Abstract
Prenatal cocaine exposure (PCE) may increase adolescent substance use through alterations of neurotransmitter systems affecting fetal brain development. The relationship between PCE and substance use at 15 and 17 years was examined. Subjects (365: 186 PCE; 179 non-cocaine exposed (NCE)) supplied biologic and self-report data using the Youth Risk Behavior Surveillance System (YRBSS) and Computerized Diagnostic Interview Schedule for Children (C-DISC 4) at ages 15 and 17. The relationship between PCE and substance use was assessed using General Estimating Equation (GEE) analyses controlling for confounding factors including violence exposure and preschool lead level. Teens with PCE vs. NCE teens were 2 times more likely to use tobacco (OR = 2.1; 95% CI 1.21–3.63; p < .001) and marijuana (OR = 1.85; CI 1.18–2.91; p < .001) and have a substance use disorder at age 17 (OR = 2.51; CI 1.00–6.28; p < .05). Evaluation of PCE status by gender revealed an association between PCE and marijuana use that was more pronounced for boys than girls at 17 years. Violence exposure was also a significant predictor of alcohol (p < .001), tobacco (p < .05), and marijuana (p < .0006) use and substance abuse/dependence (p < .01). Externalizing behavior at age 12 fully mediated the effects of PCE on substance use disorder at age 17 and partially mediated effects of PCE on tobacco use, but did not mediate effects on marijuana use. The percentage of substance use reported increased between 15 and 17 years, with no differences between the PCE and NCE groups. Data suggest specialized drug use prevention measures for children with PCE may benefit this high risk group.
Keywords: Prenatal cocaine, Adolescent, Substance use, Externalizing
1 Introduction
Adolescent substance use is a significant concern in the United States, with 6.5% of 12th graders reporting daily marijuana use (Johnston et al., 2014), 1.0% of 12–17 year-olds reporting five or more drinks at one time at least five days during the past 30 days (Center for Behavioral Health Statistics and Quality, 2015) and 20.8% of high school students reporting binge drinking five or more drinks in a row on at least one day in the past 30 days (Kann et al., 2014). In 2014, the national rate of past year substance use disorder for adolescents aged 12–17 was estimated to be 5%, with 2.7% of youth aged 12–17 having an alcohol use disorder and 3.5% having an illicit drug use disorder (Center for Behavioral Health Statistics and Quality, 2015). Earlier initiation of substance use for children and adolescents is associated with a number of problems including academic failure (Yule and Prince, 2012), not attending or completing college (King et al., 2006), and risk for development of a substance use disorder later in life (Substance Abuse Mental Health Services Administration (SAMHSA), 2013).
Problematic substance use is regarded as multi-causal and children born with prenatal cocaine-exposure (PCE) often have more risk factors than those with no PCE. Risks specific to children with PCE include direct teratological effects of cocaine on the developing brain and self-regulatory systems (King et al., 2006; Nigg et al., 2006; Thompson et al., 2009; Zucker et al., 2011; Yule and Prince, 2012; Substance Abuse Mental Health Services Administration (SAMHSA), 2013; Kann et al., 2014; Johnston et al., 2014), genetic transmission of substance use traits (Agrawal and Lynskey, 2008), and/or accumulation of negative environmental experiences such as exposure to violence (Lynskey et al., 2010).
1.1. Prenatal cocaine exposure and adolescent substance use
Prenatal cocaine exposure is hypothesized to have direct teratologic effects on fetal brain development via cocaine induced hypoxia resulting from vasoconstriction in uterine and placental blood vessels (Malanga and Kosofsky, 1999) and negative effects on monoamine neurotransmitter functions of the developing fetal brain (Kosofsky et al., 1994; McCarthy et al., 2014). Evidence suggests that PCE alters prenatal responses to stress in reaction to environmental insults (Lester and Padbury, 2009). The combination of these early alterations in brain development can result in pervasive, subtle developmental problems of self-regulation, including executive functioning deficits, behavioral problems and poor impulse control (Singer et al., 2008; Thompson et al., 2009; Minnes et al., 2010; Minnes et al., 2014a, 2014b).
Existing research on adolescents who have experienced PCE has found that they are about 2–3 times more likely to use substances or develop substance use problems than their non-cocaine exposed peers. At age 14, adolescents with PCE were twice as likely to use cocaine as non-exposed teens (Delaney-Black et al., 2011). Fifteen-year olds with PCE were found to be 1.8 times more likely to have early initiation of alcohol or marijuana use (Richardson et al., 2013), about twice as likely to have used alcohol, marijuana, or tobacco at age 15 (Minnes et al., 2014a,b), and 2.8 times more likely to have problems related to substance use than their non-exposed peers (Min et al., 2014b). The risk of developing problematic substance use appears to increase with the amount of prenatal exposure the adolescent experienced (Frank et al., 2011). To our knowledge no studies have looked beyond adolescent substance use among youth with PCE to examine the percentage of those who meet diagnostic criteria for substance use disorders.
1.2. Externalizing behavior as a mediator of cocaine’s association with adolescent substance use
There is converging evidence that an indirect pathway to adolescent substance use among children with PCE may occur through increased externalizing behavior. Externalizing typically refers to overt behavior problems that involve impulsivity and insufficient self-regulation of behavior or emotion, but can also be considered broadly as a neurodevelopmental style of self-regulation and learning that is more responsive to environmental than internal influences (Tucker et al., 2015). PCE has been associated with increased behavioral dysregulation throughout infancy and early childhood, and increased externalizing, disruptive, and risk-taking behaviors through middle childhood and into adolescence (Richardson et al., 2011; Lambert and Bauer, 2012). Problems with poor inhibitory control can manifest in behaviors that show impulsivity and poor judgment for adolescents with PCE. Examples of such behavior include earlier initiation of sexual activity (De Genna et al., 2014; Min et al., 2015), more engagement in risky sexual behavior (Min et al., 2016), and more damage, theft, and status offenses by age 15 (Richardson et al., 2015) than non-exposed adolescents.
Relatively few studies have examined the mediating effect of externalizing behavior in the relationship between prenatal cocaine exposure and teen substance use. Only the Maternal Life Style (MLS) study reported that childhood externalizing problems mediated the effects of PCE on the risk of initiating use of any substance by age 16 (Lester et al., 2012). Other studies have found direct relationships between PCE and increased risk of problematic substance use. However, they have either not directly examined the mediating effects of externalizing behaviors (Frank et al., 2011; Warner et al., 2011; Richardson et al., 2013) or have not found a significant mediating effect (Frank et al., 2011; Warner et al., 2011; Richardson et al., 2013). Additional investigation is warranted to clarify a potential pathway to substance use among prenatally drug exposed teens.
Differential relationships based on gender may exist between PCE and externalizing behaviors, as some studies have found boys with PCE to have higher levels of externalizing behaviors than girls (Greenwald et al., 2011; Bennett et al., 2013), while others have found only girls with PCE report more externalizing (Minnes et al., 2010; McLaughlin et al., 2011) and risk taking behaviors (Min et al., 2015). Increased levels of PCE are also linked to lower caregiver ratings of executive functioning at age 12 in girls but not boys, indicating dysfunction in the areas of the brain related to judgment and behavioral regulation (Minnes et al., 2014a,b). To date, it is not clear how gender may interact with PCE to predict adolescent substance use.
1.3. Other prenatal drug exposure and environmental covariates
Children with PCE also have higher levels of prenatal exposure to other drugs of abuse, including alcohol, opiates, and tobacco (Singer et al., 2000). These additional prenatal drug exposures have been associated with both neurobehavioral regulatory problems and increased risk of substance use in adolescence (Glantz and Chambers, 2006).
Environmental influences can constitute up to 70% of the variation in initiation and problematic substance use (Lynskey et al., 2010) and are important to consider when evaluating the association of PCE and substance use. A potent negative environmental influence, violence exposure, has been associated with early initiation of alcohol use (Taylor and Kliewer, 2006; Bossarte and Swahn, 2008), marijuana use (Mason, 2010; Richardson et al., 2013), cocaine use (Delaney-Black et al., 2011), and general substance use (Pinchevsky et al., 2014) for adolescents. Repeated exposure to violence is reported to set the stage for greater rates of substance abuse and other mental and physical health problems later in development (Moffitt, 2013). There is also existing evidence that violence exposure is a contributing factor to substance abuse after accounting for PCE (Frank et al., 2014; Minnes et al., 2014a, b).
