Abstract
Background/Aim
To report retinal function outcomes after ophthalmic artery chemosurgery (OAC) for advanced retinoblastoma (RB) in eyes with minimal pre-treatment retinal function.
Methods
For 72 advanced RB eyes with baseline ERGs indistinguishable from noise (“extinguished”) or flicker ERG amplitudes < 25μV (“poor”), electroretinograms were obtained prior to OAC and at 3 months, 1 year and 2 years after OAC. Presence of baseline retinal detachments (RD) and their subsequent resolution or persistence was also noted.
Results
At 3 months, 1 year and 2 years post-OAC, “extinguished” eyes showed 9/15, 4/11 and 2/6 detectable ERGs respectively, and “poor” eyes showed 19/55, 14/30 and 8/18 ERGs exceeding 25μV respectively. Correlations between baseline and post-OAC ERGs were poor; however, good correlation (R2) existed between ERGs post-OAC at 3 months and 1 year (0.749), at 3 months and 2 years (0.773) and at 1 year and 2 years (0.771). Overall, 49/70 eyes presented with retinal detachment (RD); 29 RDs resolved 3 months post-OAC, with an average ERG change of +20.6μV. Eyes with persistent RD had an average ERG change of –2.2μV. No eyes underwent ≥ 25μV change without RD resolution.
Conclusions
Minimal baseline ERGs do not preclude significant recovery of retinal function after OAC. Good correlation exists between ERG outcomes at 3 months and those at subsequent follow-ups, suggesting that ERG amplitudes at 3-months post-OAC can prognosticate longer-term retinal function, and that improvement is durable. For eyes presenting with RD, RD resolution is necessary but not sufficient for significant (≥ 25μV) increases in ERG amplitudes.
Keywords: Retinoblastoma, electrophysiology, retinal detachment
INTRODUCTION
Ophthalmic artery chemosurgery (OAC) was first introduced by our group nine years ago as a method to salvage eyes with advanced retinoblastoma that were otherwise destined for enucleation (1). The method consists of threading a micro-catheter through the femoral artery and up to the orifice of the ophthalmic artery, through which chemotherapy can be injected in a localized fashion into the circulation of the eye and orbit; this precise chemotherapeutic delivery technique has proven successful in rescuing eyes with even the most extensive cases of retinoblastoma (2–7).
OAC has proven effective in avoiding enucleation for a majority of cases. However, the concentrated delivery of chemotherapeutic drugs to the delicate structures of the retina raises the concern of OAC-induced retinal toxicity. To assess this issue in our practice, we have systematically monitored retinal function using serial electroretinography during examination under anesthesia before and after OAC. We showed in our initial small-scale study on OAC that retinal function can persist, and in some cases even improve in advanced retinoblastoma eyes after treatment with OAC (8); the possibility of improved retinal function with treatment was significant, given that retinoblastoma eyes with “no useful vision” were historically enucleated. Other studies have shown that there may be some small variation in the extent of toxicity depending on the chemotherapeutic agent used, but these differences are small at our recommended doses, and likely clinically insignificant (9).
Although evidence shows that OAC retinal toxicity is of minimal clinical concern and that retinal function can remain preserved or improve in eyes with advanced retinoblastoma after treatment, it remains unclear how eyes with very poor baseline ERGs fare after OAC, and whether OAC can promote significant retinal recovery in these eyes with ostensibly poor long-term retinal prognosis. Moreover, the correlation of baseline, pre-treatment ERGs with future post-treatment ERG outcomes is unknown, and hence the utility of the poor baseline ERG as a predictor of future retinal recovery remains to be investigated. Finally, the physiological and anatomical mechanisms underlying any observed treatment-mediated recovery in ERG after treatment remain unclear.
To address these questions, we present here a comprehensive retrospective review of our experience with retinoblastoma eyes that possess extinguished or poor electroretinograms (ERGs) at baseline, and follow the trajectory of changes in these ERGs with time at three months, one year and two years after OAC treatment. We also follow the retinal detachment status of these eyes, and document occurrences of retinal reattachment as a possible repair mechanism underlying significant ERG recovery in these eyes.
METHODS
Subjects
This study is a single center retrospective review of 72 eyes with advanced retinoblastoma managed with ophthalmic artery chemosurgery between May 2006 and October 2013. After IRB approval for the retrospective review, patients with very advanced retinoblastoma were tabulated, with very advanced retinoblastoma being defined as either Reese-Ellsworth Groups “Va” or “Vb” and ICRb Classification Groups “D” or “E” using the Children’s Oncology Group (COG) version of the ICRb.
