Abstract
Many areas of the cerebral cortex process sensory information or coordinate motor output necessary for control of movement. Disturbances in cortical cholinergic system can affect locomotor coordination. Spinal cord injury causes severe motor impairment and disturbances in cholinergic signalling can aggravate the situation. Considering the impact of cortical cholinergic firing in locomotion, we focussed the study in understanding the cholinergic alterations in cerebral cortex during spinal cord injury. The gene expression of key enzymes in cholinergic pathway - acetylcholine esterase and choline acetyl transferase showed significant upregulation in the cerebral cortex of spinal cord injured group compared to control with the fold increase in expression of acetylcholine esterase prominently higher than cholineacetyl transferase. The decreased muscarinic receptor density and reduced immunostaining of muscarinic receptor subtypes along with down regulated gene expression of muscarinic M1 and M3 receptor subtypes accounts for dysfunction of metabotropic acetylcholine receptors in spinal cord injury group. Ionotropic acetylcholine receptor alterations were evident from the decreased gene expression of alpha 7 nicotinic receptors and reduced immunostaining of alpha 7 nicotinic receptors in confocal imaging. Our data pin points the disturbances in cortical cholinergic function due to spinal cord injury; which can augment the locomotor deficits. This can be taken into account while devising a proper therapeutic approach to manage spinal cord injury.
Keywords: Cerebral cortex, Cholinergic receptors, Muscarinic, Nicotinic
Highlights
-
•
Spinal cord injury (SCI) can affect cortical cholinergic signalling.
-
•
Diminished activity of motor cortex can augment the injury severity.
-
•
Muscarinic and nicotinc acetyl choline receptors were decreased in SCI.
-
•
Disturbed expression of enzymes in cholinergic metabolism was also observed.
-
•
Proper regulation of cholinergic system can be targeted for management of SCI.
1. Introduction
Understanding the central nervous system pathways affected during spinal lesions as in the case of spinal cord injury will help to establish a proper therapy for patients with such devastating conditions. The changes at the level of neurotransmitters in the motor cortex during spinal cord injury are of special importance to understand the exact molecular mechanisms of brain- spinal cortex motor coordination. Many studies reported the involvement of various cortical regions in regulating the locomotor function during functional recovery in stroke patients [5], [12], [16], [23]. Most of these studies are insufficient in concluding the exact firing mechanisms involved in this functional recovery and hence understanding the role of neurotransmitter alterations in cerebral cortex can shed light to its role in motor control.
ACh release from the frontal cortex and hippocampus has several components, one of which is motor activity. The involvement of cholinergic receptors, both muscarinic and nicotinic, in regulating spinal locomotor network is already known [19], [20], [25]. Central muscarinic receptors are known to play key roles in memory and learning as well as in the regulation of many sensory, motor, and autonomic processes [11]. It is reported that muscarinic cholinergic effects of ACh are important in the normal function of both the sensory and motor systems [7]. In mammals, nicotinic AChR also play a crucial role in motor control [15]. Following peripheral nerve injury, the expression of numerous receptors involved in nociceptive processing is altered in the superficial dorsal horn of the spinalcord. Activation of nAChR promotes survival of chicken spinal motoneurons that would otherwise undergo apoptosis when deprived of trophic factors [17]. Among the nicotinic acetylcholine receptors, alpha 7 subunit is reported to have an important role in motor control [25].
ACh is synthesized primarily by choline acetyltransferase (ChAT) from coenzyme A and choline [24]. In neurons, ACh is transported into vesicles by the vesicular ACh transporter, the entire coding sequence of which is contained in the first intron of the ChAT gene in mammals [2], [6]. ACh is broken down by acetylcholinesterase (AChE), which is expressed in most tissues. Therefore ACh is confined to its area of synthesis and release and hence the quantification of its metabolic enzymes can be a direct index of Ach activity. Many previous studies used ChAT as a marker for the status of cholinergic transmission [9], [10], [21] and indicators of the functional stage of cholinergic neurons in the CNS [1]. Cholinergic input can be assessed by the activity of AChE and ChAT [4], [14].
The present study was designed to investigate the regulation of cholinergic function in the cerebral cortex of spinal cord injured rats. The cholinergic function was studied by evaluating the mRNA expression of its two key enzymes- AChE and ChAT. The receptor level changes were analyzed for metabotropic muscarinic receptors by receptor assays for total muscarinic, muscarinic M1 and M3 receptor subunits and gene expression studies using Real Time PCR. The ionotropic nicotinic receptors expression were also evaluated using Real Time PCR analysis and confocal imaging of alpha 7 nicotinic acetyl choline receptors using FITC tagged secondary antibodies. This study will help to open up new possibilities for a better therapy to deal with motor deficits in spinal cord injured patients.
