earthquake and tsunami, based on a preliminary dose estimation. 2013 Available at: http://apps.who.int/iris/bitstream/10665/78218/1/9789241505130_eng.pdf?ua=1.
- 21.UNSCEAR report (2013): Levels and effects of radiation exposure due to the nuclear accident after the 2011 great east-Japan earthquake and tsunami, Volume 1, Annex A. Available at: http://www.unscear.org/docs/reports/2013/1406336_Report_2013_Annex_A_Ebook_website.pdf.
- 22.IAEA report. The Fukushima Dai-ichi accident. 2015 Technical Volume 4/5. Available at: http://www-pub.iaea.org/MTCD/Publications/PDF/AdditionalVolumes/P1710/Pub1710-TV4-Web.pdf.
- 23.Yamashita S. Comprehensive health risk management after the Fukushima nuclear power plant accident. Clin Oncol (R Coll Radiol) 2016;28:000–000. doi: 10.1016/j.clon.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Nagataki S, Takamura N. Radioactive doses – predicted and actual – and likely health effects. Clin Oncol (R Coll Radiol) 2016;28:000–000. doi: 10.1016/j.clon.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa A, Ohira T, Maeda M, Yasumura S, Tanigawa K. Emergency responses and health consequences after the Fukushima accident; evacuation and relocation. Clin Oncol (R Coll Radiol) 2016;28:000–000. doi: 10.1016/j.clon.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Jones RD, Symonds RP, Habeshaw T, Watson ER, Laurie J, Lamont DW. A comparison of remote afterloading and manually inserted caesium in the treatment of carcinoma of cervix. Clin Oncol. 1990;2:193–198. doi: 10.1016/s0936-6555(05)80167-0. [DOI] [PubMed] [Google Scholar]
- 27.Albert JM. Radiation risk from CT: implications for cancer screening. AJR. 2013;201:W81–W87. doi: 10.2214/AJR.12.9226. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki S. Childhood and adolescent thyroid cancer in Fukushima after the Fukushima Daiichi nuclear power plant accident: 5 years on. Clin Oncol (R Coll Radiol) 2016;28:000–000. doi: 10.1016/j.clon.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima Medical University, Radiation and Health. http://fmu-global.jp.
- 30.Tsuda T, Tokinobu A, Yamamoto E, Suzuki E. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ando R. Reclaiming our lives in the wake of a nuclear plant accident. Clin Oncol (R Coll Radiol) 2016;28:000–000. doi: 10.1016/j.clon.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Adachi N, Adamovitch V, Adjovi Y, et al. Measurement and comparison of individual external doses of high-school students living in Japan, France, Poland and Belarus-the ’D-shuttle’ project. J Radiol Prot. 2015;36(1):49–66. doi: 10.1088/0952-4746/36/1/49. [DOI] [PubMed] [Google Scholar]
- 33.Orita M, Hayashida N, Nakayama Y, et al. Bipolarization of risk perception about the health effects of radiation in residents after the accident at Fukushima nuclear power plant. PLoS One. 2015;10(6):e0129227. doi: 10.1371/journal.pone.0129227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JT. Are passive smoking, air pollution and obesity a greater mortality risk than major radiation incidents? BMC Public Health. 2007;7:49. doi: 10.1186/1471-2458-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eurobarometer Survey 2013. http://ec.europa.eu/public_opinion/archives/ebs/ebs_340_en.pdf.