Electroretinography
ERG was performed during examination under anesthesia, as part of regularly scheduled follow-up assessments. We used an adaptation of the International Society for Clinical Electrophysiology of Vision standard ERG protocol to obtain electroretinography (ERG) recordings, wherein we utilized the 30-Hz photopic flicker amplitude data as a highly representative surrogate for the complete ISCEV protocol, as previously described (10, 11). ERGs were recorded using ERG-jet contact lens electrodes and a Diagnosys Espion-3 electrodiagnostic system, with a hand-held ColorBurst ganzfeld stimulator. The initial ERG prior to OAC was compared to follow-up ERGs at 3 months, 1 year and 2 years following completion of OAC treatment. Based on statistical analysis of the ERGs of normal eyes that were recorded under anesthesia, 30-Hz flicker responses indistinguishable from noise were considered extinguished, and those with flicker responses of 25 μV (approximately 25% of the normal value under our experimental conditions) or less were considered “poor”.
Supplemental data
The supplemental table provides data for eyes with detached retinas at baseline, including ERG amplitude changes at three months, location of tumor within the retina, total number of OAC cycles and drugs/doses used, additional local or systemic treatment, and resolution or persistence of retinal detachment at three months after OAC.
RESULTS
For the purposes of this study, we included only patients whose ERGs at baseline prior to OAC treatment were “extinguished” (indistinguishable from zero amplitude) or “poor” (less than 25 μV), and recorded follow-up ERGs at scheduled visits 3 months, 1 year and 2 years after completion of OAC. A total of 72 eyes were followed; of these eyes, 16 had an extinguished ERG at baseline, and 56 had poor ERG recordings.
“Extinguished” ERG eyes
At 3 months after OAC, 15 of 16 eyes with extinguished baseline ERGs had follow-up measurements recorded. Of these 15 eyes, 9 (60%) had detectable increases in ERG amplitude, including 4 eyes that had ERG amplitudes ranging from 19 μV to 40 μV (Figure 1A). Of the remaining 6 eyes that continued to have extinguished ERGs at 3 months, 3 remained extinguished at the 1- and 2- year follow-up visits, 2 were enucleated before the 1-year follow-up visit, and 1 was lost to follow-up.
Figure 1.

Follow-up ERG outcomes of eyes with extinguished and poor (less than 25 μV) ERGs at baseline. Patients are presented in order of ascertainment by our clinic.
Figure 1A. Follow-up 30-Hz flicker ERG measurements of eyes with extinguished (indistinguishable from zero) baseline ERGs at the 3 month follow-up.
Figure 1B. Follow-up 30-Hz flicker ERG measurements of eyes with extinguished baseline ERGs at the 1 year follow-up.
Figure 1C. Follow-up 30-Hz flicker ERG measurements of eyes with extinguished (baseline ERGs at the 2 year follow-up.
Figure 1D. Baseline and follow-up 30-Hz flicker ERG measurements of eyes with poor (<25 μV) baseline ERGs at the 3 month follow-up. Baseline and follow-up ERGs are shown for each patient in grey and black bars, respectively.
Figure 1E. Baseline and follow-up 30-Hz flicker ERG measurements of eyes with poor (<25 μV) baseline ERGs at the 1 year follow-up.
Figure 1F. Baseline and follow-up 30-Hz flicker ERG measurements of eyes with poor (<25 μV) baseline ERGs at the 2 year follow-up.
Eleven eyes were available for the 1-year follow-up. The 4 eyes that had demonstrated appreciable increases from their extinguished baseline at the 3 month visit (19 μV – 40 μV range) continued to display robust amplitudes, ranging from 37 μV to 61 μV (Figure 1B). Of the remaining 7 eyes that continued to show extinguished amplitude by 1 year, 4 continued to have an extinguished response by 2 years, and 3 were lost to follow-up. By the 2-year follow-up visit, of 6 eyes that were extinguished at baseline, 2 had amplitudes that were detected by ERG (Figure 1C). These 2 eyes already had recordable amplitudes by the 3-month follow-up visit.
“Poor” ERG eyes
55 out of 56 eyes with a “poor” ERG baseline were evaluated at the 3-month follow up. Of these eyes, 36 (65%) had a recordable increase in ERG amplitude compared to their baseline, 12 (22%) had a decrease from their initial amplitude to a new measurement above zero, and 7 (13%) became extinguished (Figure 1D). Of the 36 eyes that had a recordable improvement from their baseline, 19 had an amplitude above 25 μV, including 3 with an ERG recording above 75 μV.