Additionally, post-natal exposure to ongoing substance use by household members for children with PCE has been associated with a nearly six-fold increase in risk of problematic adolescent substance use (Frank et al., 2014). Furthermore, PCE and caregiver current substance use were both found to independently predict adolescent cocaine use by 14 years (Delaney-Black et al., 2011). Elevated blood lead levels, which often co-occur within low socioeconomic status households and, therefore, at high rates in our sample (Min et al., 2009), are also associated with increased risk of behavioral problems in childhood through early adulthood in the general population (Wasserman et al., 1998; Burns et al., 1999; Lane et al., 2008; Wright et al., 2008), and were found to be related to alcohol use in adolescents with PCE (Minnes et al., 2014a,b). Other caregiver characteristics known to independently influence behavioral outcomes include caregiver psychological distress, receptive vocabulary skills, non-verbal reasoning ability and quality of the home environment (Bennett et al., 2008; Singer et al., 2008; Singer et al., 2015). Generally, poor environmental and socioeconomic conditions are often prevalent among parents with substance use disorders and are important factors to consider in evaluating the relationship between PCE and teen substance use. These multiple factors have rarely been evaluated simultaneously in a large well-defined prospective sample.
1.4. Purpose and hypotheses
The purpose of the current study advances previous research by aiming to better understand the relationship between PCE and substance use patterns in the teen years, controlling for important confounders including early elevated lead levels and exposure to violence that often co-occur in low socioeconomic households and influence behavioral outcomes. We hypothesize that PCE will be associated with substance use, defined as use in the past 30 days, at ages 15 and 17 years, and with diagnoses of substance use disorders at age 17 based on self-report. Gender by PCE interactions will be explored based on previous behavior differences found in this cohort (Minnes et al., 2010) as well as general findings of differences in patterns of drug use by gender (Becker et al., 2016). In addition, externalizing behaviors are expected to mediate PCE’s relationship with current adolescent substance use.
2. Method
2.1. Sample
The study sample consisted of 365 adolescents (186 PCE, 179 NCE) who were followed prospectively from birth and assessed for substance use at ages 15 and 17 years. The participants and their birth mother were recruited between September 1994 and June 1996 from a large urban teaching hospital in the Midwest for a longitudinal investigation of the developmental effects of prenatal cocaine exposure.
Drug toxicology screens were administered to 647 mothers and infants at delivery who were identified to be high risk. Hospital policy specified operational criteria for detecting pregnant women suspected of substance use: lack of prenatal care, maternal behavior suggesting intoxication, self-admitted drug use, or a history of involvement with the Department of Children and Family Services. Maternal and infant urine specimens were collected immediately before or after delivery to screen for metabolites of cocaine and other illicit drugs, including opiates, cannabinoids, phencyclidine, amphetamines, and benzodiazepines. Urine specimens were analyzed using the Syva Emit method (Syva Company, Palo Alto, California), followed by gas chromatography for the confirmation of presumptive positive test results (Singer et al., 2002). Infant meconium collected by the research staff was further analyzed for cocaine and metabolites of other drugs, including cocaethylene, benzoylecgonine, and meta-hydroxybenzoylecgonine. Identification of prenatal cocaine exposure (PCE) was based on either positive results of biologic indicators (i.e., maternal and infant urine or infant meconium) or maternal self-report of cocaine use during pregnancy to medical or research staff. Non-cocaine-exposed status (NCE) was assigned if all screening measures on cocaine were negative. The NCE group, however, may have been exposed to alcohol, tobacco, and/or marijuana.
During the screening procedure for eligibility, 54 mother-infant dyads were excluded due to confounding conditions that might obscure the effects of PCE: maternal age less than 19 years (2); primary heroin use (2); maternal psychiatric history of severe depression, bipolar disorder or schizophrenia (16); maternal low intellectual functioning (1); maternal chronic illness (4); HIV positive status (5); lack of meconium (15); fetal alcohol syndrome (1); infant Down syndrome (2); infant illness (3); and other (3). Of the 593 eligible mothers, 415 (218 PCE, 197 NCE) consented for participation in the study, whereas 155 mothers (49 PCE, 106 NCE) refused to participate and 23 mothers (9 PCE, 14 NCE) were not present for the enrollment interviews. Since enrollment, 12 children (9 PCE vs. 3 NCE, x2 = 2.50, p = .11) died from sudden infant death syndrome (4 PCE, 2 NCE), respiratory distress syndrome (1 PCE, 1 NCE), cardiopulmonary arrest (1 PCE), pneumonia (1 PCE), accidental asphyxia (1 PCE), and unknown illness (1 PCE). The present study used data from 365 adolescents who completed drug use assessments at ages 15 (n = 358) and/or 17 (n = 350), representing 91% retention of the 403 active, living participants in the original study.
No difference was found by PCE status between the 365 participants and the 38 nonparticipants (20 drop-out, 17 lost contact, 1 low intellectual functioning (IQ < 50)), except that the participants were more likely to be African American (p < .008). Of the 365 adolescents who had substance use data, the 186 participating adolescents with PCE had caregivers with higher nonverbal reasoning ability than the 23 adolescents with PCE who did not participate (p < .0002). The 179 participating non-exposed adolescents were more likely to be African American (p < .02), have birth mothers who were younger (p < .02), less likely to have been married (p < .02), and had less years of education (p < .04) compared with the 15 non-exposed adolescents not included in the study.
2.2. Procedure
The Institutional Review Board of the participating hospital reviewed and approved all research procedures. The initial screenings and enrollment were performed in the hospital. The longitudinal follow-up assessments were conducted at the university-based developmental research lab at subject ages 6, 12, and 18 months and 2, 4, 6, 9, 10, 11, 12, 15, and 17 years. Parents or caregivers provided signed informed consent at each visit. Written assent was obtained from youth beginning at age 9. The consent document included a Certificate of Confidentiality number (DA-98-91) issued by U.S. Department of Health and Human Services, protecting the release of drug-related information. At each follow up visit, a trained research assistant blind to cocaine exposure status administered the cognitive and behavioral assessment protocol to the youth. A different research assistant administered caregiver interviews privately. As part of the 15- and 17-year assessments, adolescents were referred to the affiliated hospital’s National Institute of Health (NIH) supported Clinical Research Unit for collection of biologic specimens (i.e., urine, hair, and/or bloodspots) by trained research nurses. All participants were compensated with a monetary stipend and given lunch; transportation costs were provided if needed. For the 15 and 17 year assessments each subject received $100 compensation, with an additional $10 if they provided a biological specimen for drug use screening.
2.3. Measures
2.3.1. Prenatal exposure to cocaine and other substances
All biologic mothers of subjects had a retrospective (during pregnancy and 1 month prior) assessment of prenatal substance use patterns immediately post-partum. For cocaine, the number of approximately $20 “rocks” used and/or the amount of money spent per day were collected, which was converted to a standard “unit” of cocaine equivalent to 1 rock and/or $20 worth of cocaine. For alcohol, the number of drinks of beer, wine, or hard liquor consumed was recorded, with each drink equivalent to 0.5 oz. of absolute alcohol. The number of tobacco cigarettes smoked per day and the number of marijuana joints smoked per week were also acquired. Frequency of substance use was rated on a Likert-type scale with a range from 0 (not at all) to 7 (daily use), with data summarized to indicate the average number of days of substance use per week. Frequency was multiplied by the amount of use per day to yield summary measures of average cocaine units (per week), alcohol drinks (per week), cigarettes (per day), and marijuana joints (per week) used during each trimester of pregnancy and the month prior to conception and then averaged over the pregnancy. The substance use assessment was updated with the adolescent’s current caregiver at each follow-up visit to assess caregiver substance use during the last 30 days.
2.3.2. Adolescent substance use outcomes
Adolescent’s substance use was assessed at ages 15 and 17 using self-report data and biologic samples. Self-reported alcohol, tobacco, and marijuana use in the past 30 days were measured using the Youth Risk Behavior Surveillance System (YRBSS) (Centers for Disease Control and Prevention, 2009). Biologic specimens of urine, hair, and/or blood-spots were collected with the assistance of research nurses from the university’s NIH-funded Clinic Research Unit. Analysis of adolescent biologic assays for drug metabolites of amphetamines, benzodiazepines, cocaine, opiates, cannabinoids, cotinine, and ethyl glucuronide was performed by the United States Drug Testing Laboratory (USDTL) (Minnes et al., 2014a,b). Adolescents who were positive on any one self-report data or biologic sample for a particular substance were assigned a positive code (1 = yes) for that substance. Adolescents who were positive on any one substance were also coded positive (1 = yes) for a measure of “any substance use.”
