Abstract
We examined p16 expression in tumors from a population-based sample of laryngeal cancer cases diagnosed in the U.S. Samples had been previously genotyped for HPV DNA.
Overall, p16 expression was observed in laryngeal tissue from 8 of 101 (7.9%) cases. p16 expression was observed in 2 of 16 (12.5%) cases previously determined to be HPV DNA positive. The two cases dually positive for p16 and HPV DNA were non-keratinizing SCC and papillary SCC tumors that were positive for genotypes 18 and 35/89, respectively. Positivity for p16 and/or HPV DNA was not associated with 5-year survival (log-rank p value=0.55). Our findings support a limited role of HPV in laryngeal carcinogenesis. p16 is not a reliable surrogate for HPV status in laryngeal cancers and is not a predictor of laryngeal cancer survival.
Keywords: Larynx, Laryngeal cancer, Human papillomavirus, HPV, P16(INK4A), P16
1. Introduction
Human papillomavirus (HPV) plays an etiologic and prognostic role in oropharyngeal cancer [1], [2], [3]. Elevated tumor expression of p16(INK4A) (referred to as p16 hereafter), a cyclin-dependent kinase-4 inhibitor, has been well-characterized in oropharyngeal cancer patients and is strongly correlated with HPV positivity. HPV-positivity combined with expression of p16(INK4A) is strong evidence of biologically relevant infection [4].
Unlike oropharyngeal cancers, an etiologic role of HPV in laryngeal and other malignancies of the head and neck has not been definitively established [1], [3]. We recently reported the results of a population-based study to evaluate the genotype-specific prevalence of HPV in invasive laryngeal cancer cases diagnosed in the U.S. [5]. HPV DNA was detected in 31 of 148 (21%) invasive laryngeal cancers; 13 different genotypes were observed. The detection of HPV DNA in tumor tissue, however, is not definitive evidence for causation. The current report examines p16 expression in laryngeal cancer cases in order to further elucidate HPV-related laryngeal cancer development and progression.
2. Methods
This study was approved by the CDC Institutional Review Board (IRB) and the IRBs of the University of Hawaii, University of Iowa, and University of Southern California. All patients were diagnosed in 1993–2004 within the catchment area of three population-based cancer registries [5]. Laryngeal cancer cases were selected from patients with pathologically-confirmed tumors. The majority of cases were squamous cell carcinomas of all subsites including the supraglottis, glottis, and subglottis.
De-identified, clinically annotated formalin-fixed paraffin-embedded (FFPE) tissue specimens were obtained from Residual Tissue Repositories (RTR) affiliated with the National Cancer Institute׳s Surveillance, Epidemiology, and End Results (SEER) Program [6], [7]. Through linkage with registry patient and tumor data, tissue specimens were annotated with de-identified demographic, clinical, pathologic, and survival data. Tissue specimens had been previously genotyped for HPV at the CDC laboratories as previously described [5], [8] using the Linear Array HPV Genotyping Test for 37 HPV genotypes (LA, Roche Diagnostics, Indianapolis, IN). The INNO-LiPA HPV Genotyping Assay (LiPA, Innogenetics, Gent, Belgium) was also employed for specimens testing negative for HPV and human beta-globin.
2.1. Histologic subtyping by pathologic review
H&E slides of squamous cell carcinoma cases of unspecified subtype, i.e. SCC NOS, were reviewed by a study pathologist (M.R.) for subtype assignment. SCC cases were classified as keratinizing, non-keratinizing, basaloid, verrucous, papillary, and spindle cell.
2.2. p16 immunohistochemistry and pathologic review
p16 expression was evaluated via immunohistochemistry. Sections of tumor tissue were obtained from the same FFPE blocks previously used for HPV genotyping. A p16 mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (dilution 1:400) was used according to the manufacturer׳s specifications. Slides were read by a study pathologist (M.R.) who was blinded to the HPV status of cases. p16 staining was evaluated based level of staining intensity (mild/weak, moderate, strong), intracellular localization (nuclear, cytoplasmic), staining distribution (patchy, focal, diffuse), and the proportion of tumor cells stained. Specimens exhibiting strong, diffuse nuclear and cytoplasmic staining in ≥70% of tumor cells were considered to be definitively positive for p16 based on established criteria [9], [10].