Thirty eyes were available for the 1-year follow up. Twenty-three eyes (77%) had a recordable increase in ERG amplitude compared to their “poor” baseline, including 14 (47%) eyes that had amplitudes above 25 μV and 2 (7%) above 75 μV (Figure 1E). Four eyes (13%) had a decrease in ERG amplitude to a new level above zero, while 3 (10%) became extinguished (including 2 that were extinguished at the 3 month follow up).
Eighteen eyes had ERGs recorded at the 2-year follow up visit (Figure 1F). Of these eyes, 12 (67%) had an increase in their amplitude from baseline, including 8 (44%) with amplitudes above 25 μV and 5 (28%) with amplitudes above 75 μV. Three eyes (17%) had a decrease to a level above zero, and 3 (17%) became extinguished (2 of which were previously recorded as extinguished at 1 year).
Correlation between baseline and follow up ERG amplitudes
To determine whether the baseline ERG amplitude of “extinguished” and “poor” eyes could be used to prognosticate future amplitudes and retinal recovery after OAC, we correlated the baseline amplitudes with the amplitudes measured at each of the follow up visits described above (Figure 2). Correlation was poor between the baseline amplitudes and those measured at 3-month, 1-year and 2-year visits, with R2 values of 0.127, 0.108 and 0.215 respectively. Correlation between amplitudes at 3 months and 1 year after OAC, however, was more robust, with R2 = 0.749 (Figure 3A). Likewise, correlation between the 3-month and 2-year amplitudes, as well as between the 1 year and 2 year amplitudes, remained strong with R2 = 0.771 and 0.773 respectively (Figure 3C and B). These data suggest that the ERG amplitude measured at 3 months after OAC is a good predictor of ERG responses at the subsequent follow-up visits.
Figure 2.
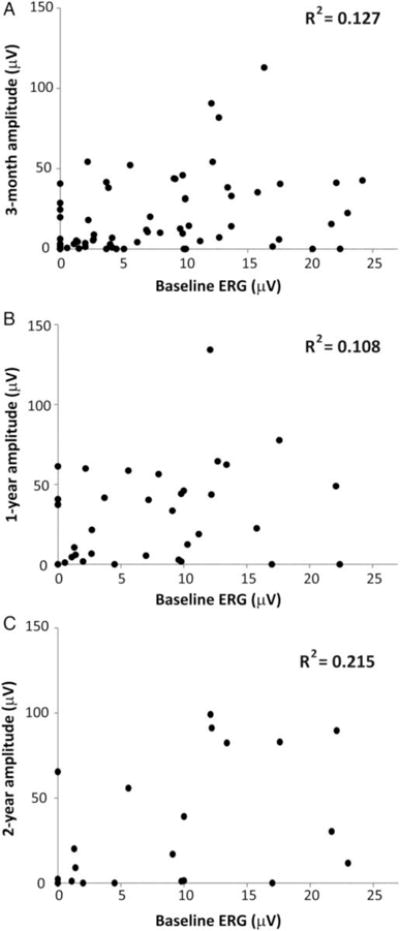
Correlation between baseline ERGs and ERG outcomes at subsequent follow-up visits. Recordings for eyes with both extinguished and poor baseline ERGs are included.
Figure 2A. Scatter plot of baseline ERGs vs 3 month ERG recordings.
Figure 2B. Scatter plot of baseline ERGs vs 1 year ERG recordings.
Figure 2C. Scatter plot of baseline ERGs vs 2 year ERG recordings.
Figure 3.

Correlation between ERG recordings at follow-up visits of 3 month and 1 year, 1 year and 2 year, and 3 month and 2 years. Recordings for eyes with both extinguished and poor baseline ERGs are included.
Figure 3A. Scatter plot of 3 month vs 1 year ERG recordings.
Figure 3B. Scatter plot of 1 year vs 2 year ERG recordings.
Figure 3C. Scatter plot of 3 month vs 2 year ERG recordings.
To control for possible improved therapeutic success of OAC with time leading to increased ERG amplitudes as a potential confounder for our data (a “learning curve” effect), we determined whether there was a correlation between ERG amplitudes and the time at which the patient was treated. No such correlation existed for ERG amplitudes at baseline, at 3 months post-OAC, or for the changes in ERG amplitude from baseline to 3 months after treatment (R2 = 0.002, 0.019 and 0.025 respectively), confirming that no “learning effect” was present.