Adolescent’s substance abuse and dependence symptoms were self-reported at age 17 via the Computerized Diagnostic Interview Schedule for Children IV (C-DISC 4) (Shaffer et al., 2000). C-DISC 4 is a reliable and valid structured interview that measures DSM-IV diagnoses for children and adolescents ages 9–17. This investigation used alcohol abuse and dependence, nicotine dependence, and marijuana abuse and dependence data from the C-DISC 4 sections on substance use disorders.
A summary variable of the number of symptoms scored positive was used as the C-DISC 4 measure of each substance use disorder. A cut-off of one or more out of the four symptoms without presence of dependence was used for a diagnosis of a substance abuse (Shaffer et al., 2000; First and Ross, 2000). A cut-off of three or more out of the seven symptoms for each substance was employed for a diagnosis of dependence for that particular substance (Shaffer et al., 2000). Adolescents who were diagnosed with either abuse or dependence on a substance were coded 1 (yes) for a composite abuse/dependence variable for that substance. Adolescents who were diagnosed with any one substance abuse/dependence were coded 1 (yes) for an “any substance abuse/dependence” variable.
2.3.3. Externalizing behavior
Externalizing behavior was assessed at age 12 using the Youth Self-Report (YSR) (Achenbach and Rescorla, 2001). The YSR is a commonly used adolescent self-report measure consisting of 105-items designed to assess emotional, behavioral and social problems in the last 6 months. T-scores of externalizing summary score (i.e., aggression and rule-breaking behavior) that were standardized for gender and age were used for this investigation. Higher scores indicated more problem behaviors with internal consistency (Cronbach’s α) of .87.
2.3.4. Adolescent, maternal, and caregiver variables
Infant and maternal demographic and medical characteristics were obtained from hospital birth records. Included were infants’ gender and race, gestational age, birth weight, height, and head circumference as well as maternal age at delivery, parity, and number of prenatal care visits. Maternal education and work history, family composition, and socioeconomic status based on Hollingshead score of IV or V (Hollingshead, 1957) were added via post-partum research interviews. At each follow-up interview, time dependent information was updated, including the child’s placement (with either biological mother/relative or adoptive/foster caregiver) and shifts, if any, in placement, determined by a change in both primary caregiver and physical setting lasting greater than one month. Concurrent assessment of current caregivers’ receptive vocabulary, non-verbal reasoning ability, psychological distress, and recent drug use were also obtained.
At infant birth, maternal and/or current caregiver receptive vocabulary and nonverbal reasoning ability were assessed using the Peabody Picture Vocabulary Test-Revised (PPVT-R) (Dunn et al., 1997) and two subscales—Picture Completion and Block Design—of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler, 1981). Maternal and/or current caregiver self-reported psychological distress was assessed using the Global Severity Index (GSI), a summary measure on the Brief Symptom Inventory (BSI) (Derogatis, 1992), at birth. At the 15 year follow-up visit, the quality of the caregiving environment was assessed using an interview format of the Home Observation for Measurement of the Environment Inventory-Early Adolescent version (EA-HOME; α= .83) (Caldwell and Bradley, 2003).
At 2 and/or 4 years, blood lead level was assessed for a subset of children (n = 294). At age 12, violence exposure was assessed using the Assessment of Liability and Exposure to Substance Use and Antisocial Behavior (ALEXSA) (Ridenour et al., 2009). The ALEXSA is an illustration-based, computerized, audio self-report measure of substance use, antisocial behavior and associated risk factors for children ages 9–12. The ALEXSA violence exposure subscale is composed of 8 items representing ever experiencing or witnessing violence, including a beating, a robbing or mugging, and a stabbing or shooting (α= .75). Items were rated on a 5-point Likert scale (0 = 0 times to 5 = 5 times or more) and averaged to form a composite violence exposure variable, with higher scores indicating more exposure to violence. At age 15, adolescents’ intelligence was assessed by an examiner unaware of the youth’s prenatal cocaine exposure status and trained by a licensed child clinical psychologist using the full Wechsler Intelligence Scales for Children-Fourth Edition (Wechsler, 2003).
2.4. Data analyses
Study variables that followed a positively skewed distribution were normalized using log transformation prior to analyses. Univariate distributional characteristics were reported based on the original data, with transformations used in bivariate and multivariate analyses. Adolescent demographics and maternal and current caregiver characteristics were compared by PCE status using t-tests or the Wilcoxon-Mann-Whitney test for continuous variables. The Chi-square tests or Fisher’s Exact test were employed for categorical variables. Zero-order Pearson correlations were estimated to examine relationships between observed variables. The associations of PCE with alcohol, tobacco, and marijuana use were assessed at 15 and 17 years using Generalized Estimating Equation (GEE) modeling procedures for correlated binary outcomes with logit link (Liang and Zeger, 1986). Since the number of adolescents who had confirmed substance abuse or dependence for alcohol, nicotine, and marijuana by 17 years was small (Table 3), logistic regression was only completed on use of any one substance.
Table 3.
Unadjusted frequencies of substance use outcomes by cocaine group status and age.
| 15 years (n = 358) | 17 years (n = 350) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| PCE (n = 183) | NCE (n = 175) | X2 | p | PCE (n = 175) | NCE (n = 175) | X2 | p | |
|
|
|
|||||||
| n (%) | n (%) | n (%) | n (%) | |||||
| Any drug usea | 66 (36.07) | 46 (26.29) | 3.98 | .05 | 76 (43.43) | 63 (36.00) | 2.02 | .16 |
| Alcohol usea | 24 (13.11) | 21 (12.00) | 0.10 | .75 | 38 (21.71) | 34 (19.43) | 0.28 | .60 |
| Nicotine usea | 34 (18.58) | 20 (11.43) | 3.57 | .06 | 51 (29.14) | 38 (21.71) | 2.55 | .11 |
| Marijuana usea | 45 (24.59) | 25 (14.29) | 6.04 | .01 | 52 (29.71) | 43 (24.57) | 1.17 | .28 |
| Any substance abuse/dependenceb | – | – | – | – | 31 (19.14) | 19 (11.59) | 3.58 | .06 |
| Alcohol abuse/dependenceb | – | – | – | – | 6 (3.70) | 2 (1.22) | 2.10 | .15 |
| Nicotine dependenceb | – | – | – | – | 15 (9.26) | 5 (3.05) | 5.46 | .02 |
| Marijuana abuse/dependenceb | – | – | – | – | 18 (11.11) | 15 (9.15) | 0.35 | .56 |
Assessed using self-report YRBSS (past 30 days) and biologic samples (urine specimens, hair, and/or bloodspots) and 15 and 17 years.
Assessed DSM-IV diagnosis of substance use disorders using self-report CDISC at 17 years.
Covariates correlated (p ≤ .2) with each substance outcome for at least onetime point and different by PCE status (p ≤ .2) were assessed one-by-one for possible inclusion in the multivariate models for each outcome variable. Covariates that were significant at an alpha of .10 or that changed the estimate for PCE by 10% were retained in the model. A gender by PCE interaction term was created and entered into the model last to examine interaction effects. Mediation was evaluated based on the logic of Baron and Kenny (Baron and Kenny, 1986). Since our previous study established PCE effects on self-reported externalizing behavior at age 12 (Min et al., 2014a), multivariate models were specified independent of and then with earlier externalizing behavior. If the PCE effects on adolescent substance outcomes were markedly reduced with statistical control for externalizing behavior at age 12, significance of the mediated effect was evaluated using the PRODCLIN program (MacKinnon et al., 2007). Adolescent gender was tested for interaction with PCE status. All analyses were conducted using SAS software, Version 9.4. Results were noted as statistically significant with a two-tailed alpha level of .05.
3. Results
3.1. Sample characteristics
Table 1 provides birth outcome and demographic characteristics of study participants by cocaine exposure status. Those with PCE had lower average gestational age, birth lengths, and head circumferences and were small for gestational age compared with non-exposed infants. Adolescents with PCE reported more externalizing behavior problems at age 12 and had lower IQ scores at age 15 than their non-exposed counterparts. Adolescents with PCE were more likely to be placed in non-kinship foster or adoptive care at both 15 and 17 years of age. Compared with the NCE group, they also had a trend for lower blood lead levels at 2 and/or 4 years old (p = .06). No group differences were found for gender, race or exposure to violence at age 12.
Table 1.