2.3. Statistical analyses
Statistical analyses were conducted using SAS version 9.2. Comparison of p16 expression used the Chi-square statistic. Survival was calculated based on the time period from date of diagnosis to date of death or date of last follow-up. Overall five-year survival by p16 and HPV DNA status was evaluated using the Kaplan–Meier method and the log-rank test. All tests were two-sided and a p value <0.05 was considered to be statistically significant.
3. Results
The laryngeal cancer study population has been previously detailed [5]. Tumor tissue specimens from 101 of 148 cases from the prior analysis with sufficient tissue for immunohistochemistry were included in the present study. SCC subtypes included 49 (48.5%) keratinizing, 17 (16.8%) non-keratinizing, 9 (8.9%) papillary, 3 (3%) basaloid, 2 (2.0%) spindle cell, and 1 (1.0%) verrucous. A total of 19 (18.8%) of cases remained classified as unspecified SCC and 1 case was a small cell carcinoma.
Eight of the 101 (7.9%) of laryngeal tumors were considered to be positive for p16 based on the criteria of strong, diffuse p16 staining of the nucleus and cytoplasm in ≥70% of tumor cells. Thirty-two cases which exhibited strong, diffuse nuclear and cytoplasmic staining in fewer than 70% of tumor cells and were not considered to be p16 positive. Table 1 compares p16 and HPV DNA status by histologic subtype. Basaloid SCC tumors exhibited the largest proportion of p16 positive tumors (2 of 3). All 3 basaloid tumors were HPV DNA negative. HPV positivity was highest in non-keratinizing (4 of 17) and papillary (2 of 9) SCC tumors. Only 2 of the 26 non-keratinizing and papillary SCC cases were positive for p16 expression.
Table 1.
p16(INK4A) expression and HPV DNA status by histology of invasive laryngeal tumors.
| Histology |
p16 |
HPV DNA |
||
|---|---|---|---|---|
| No. positive | No. negative | No. positive | No. negative | |
| SCC keratinizing (n=49) | 3 | 46 | 8 | 41 |
| SCC non-keratinizing (n=17) | 1 | 16 | 4 | 13 |
| SCC papillary (n=9) | 1 | 8 | 2 | 7 |
| SCC basaloid (n=3) | 2 | 1 | 0 | 3 |
| SCC spindle cell (n=2) | 0 | 2 | 0 | 2 |
| SCC verrucous (n=1) | 0 | 1 | 1 | 0 |
| SCC NOS (n=19) | 1 | 18 | 1 | 18 |
| Small cell carcinoma NOS (n=1) | 0 | 1 | 0 | 1 |
| Total | 8 | 93 | 16 | 85 |
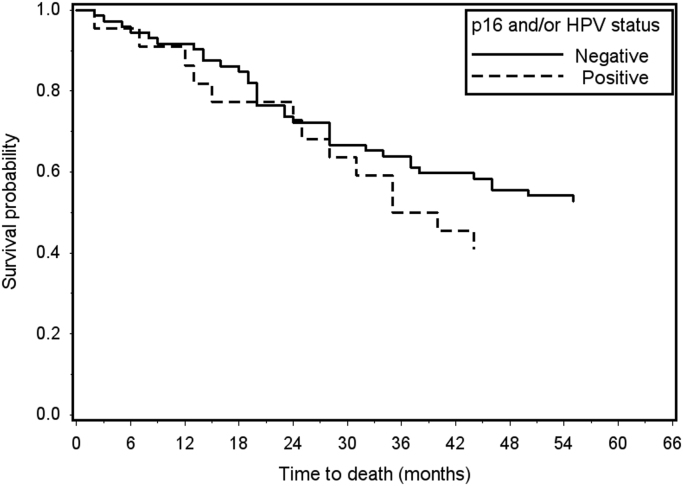
In total, p16 expression was observed in 2 of 16 (12.5%) HPV DNA positive laryngeal cancer cases (Table 2). One p16-positive case was a non-keratinizing SCC positive for HPV 18 (Fig. 1). The second p16 positive case was a papillary SCC positive for both HPV 35 and 89. Both p16/HPV DNA positive cases were localized tumors of the glottis diagnosed in males under age 50. The 14 HPV positive laryngeal cancer cases without p16 expression included glottal and supraglottal tumors of all stages diagnosed in males and females primarily aged 50 and older. Overall survival was evaluated in the 95 cases with vital status and follow-up information. p16 was not associated with 5-year survival when measured based on p16 expression alone (log-rank p value=0.84) or positivity for either p16 and/or HPV DNA (log-rank p value=0.55) (Fig. 2).