2. Materials and methods
2.1. Animals
Male adult Wistar rats of 200–250 g body weight were used for all experiments. They were housed in separate cages under 12-h light and 12-h dark periods and were maintained on standard food pellets and water ad libitum. All animal care and procedures were in accordance with Institutional and National Institute of Health guidelines and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines.
2.2. Chemicals
Biochemicals used in the present study were purchased from Sigma Chemical Co., St. Louis, USA. All other reagents of analytical grade were purchased locally. Quinuclidinyl benzilate, L-[Benzilic-4, 4′-3H], ([3H] QNB) (Sp. Activity 42 Ci/mmol) and 4-DAMP, [N-methyl-3H] (Sp. Activity 83 Ci/mmol) were from NEN Life Sciences Products Inc., Boston, USA. Atropine, Pirenzepine and 4-DAMP were from Sigma Chemical Co., USA. Tri-reagent kit was purchased from MRC, USA. Real-time-PCR Taqman probe assays on demand were from Applied Biosystems, Foster City, CA, USA.
2.3. Induction of spinal cord injury and treatment group
Adult Wistar male rats (weight) were randomly divided into the following groups (a) Control (b) Spinal cord injured (SCI). Sham operated rats were used as control. Spinal cord injury was induced in adult Wistar rats by shearing between the T12 and T13 vertebra. The monoplegic rats were kept for 21 days and sacrificed by decapitation on the 22nd day of the experiment. The cerebral cortex was dissected out quickly over ice and the tissues were stored at −80 °C for various experiments.
2.4. Behavioural analysis of motor function by rotarod test
Rotarod has been used to evaluate motor coordination by testing the ability of rats to remain on revolving rod. The apparatus has a horizontal rough metal rod of 3 cm diameter attached to a motor with variable speed. This 70 cm long rod was divided into four sections by wooden partitions. The rod was placed at a height of 50 cm to discourage the animals to jump from the rotating rod. The rate of rotation was adjusted in such a manner that it allowed the normal rats to stay on it for five minutes. Each rat was given five trials before the actual reading was taken. The readings were taken at 10, 15 and 25 rpm after 21 days of treatment in all groups of rats.
2.5. Gene expression analysis of ChAT, AChE, muscarinic M1, M3 and α7 nicotinic acetylcholine receptors subunits using Real time PCR
RNA was isolated from cerebral cortex using Tri reagent. Total cDNA synthesis was performed using ABI PRISM cDNA Archive kit. Real–Time PCR assays were performed in 96-well plates in an ABI 7300 Real–Time PCR instrument (Applied Biosystems, Foster City, CA, USA). PCR analyses were conducted with gene-specific primers and fluorescently labeled Taq probe for ChAT (Rn 01453446_m1), AChE (Rn 00596883_ m1), muscarinic M1 receptor (Rn 00589936_s1), muscarinic M3 (Rn 00560986_s1) and α7 nicotinic acetylcholine (Rn01644792_g1) receptor designed by Applied Biosystems. Endogenous control (β-actin) labeled with a reporter dye was used as internal control. All reagents were purchased from Applied Biosystems. The real-time data were analyzed with Sequence Detection Systems software version 1.7. All reactions were performed in duplicate.
The ΔΔCT method of relative quantification was used to determine the fold change in expression. This was done by first normalizing the resulting threshold cycle (CT) values of the target mRNAs to the CT values of the internal control β-actin in the same samples (ΔCT = CT Target − CT β-actin). It was further normalized with the control (ΔΔCT=ΔCT − CT Control). The fold change in expression was then obtained (2-ΔΔCT).
2.6. Total muscarinic, muscarinic M1 and muscarinic M3 receptor binding studies in cerebral cortex
Cerebral cortex was homogenised in 1 mM EDTA – Tris buffer, pH 7.4 and was centrifuged at 30,000×g for 30 min. The pellet obtained was again re-suspended in EDTA – Tris buffer and used for further experiment. Binding assay was done according to the modified procedure of Yamamura and Synder, 1974 [22]. Total muscarinic, and muscarinic M1 receptor binding parameter assays were done using [3H] QNB (0.1–2.5 nM) and M3 receptor using [3H] DAMP (0.01–5 nM). The nonspecific binding was determined using 100 μM atropine for total muscarinic, pirenzepine for muscarinic M1 and 4-DAMP mustard for M3 receptor. Total incubation volume of 250 μl contains 200–250 μg protein concentrations. Tubes were incubated at 22 °C for 60 min and filtered rapidly through GF/C filters (Whatman). The filters were washed quickly by three successive washing with 5.0 ml of ice cold 50 mM Tris–HCl buffer, pH 7.4. Bound radioactivity was counted with cocktail-T in a Wallac 1409 liquid scintillation counter. Protein was measured by the method of Lowry et al. [13], [18].