Adolescent demographics.
| PCE (n = 186)
|
NCE (n = 179)
|
p | |||
|---|---|---|---|---|---|
| n (%) | M (SD) | n (%) | M (SD) | ||
| Male | 83 (44.62) | 87 (48.60) | .45 | ||
| African-American | 152 (81.72) | 145 (81.01) | .86 | ||
| Gestational Age | 37.76 (2.86) | 38.47 (2.88) | .02 | ||
| Birth weight (grams)a | 2705 (654) | 3104 (702) | <.0001 | ||
| Birth length (cm)a | 47.27 (3.97) | 49.15 (3.76) | <.0001 | ||
| Head circumference (cm)a | 32.27 (2.17) | 33.47 (2.40) | <.0001 | ||
| Head circumference <10th% | 27 (14.75) | 8 (4.52) | .001 | ||
| Small for gestational age | 25 (13.37) | 4 (2.20) | <.0001 | ||
| 2yrs and/or 4yrs Lead Levelb | 7.07 (4.14) | 8.04 (4.66) | .06 | ||
| Violence exposure at 12yrs | 0.63 (0.77) | 0.59 (0.81) | .50 | ||
| YSR externalizing behavior at 12 years | 51.07 (10.07) | 47.79 (9.75) | .002 | ||
| WISC-IV Full Scale IQ at 15 years | 81.05 (11.57) | 83.78 (14.09) | .05 | ||
| Age at assessment | |||||
| 15 years | 15.69 (0.27) | 15.67 (0.27) | .48 | ||
| 17 years | 17.81 (0.26) | 17.79 (0.26) | .40 | ||
| Adopted/Foster care | |||||
| 15 years | 44 (24.04) | 6 (3.43) | <.0001 | ||
| 17 years | 39 (22.41) | 5 (2.89) | <.0001 | ||
P-value is adjusted for prematurity.
Sub-sample size of lead is 284, included 145 PCE and 139 NCE.
Table 2 presents the demographic and drug use characteristics of biologic mothers and current caregivers by cocaine exposure status. Birth mothers of adolescents with PCE were older, less educated, less likely to be married, and had higher parity compared with birth mothers of non-exposed adolescents. They also had fewer prenatal care visits, lower receptive vocabulary scores, and more psychological distress compared to those in the NCE group. Women who used cocaine during pregnancy reported greater use of other substances, including tobacco, alcohol, and marijuana, than women who did not use cocaine. Cocaine using women averaged 25.5 (SD 38) units of cocaine per week over the course of their pregnancy. Characteristics of current caregivers did not differ, except that caregivers of adolescents with PCE were less likely to complete high school and smoked more cigarettes per day than caregivers of non-exposed adolescents.
Table 2.
Maternal and Current Caregiver Characteristics.
| PCE (n = 186) | NCE (n = 179) | p | |||
|---|---|---|---|---|---|
|
|
|
||||
| n (%) | M (SD) | n (%) | M (SD) | ||
| Biologic mother | |||||
| Mother’s age at birth | 29.75 (5.01) | 25.45 (4.72) | <.0001 | ||
| African-American | 153 (82.26) | 146 (81.56) | .86 | ||
| Maternal years of education | 11.55 (1.67) | 11.92 (1.38) | .02 | ||
| Completion of high school | 96 (51.61) | 120 (67.04) | .003 | ||
| Married | 15 (8.06) | 28 (15.64) | .02 | ||
| Low SES | 181 (97.84) | 175 (97.77) | .96 | ||
| Parity | 3.55 (1.89) | 2.76 (1.86) | <.0001 | ||
| Number of prenatal visits | 5.19 (4.59) | 8.71 (4.83) | <.0001 | ||
| PPVT Standard Score | 73.53 (14.36) | 77.70 (14.79) | .008 | ||
| WAIS-R Block Design Scale | 6.88 (2.11) | 7.18 (2.10) | .18 | ||
| WAIS-R Picture Completion | 6.74 (2.16) | 6.95 (2.34) | .38 | ||
| BSI Global Severity Index | 0.83 (0.75) | 0.51 (0.54) | <.0001 | ||
| Substance use during pregnancya | |||||
| Drinks per weekb | 9.80 (17.64) | 1.38 (4.64) | <.0001 | ||
| Cigarettes per dayc | 11.55 (11.08) | 3.85 (7.13) | <.0001 | ||
| Marijuana per weekd | 1.35 (3.48) | 0.61 (3.55) | <.0001 | ||
| Cocaine per weeke | 22.52 (37.96) | – | – | – | |
| Current caregiver | |||||
| Years of education | 12.53 (2.29) | 12.86 (1.96) | .15 | ||
| Completion of high school | 125 (69.06) | 134 (79.29) | .03 | ||
| PPVT Standard Score | 80.05 (14.90) | 78.93 (15.18) | .50 | ||
| WAIS-R Block Design Scale | 7.11 (2.11) | 7.27 (1.95) | .49 | ||
| WAIS-R Picture Completion | 7.54 (2.49) | 7.11 (2.30) | .10 | ||
| BSI Global Severity Index | 0.34 (0.42) | 0.34 (0.46) | .43 | ||
| Substance use in the past 30 daysa | |||||
| Drinks per weekb | 1.81 (4.52) | 2.01 (4.64) | .47 | ||
| Cigarettes per dayc | 4.78 (7.14) | 3.46 (6.54) | .01 | ||
| Marijuana per weekd | 0.53 (5.43) | 0.45 (3.08) | .60 | ||
| Cocaine per week | 0 | 0 | NA | ||
| HOME environment | |||||
| 15years | 47.81 (6.73) | 48.94 (6.05) | .10 | ||
| 17years | 50.60 (4.62) | 52.80 (1.79) | .35 | ||
BSI = Brief Symptom Inventory; NCE = non cocaine exposed; PCE = prenatal cocaine exposure; PPVT = Peabody Picture Vocabulary Test; SD = standard deviation; WAIS-R = Wechsler Adult Intelligence Scale-Revised; M = mean.
P-value based on n (%).
Average drinks of beer, wine, or hard liquor per week, each equivalent to 0.5 oz. absolute alcohol.
Average number of cigarettes smoked per day.
Average joints of marijuana per week.
Average unit of cocaine equivalent to 1 rock and/or $20 worth of cocaine rocks.
3.2. Effects of PCE on substance use at ages 15 and 17
Table 3 describes unadjusted percentages of last 30 day use of alcohol, tobacco, and marijuana and any drug use by PCE group status at ages 15 and 17. Adolescents with PCE had higher percentages of alcohol, tobacco, marijuana, and any drug use than their NCE counterparts at both 15 and 17 years, with significant group difference in marijuana use (p ≤ .01) and any drug use (p ≤ .05) at age 15 years only.
A greater number of adolescents used substances by age 17 than at 15 years, regardless of PCE status (Table 4). There was a significant increase in reported past 30 day substance use from ages 15 to 17 as reflected in the significant odds ratio of alcohol (OR = 1.62, 95% CI = 1.06–2.46, p ≤ .05), tobacco (OR = 1.98, 95% CI = 1.50–2.62, p ≤ .001), and marijuana use (OR = 1.61, 95% CI = 1.18–2.18, p ≤ .01) from 15 to 17 years, with no differences in the rate of change between PCE and NCE groups.
Table 4.