Table 2.
p16(INK4A) expression in HPV-positive invasive laryngeal tumors.
| p16 | HPV DNA genotype | Subsite | SEER stage | Histology | Grade | Gender | Age group |
|---|---|---|---|---|---|---|---|
| Positive | 18 | Glottis | Localized | SCC non-keratinizing | 2 | Male | 40–49 |
| Positive | 35, 89 | Glottis | Localized | SCC papillary | 1 | Male | 40–49 |
| Negative | 16 | Supraglottis | Regional | SCC non-keratinizing | 2 | Female | 80–89 |
| Negative | 35 | Glottis | Localized | SCC papillary | 1 | Female | 70–79 |
| Negative | 39 | Supraglottis | Regional | SCC keratinizing | 2 | Female | 50–59 |
| Negative | 16, 54 | Supraglottis | Regional | SCC keratinizing | 3 | Male | 70–74 |
| Negative | 18, 33 | Glottis | Distant | SCC keratinizing | 2 | Male | 65–69 |
| Negative | 16 | Supraglottis | Localized | SCC keratinizing | 2 | Male | 65–69 |
| Negative | 11 | Supraglottis | Distant | SCC NOS | 3 | Female | 55–59 |
| Negative | Untyped | Glottis | Localized | SCC verrucous | a | Male | 65–69 |
| Negative | 16 | Supraglottis | Regional | SCC keratinizing | 3 | Male | 70–74 |
| Negative | 33 | Supraglottis | Regional | SCC keratinizing | 2 | Female | 55–59 |
| Negative | 16, 31, 33 | Glottis | Localized | SCC keratinizing | 2 | Male | 45–49 |
| Negative | 51 | Supraglottis | Regional | SCC non-keratinizing | 3 | Male | 75–79 |
| Negative | 51 | Glottis | Localized | SCC keratinizing | 2 | Male | 60–64 |
| Negative | 6 | Glottis | Regional | SCC non-keratinizing | 2 | Female | 50–54 |
Tumor grade could not be ascertained based on the registry data and secondary pathologic review.
Fig. 1.
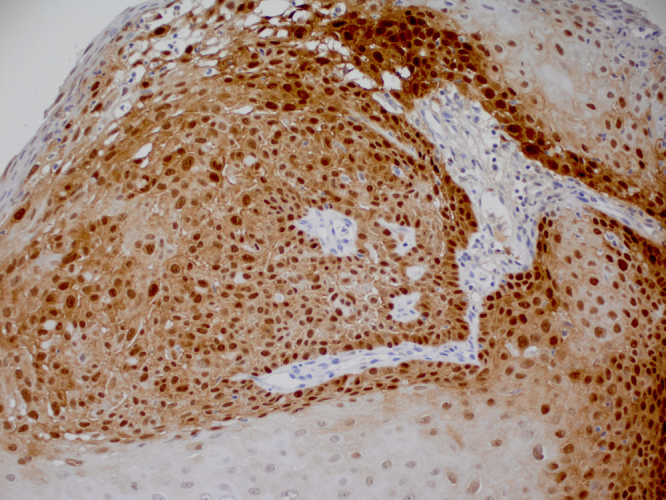
p16 expression in invasive laryngeal (glottal) non-keratinizing SCC tumor positive for HPV 18 DNA. p16 exhibits strong nuclear and cytoplasmic staining of a diffuse pattern in greater than 70% of tumor cells (20×).
Fig. 2.
p16 expression & HPV DNA status and overall 5-year survival in invasive laryngeal cancer (n=95). There was no difference in overall 5-year survival by positivity for p16 and/or HPV DNA (log-rank p value 0.55).
