Introduction
Lumbar interbody fusion (LIF) is a procedure that is indicated for various spinal disorders including degenerative disc disease, instability and deformity, neoplasia, infection, and traumatic pathologies. Degenerative pathologies include discogenic lower back pain, radiculopathy due to foraminal stenosis, lumbar degenerative spinal deformity, and spondylolisthesis (1). There are various approaches to LIF: anterior (ALIF), posterior (PLIF), transforaminal (TLIF), oblique (OLIF) and lateral (LLIF). The LIF involves placement of an implant device following discectomy and endplate preparation (2,3). ALIF was first described in the treatment of Pott’s disease (4), and over time, ALIF procedure has been studied extensively and is now a commonly performed procedure for degenerative lumbar spinal disease (3,5). Unlike the PLIF technique, the anterior approach does not require access through the spinal canal with retraction of the nerve roots and cauda equina. Furthermore, it spares potential iatrogenic injury to the posterior spinal muscles (2).
Clinical vignette
The authors report a case of a 43-year-old female presenting with multiple recurrent disc herniation, radiculopathy and discogenic lower back pain. Over the 6 months prior to presentation, the patient had undergone two posterior microdiscectomy procedures, and now presents with a further recurrent disc herniation, worsening low back pain, and severe S1 radiculopathy. Due to significant loss of disc height, Modic endplate changes and further disc sequestration (Figure 1), a decision was made to redo the discectomy and perform a fusion procedure. Removal of the disc material in the canal was performed using a microscope (not shown in Figure 2), with a standard L5/S1 ALIF technique via a retroperitoneal approach.
Figure 1.
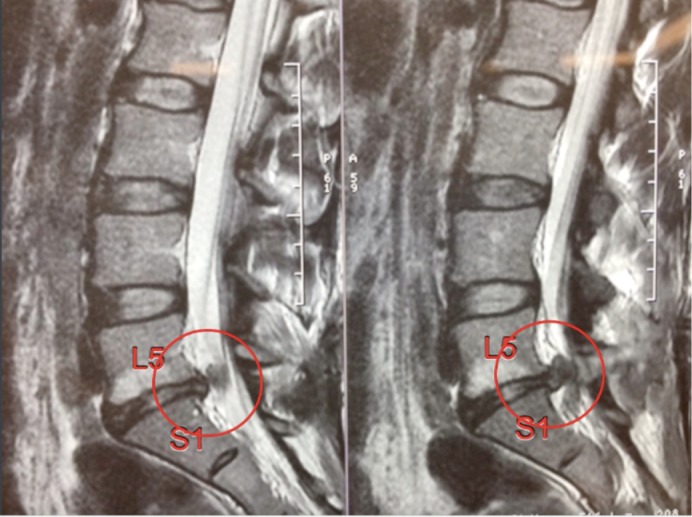
Recurrent disc herniation L5/S1 with Modic endplate changes and loss of disc height.
Figure 2.

L5/S1 anterior lumbar interbody fusion technique and workflow (6). Available online: http://www.asvide.com/articles/1750
There are multiple techniques for performing LIF. However, the L5/S1 level is particularly suitable for the ALIF approach due to the efficient vascular access below with bifurcation of the aorta and inferior vena cava. Hence, the L5/S1 discectomy with an anterior approach is suggested as the choice of treatment. The approach of this discectomy is documented in Figure 2.
Technical note
The patient was placed in the supine position. For the L5/S1 exposure, a transverse incision (mini-Pfannenstiel) is performed between the umbilicus and the symphysis pubis. Dissection of skin and soft tissue is done with the diathermy with an inferior and superior flap raised to give the vertical exposure. The exposed linea alba is divided using monopolar diathermy. Tissue forceps are used to elevate and retract the left sided rectus muscles so that the retroperitoneal plane can be entered.
The retroperitoneum is approached with blunt dissection, the inferior epigastric vessels are visualized, preserved and retracted anteriorly. The psoas muscle and the genitofemoral nerve are visualized. As the vessels are identified (left common iliac artery and vein), a low profile narrow ring-based retractor blade system is positioned (Figure 3). The iliac arteries and veins are then exposed and retracted laterally to reveal the L5/S1 disc space, with the median sacral vessels double clipped and divided.
Figure 3.