Effects of prenatal cocaine exposure on adolescent past 30-day substance use at 15 and 17 years mediated by earlier externalizing behavior.
| Alcohol usea | Tobacco use | Marijuana use | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| OR | 95% CI | Model 1 | Model 2 | Model 1 | Model 2 | |||||
|
|
|
|
|
|||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| PCE | 1.66† | 0.96–2.85 | 2.1** | 1.21–3.63 | 1.89* | 1.08–3.30 | 1.85** | 1.18–2.91 | 1.79** | 1.13–2.83 |
| Time, 17 years | 1.62* | 1.06–2.46 | 1.98*** | 1.50–2.62 | 2.01*** | 1.51–2.67 | 1.61** | 1.18–2.18 | 1.61** | 1.19–2.19 |
| Adolescent sex, male b | – | – | – | – | – | – | 1.32 | 0.85–2.07 | 1.33 | 0.85–2.08 |
| Race, African-American | 0.59† | 0.33–1.06 | 0.37*** | 0.20–0.68 | 0.4** | 0.22–0.73 | 0.61† | 0.34–1.07 | 0.63 | 0.35–1.12 |
| Mother’s age at birth b | 0.95* | 0.90–1.00 | 0.97 | 0.92–1.02 | 0.97 | 0.92, 1.02 | – | – | – | – |
| Marital status | 2.49** | 1.24–4.98 | 1.92† | 0.91–4.06 | 1.82 | 0.87–3.83 | 2.23* | 1.14–4.36 | 2.17* | 1.11–4.23 |
| Parity b | 0.83** | 0.72–0.96 | – | – | – | – | – | – | – | – |
| Maternal BSI GSI at birth b | 1.69† | 0.94–4.07 | – | – | – | – | – | – | – | – |
| HOME environmentb,c | – | – | 0.96* | 0.93–1.00 | 0.97† | 0.93–1.00 | 0.96* | 0.93–0.99 | 0.96* | 0.93–0.99 |
| Violence exposure d | 1.37** | 1.10–1.69 | 1.3* | 1.04–1.62 | 1.14 | 0.88–1.47 | 1.41*** | 1.16–1.72 | 1.34** | 1.07–1.66 |
| Externalizing behavior d | – | – | – | – | 1.03* | 1.00–1.07 | – | – | 1.01 | 0.99–1.04 |
BSI = Brief Symptom Inventory; GSI = Global Severity Index; PCE = prenatal cocaine exposure; CI = confidence interval; OR = odds ratio.
Model 1 = base model without assessing mediating effects of 12 year externalizing behavior.
Model 2 = evaluates 12 year externalizing behavior as a mediator of PCE.
p ≤ .10;
p ≤ .05;
p ≤ .01;
p ≤ .001.
Model 1, mediation was not tested for, since no statistically significant association was established between PCE and alcohol use.
Blank spaces indicate that the variable did not meet the criteria (e.g., not significant at the bivariate level) and therefore was not included in the model.
Assessed quality of home environment at 15 years.
Assessed at 12 years.
Table 4 summarizes the effects of PCE on current substance use at ages 15 and 17 after controlling for covariates. Model 1 is a base model without assessing for mediating effects of externalizing behavior at 12 years. Adolescents with PCE were approximately 2 times more likely to use tobacco (OR = 2.1, 95% CI = 1.21–3.63, p ≤ .01) and marijuana (OR = 1.85, 95% CI = 1.18–2.91, p ≤ .01) in the past 30 days than their NCE counterparts after controlling for covariates. There was a non-significant trend for PCE status to be associated with increased odds of current alcohol consumption (OR = 1.66, 95% CI = 0.96–2.85, p < .07).
Model 2 evaluated earlier externalizing behavior as a mediator of PCE. Externalizing behavior at age 12 was associated with increased odds of current tobacco use at ages 15 and 17 (OR = 1.03, 95% CI = 1.00–1.07, p =.03 and mediated PCE effects on current tobacco use at 15 and 17 years of age (mediated OR = 1.19, CI = 1.00–1.27, p < .05); PCE status remained significant even when accounting for the variance in tobacco use associated with earlier externalizing behavior. Externalizing behavior at age 12 was not significantly related to the odds of current marijuana use (OR = 1.01, 95% CI = 0.99–1.04, p = .25), and thus did not mediate the effects of PCE status. Since no significant association was established between PCE status and alcohol use, further mediation examination was not conducted.
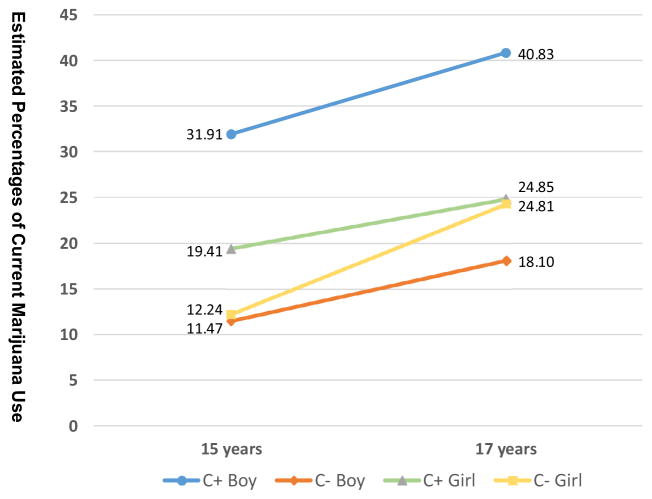
Interaction effects (PCE by gender) were evaluated. Fig. 1 reflects estimated probability (%) of marijuana use by PCE status and gender adjusted for covariates at ages 15 and 17 years. Evaluation of PCE status by gender interaction (p ≤ .05) revealed that the negative effects of PCE on marijuana use were more pronounced for boys compared to girls at age 17. Boys with PCE were approximately three times more likely than NCE boys to use marijuana both at 15 (OR = 3.62; 95% CI = 1.49–8.76; p = .004) and 17 years of age (OR = 3.12; 95% CI = 1.42–6.84; p = .004). A significant gender effect was also noted among the adolescents with PCE at age 17. Boys with PCE were 2.09 times more likely than girls with PCE to use marijuana in the past 30 days at age 17 (41% for PCE boys vs. 25% for PCE girls) (OR = 2.09; 95% CI = 1.01–4.29; p = .045). Change in percentage of marijuana use between 15 and 17 years of age was significantly different for girls with PCE only (OR = 2.32; 95% CI = 1.18–4.58, (p = .015). There were no gender or cocaine by gender interaction effects for tobacco or alcohol use.
Fig. 1.
Adjusted estimated percentages of current marijuana use by PCE status and gender at 15 and 17 years. Estimated values calculated after adjusting for PCE status*gender, race, gender, marital status, HOME score, and average prenatal marijuana exposure, violence exposure and externalizing behavior. Significant differences found between PCE boys and NCE boys at both 15 (OR = 3.62; 95% CI = 1.49–8.76; p = .004) and 17 years (OR = 3.12; 95% CI = 1.42–6.84; p = .004) and PCE boys and PCE girls at 17 years (OR = 2.09; 95% CI = 1.01–4.29; p = .045).
3.3. Effects of PCE on substance use disorders at 17 years
Adolescents with and without PCE were compared on self-reported diagnostic criteria (C-DISC 4) for substance use disorders at age 17 years (Table 3). Adolescents with PCE had higher unadjusted frequencies than the NCE controls for nicotine dependence. There were no differences by PCE group status in terms of alcohol and marijuana abuse or dependence. A greater number of adolescents with PCE reported abuse or dependence of any substance, including alcohol, nicotine, marijuana, and other illicit drugs, than NCE adolescents, although the difference was marginally significant (p ≤ .06).
As presented in Table 5 (Model 1), adolescents with PCE were 2.51 times more likely than NCE adolescents to abuse or depend on any substance (OR = 2.51, 95%CI = 1.00–6.28, p ≤ .05), after controlling for covariates. When externalizing behavior at age 12 was assessed in the model (Model 2) results indicated that it was a significant mediator of PCE’s association with any one substance abuse or dependence disorder (mediated OR = 1.43, CI = 1.14–1.79, p < .05).
Table 5.
Adjusted association of any substance abuse/dependence at 17 years with PCE status.
| Any Substance Abuse/Dependencea | ||||
|---|---|---|---|---|
|
| ||||
| Model 1 | Model 2 | |||
|
|
|
|||
| OR | 95% CI | OR | 95% CI | |
| PCE | 2.51* | 1.00–6.28 | 1.83 | 0.71–4.70 |
| Prenatal nicotine exposure b | 1.52* | 1.06–2.17 | 1.67** | 1.14–2.46 |
| Mother’s age at birth | 0.89** | 0.82–0.96 | 0.89** | 0.82–0.97 |
| Marital status | 4.46** | 1.64–12.10 | 3.24* | 1.14–9.24 |
| Maternal BSI GSI at birth | 1.35 | 0.46–3.93 | 0.68 | 0.21–2.23 |
| HOME environmentc | 1.00 | 0.94–1.05 | 1.02 | 0.96–1.08 |
| Violence exposured | 1.66** | 1.18–2.33 | 1.49* | 1.04–2.13 |
| Externalizing behaviord | 1.10*** | 1.05–1.16 | ||
BSI = Brief Symptom Inventory; GSI = Global Severity Index; NCE = non cocaine exposed; PCE = prenatal cocaine exposure; CI = confidence interval; OR = odds ratio.
Model 1 = base model without assessing mediating effects of 12 year externalizing behavior.
Model 2 = evaluates 12 year externalizing behavior as a mediator of PCE.