Reattachment of retinas detached at baseline
Of the 72 eyes examined, 49 (68%) presented with baseline retinal detachment (RD). Given that ERG outcomes at 3 months after OAC represent longer-term ERG outcomes well, we assessed for OAC-mediated retinal reattachment at this time point after treatment, and analyzed how ERG amplitude changes correlated with this anatomical recovery. Twenty-nine RDs (59%) had resolved by 3 months after initiation of OAC (Figure 4); these cases did not include any eyes in which the RD was treated with retinal reattachment surgery (e.g., vitrectomy, scleral buckling). Changes in ERG for these eyes included ranged from −8.1 mV to 78.6 μV, with 9 eyes exhibiting a change equal to or above 25 μV. For the twenty eyes (41%) that had persistent RD at 3 months, the range of ERG changes varied from −15.5 μV to 12.8 μV (Figure 4). The average ERG amplitude change for eyes with resolved RD was +20.6 μV, whereas eyes with persistent RD had a corresponding average ERG amplitude change of –2.2 μV; this difference was statistically significant (p < 0.001, Student’s t-test). In two eyes that presented with the retina definitively in place at baseline, there were no ERG changes greater than 25 μV observed with treatment. Thus, the only eyes that exhibited ERG changes > 25 μV at 3 months post-OAC were those with baseline RD that resolved with treatment. Representative images for eyes presenting with retinal detachments, with subsequent RD resolution or persistence, are shown (Figure 5.)
Figure 4.
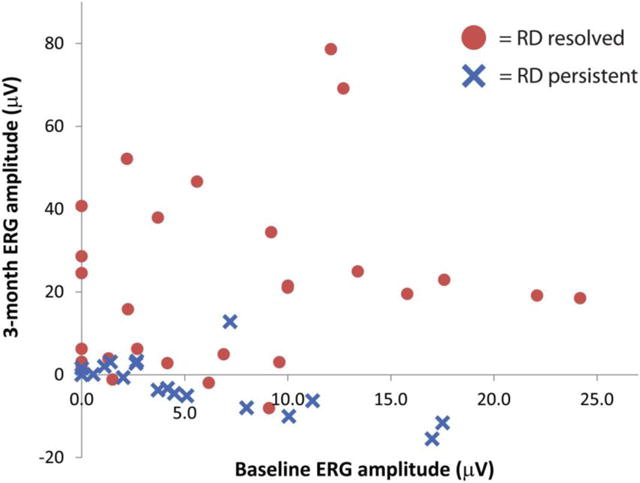
Scatter plot of 3-month ERG amplitude versus baseline ERG amplitude for eyes that undergo retinal reattachment (“RD resolved”) or with persistent retinal detachment (“RD persistent”). Red circles represent eyes that underwent retinal reattachment at the 3 month follow-up visit, whereas blue crosses represent eyes with RD that remained persistent at the same time point.
Figure 5.
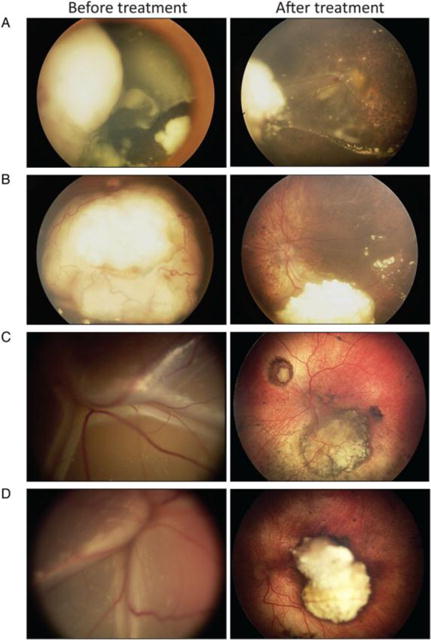
Representative fundus photographs of retinoblastoma eyes with detached retinas at baseline, before treatment,and with subsequent persistence or resolution after OAC.
Figure 5A. Example of an eye with persistent retinal detachment. ERG amplitude change for this eye 3 months after treatment was −15.5 μV.
Figure 5B. Example of an eye with resolved retinal detachment. ERG amplitude change for this eye 3 months after treatment was +46.6 μV.