4. Conclusions
Our findings support a limited role of HPV in laryngeal carcinogenesis. Fewer than 10% of all laryngeal tumors expressed p16 and p16 expression did not strongly correlate with HPV DNA status. In total, only a fraction (2%) of laryngeal cancers were positive for both p16 and HPV DNA. We previously observed HPV DNA in over 1 in 5 invasive laryngeal cancers. However, detection of HPV DNA alone is not indicative of a clinically relevant infection. In HPV-induced carcinogenesis, the E7 oncoprotein binds and inactivates the retinoblastoma tumor suppressor gene product, pRb [11]. As pRb is a negative regulator of p16, its inactivation results in overexpression of p16 [11]. Therefore, HPV-positivity combined with p16 expression is strong evidence of biologically relevant infection [4]. Our findings of limited correlation of p16 with HPV DNA status contrasts with the few studies that have examined both HPV and p16 in laryngeal cancers. In a study of patients from a single U.S. institution, 65% of p16 positive cases were also positive for HPV DNA [12]. In a pooled analysis of data from two studies, p16 expression was found in 86% of HPV-positive laryngeal cancers [13]. The predominant pattern of p16 expression of laryngeal cancers that we observed was diffuse, strong expression in both the nucleus and cytoplasm. However, for the majority of these cases, fewer than 70% of tumor cells were positive. Wide variation in p16 staining patterns has been observed in head and neck cancers [14]. In general, strong, diffuse nuclear and cytoplasmic staining in the majority (i.e., ≥70%) of tumor cells is considered to be definitively positive for p16 [9], [10]. This p16 expression pattern is seen in the majority of oropharyngeal cancers for which p16 is highly correlated with HPV DNA detection [9], [10], [15]. p16 positivity in the absence of HPV DNA—as observed in a subset of our cases—is consistent with the suggestion that p16 upregulation in laryngeal carcinogenesis may reflect somatic chromosomal alterations unrelated to HPV [16], [17], [18].
Although the sample size was limited, we observed some histologic differences by p16 and HPV status. p16 positivity was most frequently found in basaloid SCC tumors, while HPV DNA positivity was highest in non-keratinizing and papillary SCC tumors. Interestingly, non-keratinizing SCC and, to a lesser extent, basaloid and papillary SCC tumors, are the histologic variants that are most common in HPV-associated oral and oropharyngeal squamous cell carcinomas [19].
The etiologic and prognostic role of HPV in oropharyngeal cancers is well-established [1], [2], [3]. HPV tumor positivity favorably influences outcome, including overall survival, disease-free survival, and recurrence [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. Unlike oropharyngeal cancers, our findings do not support a prognostic role of HPV in laryngeal cancer. This was consistent for p16 alone and in combination with HPV DNA. We previously observed no survival advantage in HPV DNA-positive laryngeal cancers [5]. Our findings are in agreement with single institution studies that did not observe statistically significant associations of p16 overexpression with laryngeal cancer survival [12], [37], [38].
Our findings support the evidence to date which collectively suggests that, in contrast with the well-established etiologic and prognostic role of HPV in oropharyngeal malignancies, its role in laryngeal cancers is comparatively limited [17]. A major limitation of our study is the lack of information on tobacco and alcohol use, which are the predominant risk factors for laryngeal cancers. Presumably, the majority of laryngeal cancers in the present study were linked to these exposures. Our study findings indicate that any etiologic role of HPV is limited to only a fraction of laryngeal cancers.
Footnotes
This project was funded by the Centers for Disease Control and Prevention. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Brenda Y. Hernandez, Email: brenda@cc.hawaii.edu.
Mobeen Rahman, Email: mobeen@hawaii.edu.
Charles F. Lynch, Email: charles-lynch@uiowa.edu.
Wendy Cozen, Email: wcozen@usc.edu.
Elizabeth R. Unger, Email: eru0@cdc.gov.
Martin Steinau, Email: azz9@cdc.gov.
Trevor Thompson, Email: tkt2@cdc.gov.
Maria Sibug Saber, Email: sibugsab23@gmail.com.
Sean F. Altekruse, Email: altekrusesf@mail.nih.gov.
Marc T. Goodman, Email: marc.goodman@cshs.org.
Amy Powers, Email: aapowers@hawaii.edu.
Christopher Lyu, Email: Lyuc@Battelle.org.
Mona Saraiya, Email: yzs2@cdc.gov.
References
- 1.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 2005;32(Suppl. 1):S59–S66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Kreimer A.R., Clifford G.M., Boyle P., Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol. Biomark. Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. Epub 2005/03/01. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Carcinogenic Risks to Humans, Human Papillomaviruses, vol. 90, World Health Organization, Lyon, 2005.