Measured via self-report CDISC. Any substance includes alcohol, tobacco, marijuana, or any other illicit drug.
Average number of cigarettes smoked per day.
Assessed at 15 years.
Assessed at 12 years.
p ≤ .05.
p ≤ .01.
p ≤ .001.
3.4. Association of other environmental factors and substance use at ages 15 and 17
As presented in Table 4 (Model 1), other significant factors were found to be correlated with the current (past 30 days) use of alcohol, tobacco, and marijuana at ages 15 and 17 years. Older age of biologic mothers (OR = 0.95, 95% CI = 0.90–1.00, p < .04) and having more children (OR = 0.83, 95% CI = 0.72–0.96, p < .01) at the time of infant birth decreased the odds of current alcohol use. The birth mother’s being married at the time of child delivery was related to an increased odds of current alcohol consumption (OR = 2.49, 95% CI = 1.24–4.98, p < .01) and marijuana use (OR = 2.23, 95% CI = 1.14–4.36, p < .02). African American adolescents were 63% less likely to use tobacco than non-African American adolescents (OR = 0.37, 95% CI = 0.20–0.68, p ≤ .001). Higher quality of home environment was related to lower odds of tobacco use (OR = 0.96, 95% CI = 0.93–1.00, p < .02) and marijuana use (OR = 0.96, 95% CI = 0.93–0.99, p < .02) at 15 and 17 years of age. Violence exposure assessed at age 12 increased the odds of current alcohol (OR = 1.37, 95% CI = 1.10–1.69, p < .004), tobacco (OR = 1.30, 95% CI = 1.04–1.62, p < .02), and marijuana (OR = 1.41, 95% CI = 1.16–1.72, p < .0006) use at 15 and 17 years of age. Further adjustment of externalizing behavior by age 12 did not change the association of these environmental factors with substance use at ages 15 and 17, except for the associations of home environment and violence exposure with current tobacco use (Model 2 in Table 4). Quality of the home environment (p = .06) and exposure to violence (p = .32) were no longer related to tobacco use at 15 and 17 years of age when externalizing behavior was accounted for.
As reported in Table 5 (Model 1), other prenatal exposures and environmental factors were associated with abuse or dependence of any substance by age 17. Higher prenatal exposure to cigarettes (OR = 1.52, 95% CI = 1.06–2.17, p = .02), the biologic mother’s being married at the time of child birth (OR = 4.46, 95% CI = 1.62–2.17, p = .003), and exposure to violence assessed at age 12 (OR = 1.66, 95% CI = 1.18–2.33, p = .004) increased the odds of substance use disorders in terms of any substance, including alcohol, tobacco, marijuana, and other illicit drugs. Older age of birth mothers decreased the odds of any substance abuse or dependence at age 17 (OR = 0.89, 95% CI = 0.82–0.96, p = .005). Maternal psychological distress at infant delivery, ongoing caregiver substance use and quality of the home environment assessed at 12 years were not related to any substance abuse or dependence. The associations that these significant prenatal exposures and environmental factors had with any substance abuse or dependence did not change with statistical adjustment for externalizing behavior at age 12 (Model 2 in Table 5).
4. Discussion
4.1. Overall findings and relationship to existing research
Data from this study indicate that both prenatal cocaine exposure and environmental factors are associated independently with increased incidence of teen substance use in the past 30 days and self-reported diagnosis of abuse or dependence of any substance by 17 years of age after control for covariates. Specifically, at ages 15 and 17, youth with PCE reported greater tobacco and marijuana use over the past 30 days. Past 30 day use likely indicates more regular use patterns. Interestingly, the difference in marijuana use by teens with PCE was largely driven by a greater number of males compared to girls with PCE. Given findings indicating that marijuana use among teens increases risk for psychosis (Green et al., 2016), poorer visual selective attention (Nicholls et al., 2015), lower academic achievement (Arria et al., 2015; Bechtold et al., 2016), and reduction in cortical thickness (Mashhoon et al., 2015), males with PCE could be at increased risk for negative effects of marijuana use. However, additional studies considering use quantity, frequency and/or marijuana use disorder symptoms would provide important clarifying information.
Similarly, concerning results were found for self-reported diagnosis of substance abuse or dependence for any one substance among youth with PCE, supporting the hypothesis that PCE not only increases the risk for teen substance use in the past 30 days but also the percentage of adolescents that may succumb to problematic use by age 17. This data is important because intransigent substance use, primarily tobacco and marijuana use in this sample, can have persistent mental and physical health consequences (Caspers et al., 2009; Minnes et al., 2014a,b). While not directly comparable, a summary of trends for daily substance use (indicating problem use) for 10th and 12 graders in 2015 indicated usage rates at 3–5% for tobacco, 0.5–1.9% for alcohol and 3–6% for marijuana. Results from this study indicate that the sample has elevated nicotine and marijuana abuse/dependence compared to the US population and that prenatal cocaine exposure exerts additional risk (Johnston et al., 2015). These results support and extend earlier research indicating that teens with PCE are at increased risk of drug use (Glantz and Chambers, 2006; Delaney-Black et al., 2011; Frank et al., 2011; Minnes et al., 2014a,b) compared to non-exposed teens of similar race and SES. Previous studies indicating that teens with PCE are almost three times more likely to have problems related to substance use than their NCE peers (Minnes et al., 2012; Min et al., 2014b) and earlier initiation of substance use (Richardson et al., 2013), raise additional concerns for potential abuse and dependence, as well as for social problems related to substance use, for this high risk group. There was an increase in the rate of past 30-day substance use from ages 15 to 17 years, indicating that prevention and/or early intervention is important to stop potential progression to abuse and dependence.
Mounting behavioral data (Aguirre, 2003; Minnes et al., 2010; Richardson et al., 2011; Lambert and Bauer, 2012) indicate that children with PCE are, as a group, at increased risk for problems of inhibitory control (Thompson et al., 2009). Given that these weaknesses in self-regulation usually present earlier in development, they may foreshadow risk for substance use experimentation and problematic use, and indicate a common liability (Nigg et al., 2006; Lester et al., 2012) particularly among children with PCE. In this study, more self-reported externalizing behavior problems at age 12 was found to be a significant mediator of cocaine’s effect on previous 30 day tobacco use and on the presence/risk of substance abuse or dependence. These results lend support to those of Lester et al. (2012) who found that neurobehavioral disinhibition predicted initiation of substance use in prenatally cocaine exposed children. While these results differ somewhat from that reported by other researchers (Frank et al., 2011; Warner et al., 2011; Richardson et al., 2013), methodological differences between the studies, including the use of a potentially more sensitive self-report assessment of externalizing behaviors at the later age of 12 rather than at ages 9–10, may account for differences in findings. Further exploration of the pathway to substance use among adolescents with PCE is warranted to better understand the early predictors of substance use as well as the protective factors that might lead to prevention of substance use in groups at risk for problematic use in emerging adulthood and beyond.
As hypothesized, exposure to violence was associated with current teen alcohol, tobacco and marijuana use, as well as with higher rates of self-reported substance use diagnoses, supporting findings which indicate long term developmental harm related to exposure to violence (Moffitt, 2013; Howell et al., 2016). Exposure to violence has been specifically associated with various types of early substance use in the general population (Pinchevsky et al., 2014) and also for adolescents with PCE (Frank et al., 2014; Minnes et al., 2014a,b). Further studies are required to test the potentially mediating or moderating aspects of exposure to violence in adolescents with PCE. In a related finding, poorer quality of the home environment during the teen years was also found to be related to greater rates of past 30 day tobacco and marijuana use. In addition, early environmental factors such as the biologic mother being married, number of siblings, and maternal psychological distress were associated with alcohol use. Marital status, possibly related to greater access to drugs, was also related to any one substance use disorder and to both tobacco and marijuana use. These findings indicate that early childhood factors, in addition to prenatal cocaine exposure and violence exposure, also have an influence on teen substance use. Surprisingly, maternal psychological distress at birth, blood lead level, adoptive or foster care placement and most other prenatal drug exposures did not predict rates of last 30 day substance use or any one substance abuse or dependence diagnosis. The only exception was that higher prenatal tobacco exposure predicted higher rates of any substance use disorder diagnosis at age 17, consistent with a previous finding (Goldschmidt et al., 2012).