2.7. Analysis of the receptor-binding data
The data were analyzed according to Scatchard, 1946 [18]. The specific binding was determined by subtracting non-specific binding from the total. The binding parameters, maximal binding (Bmax) and equilibrium dissociation constant (Kd), were derived by linear regression analysis by plotting the specific binding of the radioligand on the X-axis and bound/free on the Y-axis. The Bmax is a measure of the total number of receptors present in the tissue and the Kd is the measure of the affinity of the receptors for the radioligand. The Kd is inversely related to receptor affinity.
2.8. Immunohistochemistry of muscarinic M1 and α7 nicotinic acetylcholine receptors in the cerebral cortex of experimental rats using confocal microscope
The experimental rats were anesthetized and were transcardially perfused with PBS, pH, 7.4, followed by 4% paraformaldehyde in PBS. After perfusion the brain was dissected and immersion fixed in 4% paraformaldehyde for 1 h and then equilibrated with 30% sucrose solution in 0.1 M PBS, pH 7.0. 10 µm sections of cerebral cortex were cut using Cryostat (Leica, CM1510 S). The sections were treated with PBST (PBS in 0.01% Triton X-100) for 20 min. Cerebral cortex slices were incubated overnight at 4 °C with primary antibody for muscarinic M1, M3 or α7 nicotinic acetylcholine receptor, diluted in PBST at 1:500 dilution (polyclonal or monoclonal). After overnight incubation, the slices were rinsed with PBST and then incubated with secondary antibody labeled with FITC. The sections were observed and photographed using confocal imaging system (Leica SP 5). Expressions of muscarinic M1 and α7 nicotinic acetylcholine receptor were analyzed using pixel intensity method.
2.9. Statistical analysis
Statistical evaluations were done by ANOVA using InStat (Ver.2.04a) computer program. Linear regression Scatchard plots were made using Sigma Plot (Ver 2.03) Sigma Plot software (version 2.0, Jandel GmbH, Erkrath, Germany). Relative Quantification Software was used for analyzing real-time PCR results.
3. Results
3.1. Behavioural analysis of motor function by rotarod test
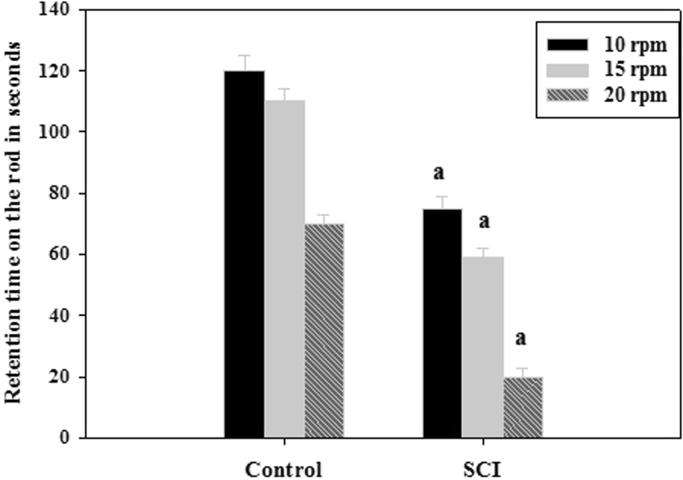
To assess voluntary motor coordination, a rotarod test was performed. Rotarod experiment showed a significant (p<0.001) decrease in the retention time on the rotating rod in the SCI rats at 10, 15 and 25 rpm when compared to control (Fig. 1). The sham operated control rats successfully executed the test, showing that their motor coordination was not affected by the surgical procedure.
Fig. 1.