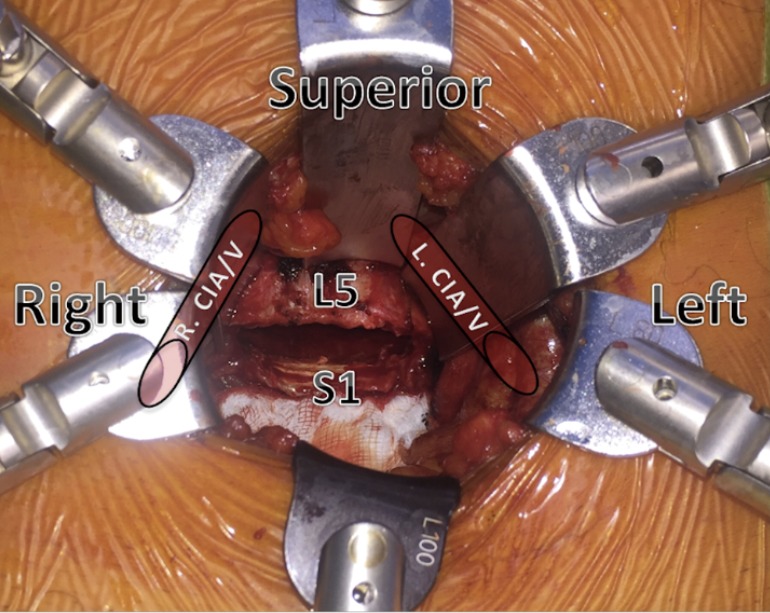
Top view demonstrating the position of the iliac vessel in relation to the disc space. R CIA/V, right common iliac artery and vein; L CIA/V, left common iliac artery and vein.
The anterior disc space dissection is performed with peanut dissectors to avoid diathermy injury to the sympathetic nerves that cross the L5/S1 disc to reduce the risk of retrograde ejaculation. The discectomy is approached with an annulotomy spanning the full anterior aspect of the L5/S1 disc. Using a Cobb elevator, the plane between the bony and cartilaginous endplate is developed. Using a rotatable distractor the disc height elevation is provided for efficient disc removal with a piecemeal approach using a pituitary rongeur. A microscope can now be used for visualization of the Posterior Longitudinal Ligament, with further disc removal of sequestered fragments in the canal to complete the decompression.
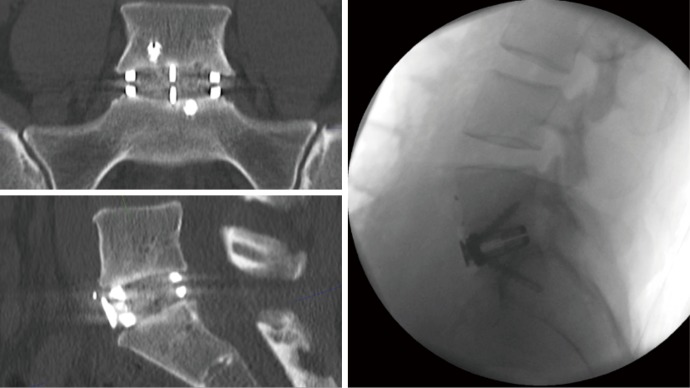
During preparation of the disc space and decompression of the neural elements, the bone graft material is prepared. To facilitate bone growth and fusion, bone graft is added to the implant device; the bone graft is prepared with allograft (Allovance, Ausbiotechnologies) and BMP (Infuse, Medtronic). Prior to the disc prosthesis implant, a trial prosthesis is inserted to select for the best implant fit. X-ray is performed to confirm depth, position, and lordosis. The appropriate implant is chosen (Redmond ALIF, A-Spine ASIA) and is packed with bone graft, and ready for implantation. Implant device is secured with integral screw fixation. Following haemostasis, the retractors are removed the peritoneum returns to its position. The linea alba is closed with heavy PDS, with standard subcutaneous and skin closure. Three months post-surgery the patient recovered with acceptable cosmesis from the mini-pfannenstiel excision. Postoperative X-ray (day 1) and CT scan (3 months) demonstrated excellent implant position, restoration of disc height and focal lordosis (Figure 4).
Figure 4.
Postoperative X-ray (day 1) and CT scan (3 months) demonstrating excellent implant position, restoration of disc height and focal lordosis.