4.2. Strengths and limitations
There are some limitations to consider in this study. These data do not tell us much about the patterns of drug use in the past 30 days, for example whether or not the alcohol or marijuana use was regular. In addition, it does not indicate how teen polydrug use or problems associated with drug use relate to later rates of drug and alcohol abuse or dependence. Because the rates of self-reported diagnoses of each substance at age 17 were generally too small to complete logistic regression, the information gleaned from that finding is not specific. In addition, the strength and inclusiveness of the violence exposure finding is very compelling but it too is a very general measure of violence exposure collected retrospectively at 12 years of age. Given the consistency of this predictor variable in different risk populations, additional research investigating the interaction of PCE and violence exposure could lead to a more specific understanding of how violence exposure leads to substance use and abuse/dependence, and to development of potentially targeted prevention strategies. The findings of this study apply to a group of high risk, low SES, minority individuals that were prenatally exposed to crack cocaine, limiting the ability to generalize these findings beyond this high risk population.
This study extends our knowledge of risk for substance use and abuse among prenatally cocaine-exposed teens through the age of 17, beyond the age that most studies have assessed. The study was one of the few that directly explored self-reported externalizing behavior at age 12 as a mediator. Support for this indirect pathway toward substance use among prenatally cocaine exposed children could be helpful in designing targeted drug use prevention programs. An added strength of this study is that the data come from a prospectively designed longitudinal study and very well characterized sample with a very low attrition rate, therefore increasing the validity of the findings. In addition, early blood lead levels, time varying caregiver and environmental conditions, and assessment of violence exposure have not been controlled for simultaneously in other studies and strengthen the current findings that support a specific association of prenatal cocaine exposure and teen substance use. In addition, this study utilized infant meconium to examine cocaine metabolites, ensuring that group status was correct and reducing error that may have come from sole reliance on maternal retrospective self-report. Multiple assessment methods, including biologic samples (teen hair, blood spot and urine drug screening and infant meconium to ensure maternal substance use during pregnancy) in addition to standardized and privately administered substance use questionnaires, were utilized. This ensured the most accurate account of teen drug use as well as assessment of biologic mothers’ drug use during pregnancy. Lastly, this study is the only one to our knowledge that examined both use of drugs in the last 30 days and standard diagnostic criteria indicating problematic use.
4.3. Implications
Study findings indicate that adolescents with PCE are at increased risk for drug use and self-reported diagnoses of substance abuse/dependence. Given the serious disabling aspects of chronic substance use and potential exposure to the next generation if use continues during pregnancy, every effort should be made to develop prevention and early intervention programs for at risk youth. Findings also suggest that a path toward substance use in those with PCE may be indicated by early externalizing behavior problems and therefore those children manifesting problems with inhibitory control should be considered at special risk. This may be especially true for those children known to have been exposed to violence. Continued studies into adulthood will elucidate whether the childhood behavioral problems and early indications of substance use in adolescence persist through early adulthood and affect social and vocational adjustment.
Acknowledgments
Role of funding source
This research was supported by the National Institute on Drug Abuse (NIDA)R01 07957. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Drug Abuse or the National Institutes of Health. This publication was also made possible by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research.
Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors would like to thank all of our families who participated in our research for 17 years. We would also like to thank Miaoping Wu, MS, Laurie Ellison, LISW, and Paul Weishampel, M.A. for research assistance, and Terri Lotz-Ganley for manuscript preparation and editorial assistance.
Footnotes
Contributors
Dr. Meeyoung Min performed the statistical analyses, and drafted the manuscript. Dr. Sonia Minnes conceptualized the paper, designed the study and interpreted the data. Dr. Adelaide Lang coordinated the study, performed the measurements, and proofread the manuscript. Ms. Miaoping Wu participated in the interpretation of data. Ms. June-Yung Kim assisted in the literature review, drafting, and proofing the manuscript. Ms. Meredith Francis assisted in the literature review, drafting, and proofing the manuscript. Dr. Lynn Singer participated in the study’s conception and design, interpretation of data, and reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of interest
No conflict declared
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School Age Forms and Profiles. University of Vermont. Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Aguirre GK. Functional imaging in behavioral neurology and cognitive neuropsychology. In: Feinberg TE, Farah J, editors. Behavioral Neurology and Cognitive Neuropsychology. McGraw Hill; New York, NY: 2003. [Google Scholar]
- Arria AM, Caldeira KM, Bugbee BA, Vincent KB, O’Grady KE. The academic consequences of marijuana use during college. Psychol Addict Behav. 2015;29:564–575. doi: 10.1037/adb0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bechtold J, Hipwell A, Lewis DA, Loeber R, Pardini D. Concurrent and sustained cumulative effects of adolescent marijuana use on subclinical psychotic symptoms. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.15070878. Epub, appiajp201615070878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan M, Reed BG. Sociocultural context for sex differences in addiction. Addict Biol. 2016;21:1052–1059. doi: 10.1111/adb.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Bendersky M, Lewis M. Children’s cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol. 2008;44:919–928. doi: 10.1037/0012-1649.44.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Marini VA, Berzenski SR, Carmody DP, Lewis M. Externalizing problems in late childhood as a function of prenatal cocaine exposure and environmental risk. J Pediatr Psychol. 2013;38:296–308. doi: 10.1093/jpepsy/jss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossarte RM, Swahn MH. Interactions between race/ethnicity and psychosocial correlates of preteen alcohol use initiation among seventh grade students in an urban setting. J Stud Alcohol Drugs. 2008;69:660–665. doi: 10.15288/jsad.2008.69.660. [DOI] [PubMed] [Google Scholar]
- Burns JM, Baghurst PA, Sawyer MG, McMichael AJ, Tong SL. Lifetime low-level exposure to environmental lead and children’s emotional and behavioral development at ages 11–13 years: the Port Pirie Cohort Study. Am J Epidemiol. 1999;149:740–749. doi: 10.1093/oxfordjournals.aje.a009883. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. HOME Inventory Early Adolescent Version. University of Arkansas for Medical Sciences; Little Rock, AR: 2003. [Google Scholar]
- Caspers KM, Yucuis R, McKirgan LM, Spinks R, Arndt S. Lifetime substance misuse and 5-year incidence rates of emergent health problems among middle-aged adults. J Addict Dis. 2009;28:320–331. doi: 10.1080/10550880903182796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Health and Human Services; 2015. (HHS Publication No. SMA 15–4927, NSDUH Series H-50), Retrieved from https://www.samhsa.gov/data/ on December 22, 2016. [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed on 12.29.2009];YRBSS: Youth Risk Behavior Surveillance System. 2009 http://www.cdc.gov/HealthyYouth/yrbas/index.htm.