Retention time of experimental rats in rotarod. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. a p<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
3.2. Gene expression analysis of ChAT and AChE in the cerebral cortex using Real Time PCR
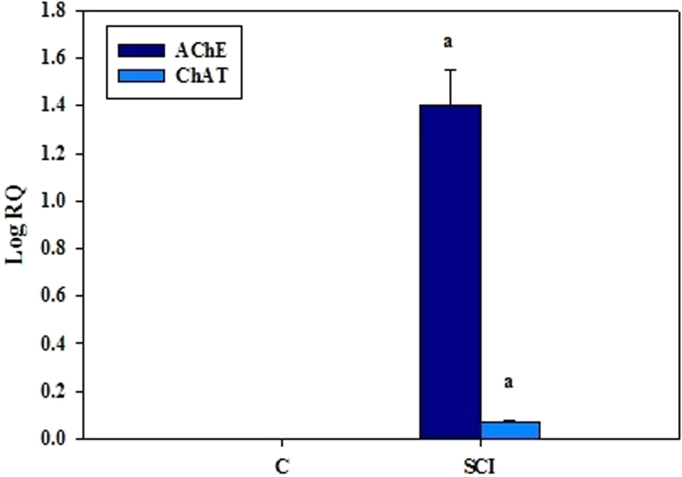
Gene expression of both acetylcholine esterase and choline acetyl transferase mRNA showed significant (p<0.001) up regulation in the cerebral cortex of SCI rats compared to control with the fold increase in expression of AChE is prominently higher than ChAT. (Fig. 2).
Fig. 2.
Real Time PCR amplification of acetylcholine esterase and choline acetyl transferase mRNA in the cerebral cortex of control and experimental rats. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. a p<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
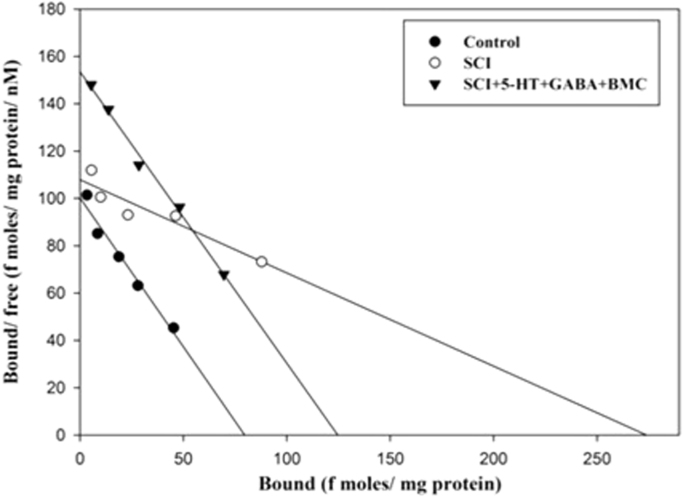
3.3. Scatchard analysis of [3H] QNB binding against atropine to total muscarinic receptor in the cerebral cortex
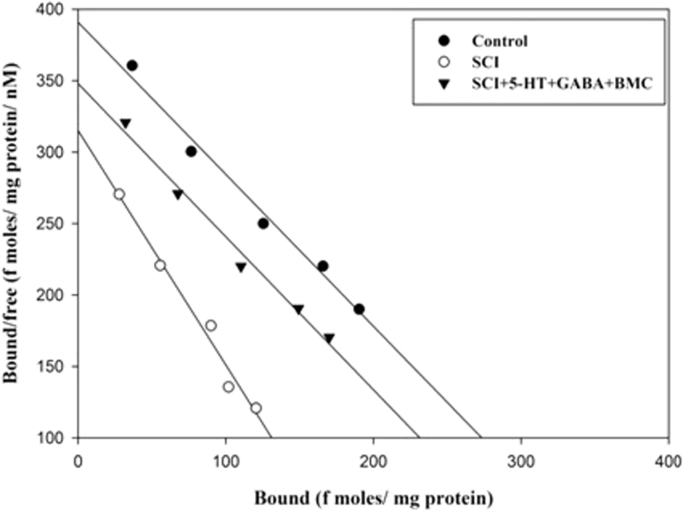
The total muscarinic receptor was assayed using the specific ligand, [3H] QNB and muscarinic general antagonist atropine. The Scatchard analysis showed that the Bmax and Kd decreased significantly (p<0.001) in SCI rats compared to control group. (Fig. 3, Table 1).
Fig. 3.
Scatchard analysis of [3H] QNB binding against atropine to total muscarinic receptor in the cerebral cortex. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. ap<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
Table 1.