- 4.Langendijk J.A., Psyrri A. The prognostic significance of p16 overexpression in oropharyngeal squamous cell carcinoma: implications for treatment strategies and future clinical studies. Ann. Oncol. 2010;21(10):1931–1934. doi: 10.1093/annonc/mdq439. Epub 2010/08/19. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez B.Y., Goodman M.T., Lynch C.F., Cozen W., Unger E.R., Steinau M. Human papillomavirus prevalence in invasive laryngeal cancer in the United States. PLoS One. 2014;9(12):e115931. doi: 10.1371/journal.pone.0115931. Epub 2014/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.M.T. Goodman, B.Y. Hernandez, S. Hewitt, C.F. Lynch, T.R. Cote, H.F. Frierson Jr., et al., Tissues from population-based cancer registries: a novel approach to increasing research potential, Hum. Pathol. 36 (7) (2005) 812–820. Epub 2005/08/09. [DOI] [PubMed]
- 7.Altekruse S.F., Petrick J.L., Rolin A.I., Cuccinelli J.E., Zou Z., Tatalovich Z. Geographic variation of intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and hepatocellular carcinoma in the United States. PLoS One. 2015;10(3):e0120574. doi: 10.1371/journal.pone.0120574. Epub 2015/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gargano J.W., Wilkinson E.J., Unger E.R., Steinau M., Watson M., Huang Y. Prevalence of human papillomavirus types in invasive vulvar cancers and vulvar intraepithelial neoplasia 3 in the United States before vaccine introduction. J. Low. Genit. Tract. Dis. 2012;16(4):471–479. doi: 10.1097/LGT.0b013e3182472947. Epub 2012/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Naggar A.K., Westra W.H. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459–461. doi: 10.1002/hed.21974. Epub 2011/12/20. [DOI] [PubMed] [Google Scholar]
- 10.Lassen P., Primdahl H., Johansen J., Kristensen C.A., Andersen E., Andersen L.J. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother. Oncol. 2014;113(3):310–316. doi: 10.1016/j.radonc.2014.11.032. Epub 2014/12/30. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson K., Svensson S., Landberg G. Retinoblastoma protein function and p16INK4a expression in actinic keratosis, squamous cell carcinoma in situ and invasive squamous cell carcinoma of the skin and links between p16INK4a expression and infiltrative behavior. Mod. Pathol. 2004;17(12):1464–1474. doi: 10.1038/modpathol.3800220. Epub 2004/07/17. [DOI] [PubMed] [Google Scholar]
- 12.Chernock R.D., Wang X., Gao G., Lewis J.S., Jr., Zhang Q., Thorstad W.L. Detection and significance of human papillomavirus, CDKN2A(p16) and CDKN1A(p21) expression in squamous cell carcinoma of the larynx. Mod. Pathol. 2013;26(2):223–231. doi: 10.1038/modpathol.2012.159. Epub 2012/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ndiaye C., Mena M., Alemany L., Arbyn M., Castellsague X., Laporte L. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. doi: 10.1016/S1470-2045(14)70471-1. Epub 2014/12/03. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z.W., Weinreb I., Kamel-Reid S., Perez-Ordonez B. Equivocal p16 immunostaining in squamous cell carcinoma of the head and neck: staining patterns are suggestive of HPV status. Head Neck Pathol. 2012;6(4):422–429. doi: 10.1007/s12105-012-0382-3. Epub 2012/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis J.S., Jr. p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl. 1):S75–S82. doi: 10.1007/s12105-012-0369-0. Epub 2012/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klingenberg B., Hafkamp H.C., Haesevoets A., Manni J.J., Slootweg P.J., Weissenborn S.J. p16 INK4A overexpression is frequently detected in tumour-free tonsil tissue without association with HPV. Histopathology. 2010;56(7):957–967. doi: 10.1111/j.1365-2559.2010.03576.x. Epub 2010/07/20. [DOI] [PubMed] [Google Scholar]
- 17.Combes J.D., Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral. Oncol. 2014;50(5):370–379. doi: 10.1016/j.oraloncology.2013.11.004. Epub 2013/12/18. [DOI] [PubMed] [Google Scholar]
- 18.Larque A.B., Conde L., Hakim S., Alos L., Jares P., Vilaseca I. P16(INK(4)a) overexpression is associated with CDKN2A mutation and worse prognosis in HPV-negative laryngeal squamous cell carcinomas. Virchows Arch. 2015;466(4):375–382. doi: 10.1007/s00428-015-1725-8. Epub 2015/02/06. [DOI] [PubMed] [Google Scholar]
- 19.El-Mofty S.K. Histopathologic risk factors in oral and oropharyngeal squamous cell carcinoma variants: an update with special reference to HPV-related carcinomas. Med. Oral. Patol. Oral. Cirugia Bucal. 2014;19(4):e377–e385. doi: 10.4317/medoral.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakhry C., Westra W.H., Li S., Cmelak A., Ridge J.A., Pinto H. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. Epub 2008/02/14. [DOI] [PubMed] [Google Scholar]