Figure 5C. Example of an eye with resolved retinal detachment. ERG amplitude change for this eye 3 months after treatment was +24.5 μV.
Figure 5D. Example of an eye with resolved retinal detachment. ERG amplitude change for this eye 3 months after treatment was +3.0 μV.
We could not clearly identify a factor to predict whether a detached retina would reattach with ophthalmic artery chemosurgical treatment. Patients with baseline detached retinas that eventually reattached and gained appreciable increases in ERG amplitude were on average younger (11.2 months) than patients whose retinas remained persistently detached (18.0 months). However, this difference did not reach statistical significance (p = 0.0756 by Student t-test). Presence of vitreous and/or subretinal seeds, two other ostensibly poor prognostic factors, also did not appear to correlate with whether detached retinas reattached with OAC treatment.
DISCUSSION
In this study, we show that in eyes with retinoblastoma, extinguished or poor retinal function prior to OAC does not preclude significant recovery of retinal function after the procedure, as measured by flicker ERG amplitude. While we have previously shown that persistence and recovery of retinal function was possible after OAC, our sample size was much smaller, consisting of 10 patients and with a shorter average follow-up period (3–14 months) (8). Prior to the use of OAC, enucleation, and thus permanent vision loss, was considered the standard of care for many of these cases. Our findings that ERG amplitudes in these “poor starter” eyes can recover to levels that approach or equal normal retinal function establishes an additional dimension to the spectrum of recovery: in addition to patient survival, cancer-free survival and ocular survival, OAC offers the possibility of improved or restored retinal function compared to baseline as an additional measurable outcome of recovery.
Our larger sample size allows us not only to more precisely appraise the range of possible retinal recovery outcomes after OAC, but also to correlate these final outcomes with initial pre-OAC and subsequent post-OAC ERG amplitudes. We find that there is poor correlation between the initial pre-OAC ERGs in these “poor starters” and post-OAC ERG amplitude at 3 months, 1 year and 2 years. However, ERG amplitudes at the 3 months after completion of OAC appear to correlate well with those measured at 1 year, and similarly, 1-year ERG amplitudes correlate well with those measured at 2 years. This implies that the ERG amplitudes measured at the 3 month follow-up visit are a useful indicator of retinal function to be expected in post-OAC eyes until at least 2 years after the procedure. In particular, poor ERG function at baseline is not a valid predictor of a poor retinal function outcome, and does not per se justify withholding of OAC treatment.
In this study, we also establish retinal reattachment as the anatomical mechanism underlying significant (> 25 μV) changes in ERG amplitude. Occurrence of retinal reattachment with treatment thus increases the probability of a favorable outcome with regards to regaining visual function as measured by ERG in these patients who are pre-verbal. Conversely, persistence of a retinal detachment predicts a poor outcome with regards to increases in ERG amplitude. Based on our analysis, factors that may at first glance seem to predict a poor prognosis, such as older age or presence of vitreous seeds at time of presentation, do not predict whether a detached retina will eventually reattach with treatment, and thus the presence of these factors should not weigh into the decision to administer OAC.
While flicker ERG amplitudes provide a convenient method for measuring retinal function in patients who are pre-verbal, we recognize that a limitation of this study is the use of the electroretinogram value as a surrogate for subjective visual acuity. Nevertheless, given the correlation between flicker ERG amplitude and retinal function, as well as the massive improvement in ERG amplitude that some “poor starter” eyes undergo, these measurements may be of value in the documentation of the recovery of visual potential in any one patient.
In this study, between 23% and 35% of eyes with recordable ERGs at baseline experienced a decline in ERG amplitude over the course of treatment, depending on the time point of the observation. Possible explanations for the loss of retinal function in these eyes include toxicity of therapy to retinal tissue or to the retinal pigment epithelium (mediated by both intra-arterial and intravitreal treatment routes); retinal ischemia, as has been noted following intra-arterial chemotherapy and suggested by thinning of the choroid as seen in previous OCT studies (12–14); development of retinal detachment following treatment; and possible paraneoplastic phenomena. Clarification of these effects should be an important question for further study.
While baseline ERG amplitude cannot predict the extent of recovery in a specific eye, an interesting question that remains to be answered is which characteristics contribute to retinal reattachment in some retinoblastoma eyes with extinguished or poor ERGs at baseline before OAC. Possible factors that may play into the recovery curve include duration of the detachment prior to treatment, retinal location of the detachments, presence and type of vitreous seeding, and treatments received prior to OAC.
Supplementary Material
SUB-TITLE.