Scatchard analysis of muscarinic receptor subtypes in the cerebral cortex.
| Experimental groups |
Total muscarinic receptor |
Muscarinic M1 receptor |
Muscarinic M3 receptor |
|||
|---|---|---|---|---|---|---|
| Bmax (fmoles/mg protein) | Kd (nM) | Bmax (fmoles/mg protein) | Kd (nM) | Bmax (fmoles/mg protein) | Kd (nM) | |
| Control | 273.44±26.34 | 0.72±0.04 | 141.09±11.02 | 1.15±0.07 | 80.38±6.6 | 0.81±0.06 |
| SCI | 131.57±12.3a | 0.45±0.03a | 91.14±6.50a | 1.37±0.08a | 272.49±22.8a | 2.5±0.02a |
Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats.
p<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
3.4. Scatchard analysis of [3H] QNB binding against pirenzepine to muscarinic M1 receptor subunit in the cerebral cortex
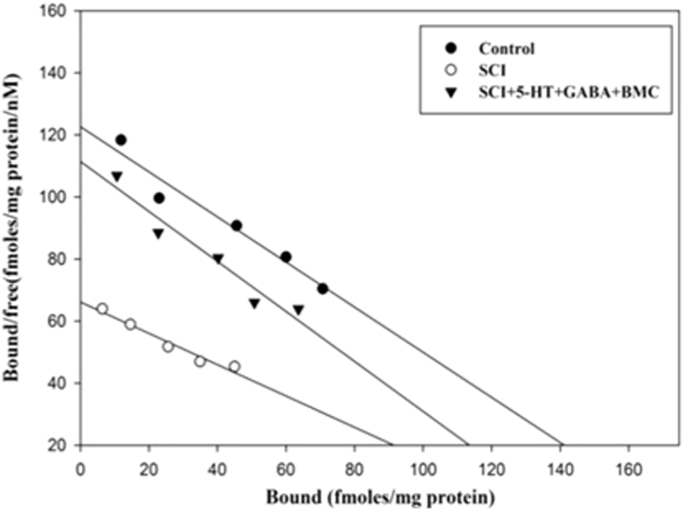
Binding analysis of muscarinic M1 receptor was done using [3H] QNB and M1 subtype specific antagonist pirenzepine. The Bmax decreased and Kd increased significantly (p<0.001) in SCI group when compared to control group. (Fig. 4, Table 1).
Fig. 4.
Scatchard analysis of [3H] QNB binding against pirenzepine to muscarinic M1 receptor subunit in the cerebral cortex. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. a p<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
3.5. Scatchard analysis of [3H] DAMP binding against 4-DAMP mustard to muscarinic M3 receptor subunit in the cerebral cortex
Binding analysis of muscarinic M3 receptors was done using [3H] DAMP and M3 subtype specific antagonist 4-DAMP mustard. The Bmax and Kd were increased significantly (p<0.001) in SCI group when compared to control. (Fig. 5, Table 1).
Fig. 5.
Scatchard analysis of [3H] DAMP binding against 4- DAMP mustard to muscarinic M3 receptor subunit in the cerebral cortex. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. a p<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
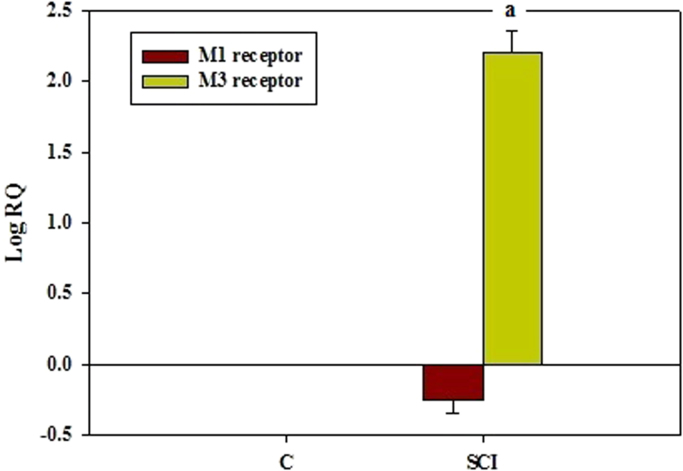
3.6. Gene expression analysis of muscarinic M1 and M3 receptor subunits in the cerebral cortex using Real Time PCR
Real Time-PCR analysis showed that the muscarinic M1 receptor subunit mRNA was down regulated and M3 receptor subunits was up regulated significantly (p<0.001) in SCI compared to control rats (Fig. 6). Even though M3 receptor subunit showed an upregulation of its gene with an increase in its receptor density, the receptor assay for total muscarinic receptors showed a significant decrease (Table 1), pointing to an overall decrease in muscarinic receptor function.
Fig. 6.
Real Time PCR amplification of muscarinic M1 and M3 receptor subunits in the cerebral cortex. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. a p<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
3.7. Confocal imaging of Muscarinic M1 receptor subunits in the cerebral cortex of control and spinal cord injured rats
Muscarinic M1 receptor subunit antibody staining in the cerebral cortex showed a significant (p<0.001) decrease in the mean pixel value in SCI rats compared to control. (Fig. 7, Table 2).