- 21.Monk B.J., Burger R.A., Lin F., Parham G., Vasilev S.A., Wilczynski S.P. Prognostic significance of human papillomavirus DNA in vulvar carcinoma. Obstet. Gynecol. 1995;85(5 Pt 1):709–715. doi: 10.1016/0029-7844(95)00045-s. Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- 22.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tan P.F. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. Epub 2010/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansink A.C., Krul M.R., De Weger R.A., Kleyne J.A., Pijpers H., Van Tinteren H. Human papillomavirus, lichen sclerosus, and squamous cell carcinoma of the vulva: detection and prognostic significance. Gynecol. Oncol. 1994;52(2):180–184. doi: 10.1006/gyno.1994.1028. Epub 1994/02/01. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie J.M., Smith E.M., Summersgill K.F., Hoffman H.T., Wang D., Klussmann J.P. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int. J. Cancer. 2003;104(3):336–344. doi: 10.1002/ijc.10960. Epub 2003/02/06. [DOI] [PubMed] [Google Scholar]
- 25.Gillison M.L., Koch W.M., Capone R.B., Spafford M., Westra W.H., Wu L. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. Epub 2000/05/04. [DOI] [PubMed] [Google Scholar]
- 26.Li W., Thompson C.H., O׳Brien C.J., McNeil E.B., Scolyer R.A., Cossart Y.E. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int. J. Cancer. 2003;106(4):553–558. doi: 10.1002/ijc.11261. Epub 2003/07/08. [DOI] [PubMed] [Google Scholar]
- 27.Lont A.P., Kroon B.K., Horenblas S., Gallee M.P., Berkhof J., Meijer C.J. Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int. J. Cancer. 2006;119(5):1078–1081. doi: 10.1002/ijc.21961. Epub 2006/03/30. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz S.R., Yueh B., McDougall J.K., Daling J.R., Schwartz S.M. Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol. Head Neck Surg. 2001;125(1):1–9. doi: 10.1067/mhn.2001.116979. Epub 2001/07/18. [DOI] [PubMed] [Google Scholar]
- 29.Haraf D.J., Nodzenski E., Brachman D., Mick R., Montag A., Graves D. Human papilloma virus and p53 in head and neck cancer: clinical correlates and survival. Clin. Cancer Res. 1996;(4):755–762. Epub 1996/04/01. [PubMed] [Google Scholar]
- 30.Gillison M.L., Koch W.M., Shah K.V. Human papillomavirus in head and neck squamous cell carcinoma: are some head and neck cancers a sexually transmitted disease? Curr. Opin. Oncol. 1999;11(3):191–199. doi: 10.1097/00001622-199905000-00010. Epub 1999/05/18. [DOI] [PubMed] [Google Scholar]
- 31.Chiba I., Shindoh M., Yasuda M., Yamazaki Y., Amemiya A., Sato Y. Mutations in the p53 gene and human papillomavirus infection as significant prognostic factors in squamous cell carcinomas of the oral cavity. Oncogene. 1996;12(8):1663–1668. Epub 1996/04/18. [PubMed] [Google Scholar]
- 32.Mellin H., Friesland S., Lewensohn R., Dalianis T., Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int. J. Cancer. 2000;89(3):300–304. Epub 2000/06/22. [PubMed] [Google Scholar]
- 33.Kumar B., Cordell K.G., Lee J.S., Worden F.P., Prince M.E., Tran H.H. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J. Clin. Oncol. 2008;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. Epub 2008/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licitra L., Perrone F., Bossi P., Suardi S., Mariani L., Artusi R. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2006;24(36):5630–5636. doi: 10.1200/JCO.2005.04.6136. Epub 2006/12/21. [DOI] [PubMed] [Google Scholar]
- 35.Reimers N., Kasper H.U., Weissenborn S.J., Stutzer H., Preuss S.F., Hoffmann T.K. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int. J. Cancer. 2007;120(8):1731–1738. doi: 10.1002/ijc.22355. Epub 2007/01/20. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E. Human papillomavirus (HPV) and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. Epub 2011/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morshed K. Association between human papillomavirus infection and laryngeal squamous cell carcinoma. J. Med. Virol. 2010;82(6):1017–1023. doi: 10.1002/jmv.21749. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 38.Young R.J., Urban D., Angel C., Corry J., Lyons B., Vallance N. Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br. J. Cancer. 2015;112(6):1098–1104. doi: 10.1038/bjc.2015.59. Epub 2015/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]