- De Genna N, Goldschmidt L, Richardson GA. Prenatal cocaine exposure and age of sexual initiation: direct and indirect effects. Drug Alcohol Depend. 2014;145:194–200. doi: 10.1016/j.drugalcdep.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Partridge RT, Ager J, Sokolc RJ. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol Teratol. 2011;33:110–119. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. The Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual—II. Clinical Psychometric Research; Towson, MD: 1992. [Google Scholar]
- Dunn L, Dunn L, Williams KT, Wang JJ, Booklets N. Peabody Picture Vocabulary Test, (PPVT-III): Form IIA. American Guidance Service, Inc; Circle Pines, MN: 1997. [Google Scholar]
- First MB, Ross R, editors. Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- Frank DA, Rose-Jacobs R, Crooks D, Cabral HJ, Gerteis J, Hacker KA, Martin B, Weinstein ZB, Heeren T. Adolescent initiation of licit and illicit substance use: impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicol Teratol. 2011;33:100–109. doi: 10.1016/j.ntt.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Kuranz S, Appugliese D, Cabral H, Chen C, Crooks D, Heeren T, Liebschutz J, Richardson M, Rose-Jacobs R. Problematic substance use in urban adolescents: role of intrauterine exposures to cocaine and marijuana and post-natal environment. Drug Alcohol Depend. 2014;142:181–190. doi: 10.1016/j.drugalcdep.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz MD, Chambers JC. Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Dev Psychopathol. 2006;18:893–922. doi: 10.1017/s0954579406060445. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Cornelius MD, Day NL. Prenatal cigarette smoke exposure and early initiation of multiple substance use. Nicotine Tob Res. 2012;14:694–702. doi: 10.1093/ntr/ntr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Musci RJ, Johnson RM, Matson PA, Reboussin BA, Ialongo NS. Outcomes associated with adolescent marijuana and alcohol use among urban young adults: a prospective study. Addict Behav. 2016;53:155–160. doi: 10.1016/j.addbeh.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Chiodo LM, Hannigan JH, Sokol RJ, Janisse J, Delaney-Black V. Teens with heavy prenatal cocaine exposure respond to experimental social provocation with escape not aggression. Neurotoxicol Teratol. 2011;33:198–204. doi: 10.1016/j.ntt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. Yale University; New Haven, CT: 1957. [Google Scholar]
- Howell KH, Barnes SE, Miller LE, Graham-Bermann SA. Developmental variations in the impact of intimate partner violence exposure during childhood. J Inj Violence Res. 2016;8:43–57. doi: 10.5249/jivr.v8i1.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Miech RA. Monitoring the Future National Survey Results on Drug Use: 1975–2013. The University of Michigan Institute for Social Research; 2014. Sponsored By: The National Institute on Drug Abuse National Institutes of Health. [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Sponsored by The National Institute on Drug Abuse at The National Institutes of Health. 2015. Monitoring the Future National Survey Results on Drug Use 1975–2015, Key Findings on Adolescent Drug Use. [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Hawkins J, Harris WA, Zaza S. Youth risk behavior surveillance — United States, 2013. MMWR Surveill Summ. 2014;63:1–168. [PubMed] [Google Scholar]
- King KM, Meehan BT, Trim RS, Chassin L. Research report: marker or mediator? The effects of adolescent substance use on young adult educational attainment. Addiction. 2006;101:1730–1740. doi: 10.1111/j.1360-0443.2006.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: a mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994;9:234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- Lambert B, Bauer C. Developmental and behavioral consequences of prenatal cocaine exposure: a review. J Perinatol. 2012;32:819–828. doi: 10.1038/jp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Webster NJ, Levandowski BA, Rubinstein RA, Keefe RH, Wojtowycz MA, et al. Environmental injustice: childhood lead poisoning, teen pregnancy, and tobacco. J Adolesc Health. 2008;42:43–49. doi: 10.1016/j.jadohealth.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Lester B, Lin H, DeGarmo D, Fisher P, LaGasse L, Levine T, et al. Neurobehavioral disinhibition predicts initiation of substance use in children with prenatal cocaine exposure. Drug Alcohol Depend. 2012;126:80–86. doi: 10.1016/j.drugalcdep.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lynskey MT, Agrawal A, Heath AC. Genetically informative research on adolescent substance use: methods, findings, and challenges. J Am Acad Child Adolesc Psychiatry. 2010;49:1202–1214. doi: 10.1016/j.jaac.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39:384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Mechanisms of action of drugs of abuse on the developing fetal brain. Clin Perinatol. 1999;26:17–37. v–vi. [PubMed] [Google Scholar]
- Mashhoon Y, Sava S, Sneider JT, Nickerson LD, Silveri MM. Cortical thinness and volume differences associated with marijuana abuse in emerging adults. Drug Alcohol Depend. 2015;155:275–283. doi: 10.1016/j.drugalcdep.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ. An exploratory study of the effects of neighborhood characteristics on adolescent substance use. Addict Res Theory. 2010;18:33–50. [Google Scholar]
- McCarthy DM, Kabir ZD, Bhide PG, Kosofsky BE. Effects of prenatal exposure to cocaine on brain structure and function. Prog Brain Res. 2014;211:277–289. doi: 10.1016/B978-0-444-63425-2.00012-X. [DOI] [PubMed] [Google Scholar]
- McLaughlin AA, Minnes S, Singer LT, Min M, Short EJ, Scott TL, Satayathum S. Caregiver and self-report of mental health symptoms in 9-year old children with prenatal cocaine exposure. Neurotoxicol Teratol. 2011;33:582–591. doi: 10.1016/j.ntt.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Singer LT, Kirchner HL, Minnes S, Short E, Hussain Z, Nelson S. Cognitive development and low-level lead exposure in poly-drug exposed children. Neurotoxicol Teratol. 2009;31:225–231. doi: 10.1016/j.ntt.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Short EJ, Yoon S, Singer LT. Self-reported adolescent behavioral adjustment: effects of prenatal cocaine exposure. J Adolesc Health. 2014a;55:167–174. doi: 10.1016/j.jadohealth.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Weishampel P, Short EJ, Yoon S, Singer LT. Externalizing behavior and substance use related problems at 15 years in prenatally cocaine exposed adolescents. J Adolesc. 2014b;37:269–279. doi: 10.1016/j.adolescence.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Yoon S, Singer L. Effects of prenatal cocaine exposure on early sexual behavior: gender difference in externalizing behavior as a mediator. Drug Alcohol Depend. 2015;153:59–65. doi: 10.1016/j.drugalcdep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Albert JM, Kim JY, Singer LT. Pathways to adolescent sexual risk behaviors: effects of prenatal cocaine exposure. Drug Alcohol Depend. 2016;161:284–291. doi: 10.1016/j.drugalcdep.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Queh D. The effects of prenatal cocaine exposure on problem behavior in children 4–10 years. Neurotoxicol Teratol. 2010;32:443–451. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Min MO, Singer LT, Edguer M, Wu M, Thi P. Cocaine use during pregnancy and health outcome after 10 years. Drug Alcohol Depend. 2012;126:71–79. doi: 10.1016/j.drugalcdep.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Min M, Wu M, Lang A, Short E, Singer LT. Executive function in adolescents with prenatal exposure to cocaine. Alcohol. 2014a;27:647–656. [Google Scholar]
- Minnes S, Singer L, Min MO, Wu M, Lang A, Yoon S. Effects of prenatal cocaine/polydrug exposure on substance use by age 15. Drug Alcohol Depend. 2014b;134:201–210. doi: 10.1016/j.drugalcdep.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Childhood exposure to violence and lifelong health: clinical intervention science and stress-biology research join forces. Dev Psychopathol. 2013;25:1619–1634. doi: 10.1017/S0954579413000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls C, Bruno R, Matthews A. Chronic cannabis use and ERP correlates of visual selective attention during the performance of a flanker go/nogo task. Biol Psychol. 2015;110:115–125. doi: 10.1016/j.biopsycho.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Pinchevsky GM, Fagan AA, Wright EM. Victimization experiences and adolescent substance use: does the type and degree of victimization matter? J Interpers Violence. 2014;29:299–319. doi: 10.1177/0886260513505150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Leech S, Willford J. Prenatal cocaine exposure: effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicol Teratol. 2011;33:69–77. doi: 10.1016/j.ntt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL. Adolescent initiation of drug use: effects of prenatal cocaine exposure. J Am Acad Child Adolesc Psychiatry. 2013;52:37–46. doi: 10.1016/j.jaac.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Larkby C, Day NL. Effects of prenatal cocaine exposure on adolescent development. Neurotoxicol Teratol. 2015;49:41–48. doi: 10.1016/j.ntt.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Clark DB, Cottler LB. The illustration-based assessment of liability and exposure to substance use and antisocial behavior for children. Am J Drug Alcohol Abuse. 2009;35:242–252. doi: 10.1080/00952990902998715. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicol Teratol. 2000;22:653–666. doi: 10.1016/s0892-0362(00)00092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, Kliegman R. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–111. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Minnes S, Min MO, Lewis BA, Short EJ. Prenatal cocaine exposure and child outcomes: a conference report based on a prospective study from Cleveland. Hum Psychopharmacol. 2015;30:285–289. doi: 10.1002/hup.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. NSDUH Series H-46, HHS Publication No. SMA 13-4795. [Google Scholar]
- Taylor K, Kliewer W. Violence exposure and early adolescent alcohol use: an exploratory study of family risk and protective factors. J Child Fam Stud. 2006;15:201–215. [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM, Poulsen C, Luu P. Critical periods for the neurodevelopmental processes of externalizing and internalizing. Dev Psychopathol. 2015;27:321–346. doi: 10.1017/S0954579415000024. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Szabo NJ. Early adolescent cocaine use as determined by hair analysis in a prenatal cocaine exposure cohort. Neurotoxicol Teratol. 2011;33:88–99. doi: 10.1016/j.ntt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Staghezza-Jaramillo B, Shrout P, Popovac D, Graziano J. The effect of lead exposure on behavior problems in preschool children. Am J Public Health. 1998;88:481–486. doi: 10.2105/ajph.88.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. WISC-IV: Administration and Scoring Manual. Psychological Corp; San Antonio, TX: 2003. [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule AM, Prince JB. Adolescent substance use disorders in the school setting. Child Adolesc Psychiatr Clin N Am. 2012;21:175–186. doi: 10.1016/j.chc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol/disinhibition pathway to substance use disorders: a multilevel developmental problem. Child Dev Perspect. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]