Fig. 7.
Confocal imaging of Muscarinic M1 receptor subunit in the cerebral cortex. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. C – Control, SCI – Spinal cord injury group Scale bar =150 µm.
Table 2.
Mean Pixel intensity of control and Spinal cord injured rats.
| CONDITION | MEAN PIXEL INTENSITY |
|
|---|---|---|
| Muscarinic M1 Receptor | α7 nicotinic acetylcholine receptor | |
| Control | 46.31±3.76 | 49.54±3.23 |
| SCI | 19.14±1.27a | 17.83±1.32a |
Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. C – Control, SCI – Spinal cord injury.
p<0.001 when compared to control.
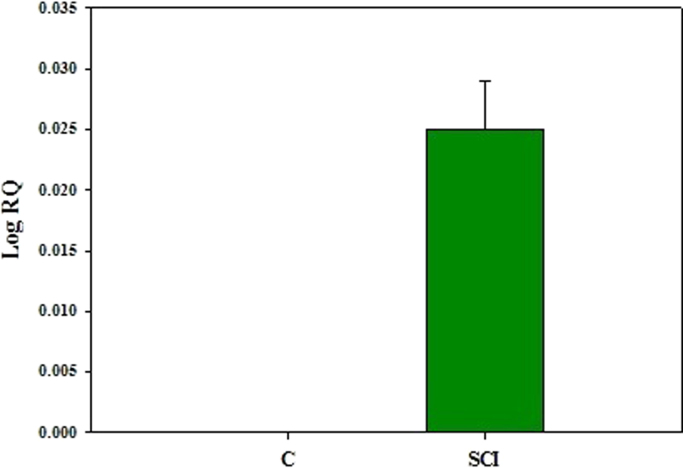
3.8. Gene expression analysis of α7 nicotinic acetylcholine receptor subunit in the cerebral cortex using Real Time PCR
Real Time-PCR analysis showed that α7 nicotinic acetylcholine receptor gene expression was significantly (p<0.001) up regulated in SCI rats when compared to control. (Fig. 8).
Fig. 8.
Real Time PCR amplification of α7 nicotinic acetylcholine receptor subunit in the cerebral cortex. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. a p<0.001 when compared to control. C – Control, SCI – Spinal cord injury group.
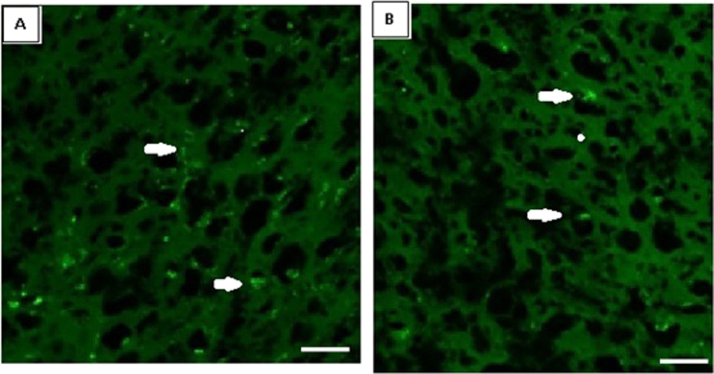
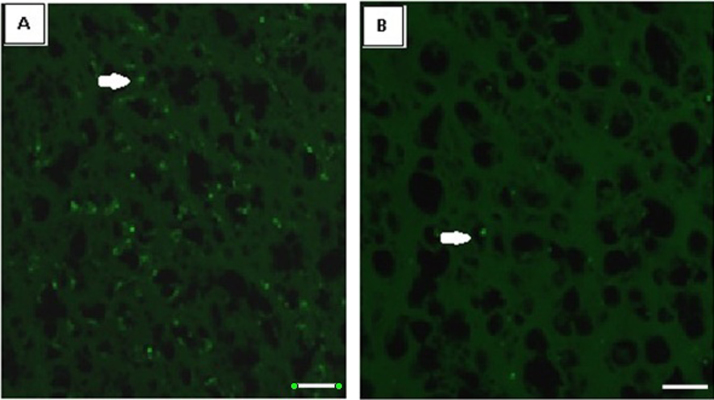
3.9. Confocal imaging of α7 nicotinic acetylcholine receptor subunit in the cerebral cortex of control and spinal cord injured of rats
α7 nicotinic acetylcholine receptor subunit antibody staining in the cerebral cortex showed a significant (p<0.001) decrease in the mean pixel value in SCI rats compared to control. (Fig. 9, Table 2). Even though the gene expression of α7 nicotinic acetylcholine receptor subunit showed a slight increase, the protein expression was decreased indicating a decreased activity of nicotinic receptor in SCI group.