ERG analysis of retinal function recovery after ophthalmic artery chemosurgery in retinoblastoma eyes with minimal baseline retinal function shows that retinal recovery is common, and that significant recovery requires reattachment of retinas detached at baseline.
Acknowledgments
Funding statement
This research was funded in part through The Fund for Ophthalmic Knowledge, Inc, New York (no grant number; philanthropic fund) and in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. These funding organizations had no role in the design or conduct of this research.
Footnotes
Competing interests statement
The authors of this manuscript have the following competing interests: Brian P. Marr MD is a consultant for Aura Biosciences. All other authors declare no competing interests.
Contributorship statement
SEB, JHF, DHA and AHA conceived and designed the study. JHF, BPM, YPG, DHA and SEB collected the data. AHA, JHF and SEB analyzed the data. AHA performed statistical analyses. AHA produced an initial draft of the paper, and all authors contributed to revised drafts and preparation of the manuscript.
References
- 1.Abramson DH, Dunkel IJ, Brodie SE, et al. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115(8):1398–404. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH, Dunkel IJ, Brodie SE, et al. Bilateral superselective ophthalmic artery chemotherapy for bilateral retinoblastoma: tandem therapy. Archives of Ophthalmology. 2010;128(3):370–2. doi: 10.1001/archophthalmol.2010.7. [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. The British journal of Ophthalmology. 2012;96(4):499–502. doi: 10.1136/bjophthalmol-2011-300498. [DOI] [PubMed] [Google Scholar]
- 4.De Francesco S, Galluzzi P, Bracco S, et al. Alternated intra-arterial and intravitreal chemotherapy for advanced intraocular retinoblastoma: preliminary successful results without systemic chemotherapy. International Ophthalmology. 2015;35(6):887–95. doi: 10.1007/s10792-015-0129-8. [DOI] [PubMed] [Google Scholar]
- 5.Palioura S, Gobin YP, Brodie SE, et al. Ophthalmic artery chemosurgery for the management of retinoblastoma in eyes with extensive (>50%) retinal detachment. Pediatric Blood & Cancer. 2012;59(5):859–64. doi: 10.1002/pbc.24170. [DOI] [PubMed] [Google Scholar]
- 6.Schaiquevich P, Ceciliano A, Millan N, et al. Intra-arterial chemotherapy is more effective than sequential periocular and intravenous chemotherapy as salvage treatment for relapsed retinoblastoma. Pediatric Blood & Cancer. 2013;60(5):766–70. doi: 10.1002/pbc.24356. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121(7):1453–60. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Brodie SE, Pierre Gobin Y, Dunkel IJ, et al. Persistence of retinal function after selective ophthalmic artery chemotherapy infusion for retinoblastoma. Documenta Ophthalmologica. 2009;119(1):13–22. doi: 10.1007/s10633-008-9164-3. [DOI] [PubMed] [Google Scholar]
- 9.Francis JH, Abramson DH, Gobin YP, et al. Electroretinogram monitoring of dose-dependent toxicity after ophthalmic artery chemosurgery in retinoblastoma eyes: six year review. PLoS ONE. 2014;9(1):e84247. doi: 10.1371/journal.pone.0084247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CY, Jonna G, Francis JH, et al. Non-selectivity of ERG reductions in eyes treated for retinoblastoma. Documenta Ophthalmologica. 2014;128(1):13–23. doi: 10.1007/s10633-013-9416-8. [DOI] [PubMed] [Google Scholar]
- 11.Brodie SE. Electroretinogram Monitoring of Retinoblastoma Treatment. In: Francis JH, Abramson DA, editors. Recent Advances in Retinoblastoma Treatment. Springer; New York: 2015. pp. 47–59. [Google Scholar]
- 12.Maidana DE, Pellegrini M, Shields JA, et al. Choroidal thickness after intraarterial chemotherapy for retinoblastoma. Retina. 2014;34(10):2103–9. doi: 10.1097/IAE.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 13.Tse BC, Kaste SC, Brennan R, et al. Enophthalmos and choroidal atrophy after intraophthalmic artery chemotherapy for retinoblastoma. Ophthalmology. 2015;122(2):435–7. doi: 10.1016/j.ophtha.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Parareda A, Català J, Carcaboso AM, et al. Intra-arterial chemotherapy for retinoblastoma. Challenges of a prospective study Acta Ophthalmol. 2014;92(3):209–15. doi: 10.1111/aos.12295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