Comments
ALIF is particularly useful for procedures at the L4/L5 & L5/S1 level as it allows excellent visualization and access to ventral surface of the disc space. The ALIF approach has demonstrated high fusion rates, good radiological outcomes, good restoration of disc height & lordosis, and also a reduced risk for dural injuries (3,7,8). However, with the anterior approach there is the need to access through the abdomen via a retroperitoneal approach, with the potential risk for vascular and visceral injuries; and retrograde ejaculation (2,3,7,8). The vascular injury is a major concern, however when the ALIF procedure is performed by a team of a vascular surgeon and spine surgeon—there is a reduction in vascular injuries, operation time and duration of hospital stay (9,10). Furthermore, there is a lower complication rate with regards to harvest of autograft, and a higher fusion rate when bone graft substitutes such as allograft and bone morphogenetic protein is added to the implant device for the ALIF procedure (11).
There are several patient characteristics and risk factors which significantly influence the clinical outcomes and complication rates of ALIF surgery. These include elderly age (12), worker’s compensation status (13) and patient frailty (14). We did not find overweight or obesity (15), nor the presence of an “access surgeon” (16) as a significant factor contributing to ALIF outcomes.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine 2005;2:670-2. 10.3171/spi.2005.2.6.0670 [DOI] [PubMed] [Google Scholar]
- 2.Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teng I, Han J, Phan K, et al. A meta-analysis comparing ALIF, PLIF, TLIF and LLIF. J Clin Neurosci 2017; 44:11-7. 10.1016/j.jocn.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 4.Ito H, Tsuchiya J, Asami G. Anew radical operation for Pott’s disease. J Bone Joint Surg Br 1934;16:499-515. [Google Scholar]
- 5.Mobbs RJ, Loganathan A, Yeung V, et al. Indications for anterior lumbar interbody fusion. Orthop Surg 2013;5:153-63. 10.1111/os.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mobbs RJ, Lennox A, Ho YT, et al. L5/S1 anterior lumbar interbody fusion technique and workflow. Asvide 2017;4:434. Available online: http://www.asvide.com/articles/1750
- 7.Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 2015;29:705-11. 10.3109/02688697.2015.1036838 [DOI] [PubMed] [Google Scholar]
- 8.Giang G, Mobbs R, Phan S, et al. Evaluating Outcomes of Stand-Alone Anterior Lumbar Interbody Fusion: A Systematic Review. World Neurosurg 2017;104:259-71. 10.1016/j.wneu.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 9.Mobbs RJ, Phan K, Daly D, et al. Approach-Related Complications of Anterior Lumbar Interbody Fusion: Results of a Combined Spine and Vascular Surgical Team. Global Spine J 2016;6:147-54. 10.1055/s-0035-1557141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asha MJ, Choksey MS, Shad A, et al. The role of the vascular surgeon in anterior lumbar spine surgery. Br J Neurosurg 2012;26:499-503. 10.3109/02688697.2012.680629 [DOI] [PubMed] [Google Scholar]
- 11.Malham GM, Parker RM, Ellis NJ, et al. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine 2014;21:851-60. 10.3171/2014.8.SPINE13524 [DOI] [PubMed] [Google Scholar]
- 12.Phan K, Ramachandran V, Tran T, et al. Impact of Elderly Age on Complications and Clinical Outcomes Following Anterior Lumbar Interbody Fusion Surgery. World Neurosurg 2017;105:503-9. 10.1016/j.wneu.2017.05.056 [DOI] [PubMed] [Google Scholar]
- 13.Phan K, Davies S, Rao PJ, et al. Worker's Compensation Status and Outcomes Following Anterior Lumbar Interbody Fusion: Prospective Observational Study. World Neurosurg 2017;103:680-5. 10.1016/j.wneu.2017.04.123 [DOI] [PubMed] [Google Scholar]
- 14.Phan K, Kim JS, Lee NJ, et al. Frailty is associated with morbidity in adults undergoing elective anterior lumbar interbody fusion (ALIF) surgery. Spine J 2017;17:538-44. 10.1016/j.spinee.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 15.Phan K, Rogers P, Rao PJ, et al. Influence of obesity on complications, clinical outcome and subsidence following anterior lumbar interbody fusion (ALIF): prospective observational study. World Neurosurg 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Phan K, Xu J, Scherman DB, et al. Anterior Lumbar Interbody Fusion With and Without an "Access Surgeon": A Systematic Review and Meta-analysis. Spine (Phila Pa 1976) 2017;42:E592-E601. 10.1097/BRS.0000000000001905 [DOI] [PubMed] [Google Scholar]