Fig. 9.
Confocal imaging of α7 nicotinic acetylcholine receptor subunit in the cerebral cortex sections. Values are mean±S.E.M of 4–6 separate experiments. Each group consists of 4–6 rats. C – Control, SCI – Spinal cord injury group Scale bar =150 µm.
4. Discussion
The present study indicates a drastic reduction in cholinergic function in SCI condition demonstrated by the decreased muscarinic and nicotinic receptors along with functional alterations in the enzymes in cholinergic pathway. The results from our study showed the metabolic disturbance in cholinergic pathway as ChAT and AChE is up regulated in spinal cord injury group with the higher fold increase in expression of AChE. AChE, the enzyme involved in the breakdown of acetyl choline, is reported to show increased expression during stress condition [15]. Increase in AChE cause a decrease in acetylcholine content. The increased expression of AChE observed in our study will add on to the cholinergic deficits in the cerebral cortex of spinal cord injured rats. AChE is also considered as a marker of apoptosis. It is increased by the activation of members of caspase family [8]. Increased AChE has been observed during progressive disease conditions in Alzheimers condition [18]. This points to the possibility of activation of apoptotic pathways in spinal cord injured condition in addition to the motor deficits contributed by the lack of cholinergic transmission.
From our study it is evident that the total muscarinic receptors along with muscarinic M1 receptor subunits showed a reduction in the receptor density and receptor expression in spinal cord injury group. Also, in SCI rats, the mRNA level and binding parameters of muscarinic M3 receptors showed an increase in the cerebral cortex when compared to control. Muscarinic acetylcholine receptors are known to regulate numerous fundamental physiological processes, including central sensory, vegetative, and motor functions [3]. The reduction in muscarinic receptor function in SCI can augment the locomotor disability and may act as one of the molecular mechanism which contributes to the severity of monoplegic condition.
The immuno histochemical studies revealed a reduced expression of α7 nicotinic acetylcholine receptors in the cerebral cortex of spinal cord injured rats, even though gene expression level was not altered significantly. Along with a functional alteration in the metabotropic muscarinic cholinergic receptors spinal cord injury resulted in a reduction in the ionotropic nicotinic cholinergic receptors too. In mammals, nicotinic acetylcholine receptors play a crucial role in motor control. Nicotinic acetylcholine receptors could promote fast synaptic coupling between motoneurons, and thus play a role in somatic and visceral motor functions [26]. Hence, the reduction in fast acting nicotinic cholinergic receptors can affect the motor functions in spinal cord injured condition.
Our studies demonstrated the cholinergic impairment mediated through muscarinic and nicotinic acetyl choline receptors and the enzymes in acetyl choline metabolism in spinal cord injured rats. Our results points to the fact that the overall reduction in the cortical cholinergic function might have acted as one of the major contributors of the motor deficits exhibited in spinal cord injured condition.
Acknowledgements
Dr. Anju T R thanks Department of Biotechnology grant number (Ac.C1/11889/DBT-RA/2012) for Research Associateship. This work was supported by research grants from UGC, DBT, DST, ICMR, Govt. of India and KSCSTE, Govt. of Kerala to Dr. C.S. Paulose.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.02.003.
Appendix A. Transparency document
Supplementary material
.
References
- 1.Bakhit C., Armanini M., Wong W.L., Bennett G.L., Wrathall J.R. Increase in nerve growth factor-like immunoreactivity and decrease in choline acetyltransferase following contusive spinal cord injury. Brain Res. 1991;554:264–271. doi: 10.1016/0006-8993(91)90199-6. [DOI] [PubMed] [Google Scholar]
- 2.Bejanin S., Cervini R., Mallet J., Berrard S. A unique gene organization for two cholinergic markers, choline acetyltransferase and a putative vesicular transporter of acetylcholine. J. Biol. Chem. 1994;269:21944–21947. [PubMed] [Google Scholar]
- 3.Brown J.H., Taylor P. In: The Pharmacological Basis of Therapeutics. Hardman J.G., Limbird L.E., editors. McGraw–Hill; New York: 1996. pp. 141–160. [Google Scholar]
- 4.Cummings J.L. Cholinesterase inhibitors: a new class of psychotropic compounds. Am. J. Psychiatry. 2000;157:4–15. doi: 10.1176/ajp.157.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Dong Y., Dobkin B.H., Cen S.Y., Wu A.D., Winstein C.J. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 6.Erickson J.D., Varoqui H., Schäfer M.K., Modi W., Diebler M.-F., Weihe E., Rand J., Eiden L.E., Bonner T.I., Usdin T.B. Functional identification of a vesicular acetylcholine transporter and its expression from a “cholinergic” gene locus. J. Biol. Chem. 1994;269:21929–21932. [PubMed] [Google Scholar]
- 7.James K., Wamsley M.S., Lewis W., Scott Y., Michael J.K. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J. Neurosci. 1981;1:176–191. doi: 10.1523/JNEUROSCI.01-02-00176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H., Zhang J., Zhu H., Li H., Zhang X. Nerve growth factor prevents the apoptosis-associated increase in acetylcholinesterase activity after hydrogen peroxide treatment by activating Akt. Acta Biochim. Biophys. Sin. 2007;39:46–56. doi: 10.1111/j.1745-7270.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuhar M.J., Murrin L.C. Sodium-dependent, high affinity choline uptake. J. Neurochem. 1978;30:15–21. doi: 10.1111/j.1471-4159.1978.tb07029.x. [DOI] [PubMed] [Google Scholar]
- 10.Kus L., Borys E., Ping Chu Y., Ferguson S.M., Blakely R.D., Emborg M.E., Kordower J.H. Distribution of high affinity choline transporter immunoreactivity in the primate central nervous system. J. Comp. Neurol. 2003;463:341–357. doi: 10.1002/cne.10759. [DOI] [PubMed] [Google Scholar]
- 11.R.R. Levine, N.J.M. Birdsall, eds., Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Life Sci. 60: 963–1207, 1997. [DOI] [PMC free article] [PubMed]
- 12.Lotze M., Markert J., Sauseng P., Hoppe J., Plewnia C., Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J. Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry O.H., Roserbbrough N., Farr A.L., Randall R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Mancama D., Arranz M.J., Landau S., Kerwin R. Reduced expression of the muscarinic 1 receptor cortical subtype in schizophrenia. Am. J. Med. Genet. 2003;119:2–6. doi: 10.1002/ajmg.b.20020. [DOI] [PubMed] [Google Scholar]
- 15.Marc D., Girard M., Van der Werf S. A Gly1 to Ala substitution in poliovirus capsid protein VP0 blocks its myristoylation and prevents viral assembly. J. Gen. Virol. 1991;72(5):1151–1157. doi: 10.1099/0022-1317-72-5-1151. [DOI] [PubMed] [Google Scholar]
- 16.Marshall R.S., Perera G.M., Lazar R.M., Krakauer J.W., Constantine R.C., DeLaPaz R.L. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- 17.Messi M.L., Renganathan M., Grigorenko E., Delbono O. Activation of α7 nicotinic acetylcholine receptor promotes survival of spinal cord motoneurons. FEBS Lett. 1997;411(1):32–38. doi: 10.1016/s0014-5793(97)00600-5. [DOI] [PubMed] [Google Scholar]
- 18.Mooradian A.D. Effect of aging on the blood-brain barrier. Neurobiol. Aging. 1988;9(1):31–39. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- 19.Myers C.P., Lewcock J.W., Hanson M.G., Gosgnach S., Aimone J.B., Gage F.G., Lee K.F. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron. 2005;46:37–49. doi: 10.1016/j.neuron.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Rosario Gu, Tiziana C., Fiorella C., Giancarlo P., Stefania S., Giampiero L. Acetylcholine release from fetal tissue homotopically grafted to the motoneuron-depleted lumbar spinal cord. An in vivo microdialysis study in the awake rat. Exp. Neurol. 2007;204(1):326–338. doi: 10.1016/j.expneurol.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Saltarelli M.D., Lowenstein P.R., Coyle J.T. Rapid in vitro modulation of [3H]hemicholinium-3 binding sites in ratstriatal slices. Eur. J. Pharmacol. 1987;135:35–40. doi: 10.1016/0014-2999(87)90754-0. [DOI] [PubMed] [Google Scholar]
- 22.Scatchard G. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949;51:660–672. [Google Scholar]
- 23.Ward N.S., Brown M.M., Thompson A.J., Frackowiak R.S. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessler I., Kirkpatrick C.J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamura H.I., Synder G. Muscarinic cholinergic binding in rat brain. Proc. Natl. Acad. Sci. USA. 1974;71:1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaninetti M., Tribollet E., Bertrand D., Raggenbass M. Presence of functional neuronal nicotinic acetylcholine receptors in brainstem motoneurons of the rat. Eur. J. Neurosci. 1999;11:2737–2748. doi: 10.1046/j.1460-9568.1999.00689.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material