Abstract
Background
A patency capsule (PC) can help predict capsule endoscope (CE) retention; however, PC tolerability is unknown in children. We retrospectively evaluated PC tolerability in school-aged children.
Methods
Sixty-one patients (median age, 12.9 years; range 7.4–17.3 years) who underwent PC examination were analyzed for occurrence and determinants of ingestion difficulty and relationships between ingestion of the 2 capsules. We defined ingestion difficulty as taking 30 min or more, or failure, to ingest the PC.
Results
Thirty-nine patients (64%) successfully ingested the PC without ingestion difficulty. The other 22 had ingestion difficulty and were significantly younger (11.7 ± 2.2 vs. 13.0 ± 1.8 years; p = 0.04) and shorter (143.3 ± 14.0 vs. 154.6 ± 12.5 cm; p = 0.003) than those without ingestion difficulty. Multivariate analysis showed that the most significant factor for predicting PC ingestion difficulty was height (cutoff value, 152 cm). Time to ingest the CE was significantly shorter than that for PC ingestion (8 ± 32 vs. 20 ± 58 min; p = 0.01). All patients indicated that ingestion of the CE was easier because of its smooth surface compared with the PC.
Conclusions
PC ingestion is not guaranteed in school-aged children. PC ingestion ability should be evaluated by considering the child's height and lack of experience ingesting capsules prior to PC examination.
Keywords: Patency capsule, Children, Capsule endoscopy, Ingestion
Introduction
The capsule endoscope (CE) is a swallowable device used to evaluate small intestinal diseases noninvasively in both adults and children [1, 2, 3, 4]. A serious complication of capsule endoscopy, however, is retention of the capsule in the intestine [4, 5, 6, 7], especially at stenoses [8]. Because capsule retention requires a surgical or endoscopic procedure to remove the capsule, assessing the risk of capsule retention, that is, confirming intestinal patency, is desirable before capsule endoscopy, especially in patients with suspected stenosis [5, 6, 7, 8, 9]. However, a conventional barium small-bowel series does not always reveal stenosis, leading to capsule retention [4, 10]; therefore, a novel method for assessing intestinal patency has been developed that uses a soluble capsule, a patency capsule (PC), that has the same dimensions as those of the CE (length, 26 mm; diameter, 11 mm) [11]. When a patient does not excrete an ingested PC or excretes it in a dissolved state, the physician learns the potential for capsule retention and can avoid using the CE [10, 11]. Previous studies have demonstrated the utility of a PC to verify the safety of using the CE in adults [10, 11]; however, because the number of studies on PC use in children is limited [12], PC tolerability in children is not known. Ingestion difficulty and aspiration of the CE have been reported as serious adverse events in elderly patients [13, 14], but such events have not yet been studied well in children. Because body size and swallowing ability differ between adults and children, capsule ingestion is key to the use of both the PC and CE in children [1, 2]. Therefore, here, we evaluated PC tolerability in children scheduled for CE, focusing on their ability to ingest the capsules.
Methods
Study Design
We retrospectively analyzed medical records from March 1, 2012, through March 31, 2016, of pediatric patients whose intestinal patency had been evaluated by using a PC (Given Imaging, Ltd., Yoqneam, Israel) prior to CE to investigate small intestinal diseases. PC and CE examinations were performed after written informed consent was obtained from each patient and/or each child's legal guardian. The children and their parents or legal guardians were given, in writing, a full explanation of the aims of the PC and CE examinations, their possible hazards, and inconveniences that could be faced. From the patient records, we collected physical characteristics, indications for CE, time taken to ingest the PC and/or CE, and outcomes of the PC and CE evaluations. We defined ingestion difficulty as taking 30 min or more, or failure, to ingest the PC. From these data, we analyzed the occurrence rate of and factors contributing to PC ingestion difficulty, as well as relationships between the outcomes of PC and CE ingestion. This retrospective study protocol conformed to the principles of the Declaration of Helsinki and was approved by our institutional Ethics Committee (approval number: 3416).
PC Examination
The PC used in this study is licensed in Japan; it is coated with an impermeable membrane and contains 10% barium sulfate for radiopacity [15]. Unlike the PCs licensed in the United States and Europe [12], it contains no radiofrequency identification tag. Because the identification tag has been reported to cause impaction at stenoses, leading to small-bowel ileus [16], the tagless PC was developed and licensed in Japan [15].
Patients were given the PC orally and the time taken for ingestion was recorded. Some patients who failed to ingest the PC on the first attempt retried on another day. After the PC had been ingested successfully, its excretion or location in the abdomen was evaluated. The time for PC excretion was recorded by the patients and/or their legal guardians. Abdominal radiograms were obtained when required to confirm the location of the PC. Once the PC was confirmed to have reached the colon or had been excreted without dissolution, the patients underwent CE. After the CE exam, patients were asked to judge whether it was easier to ingest the PC or the CE.
Capsule Endoscopic Examination
Patients fasted overnight or for at least 8 h before swallowing the CE (Pillcam SB2, Given Imaging, Ltd., Yoqneam, Israel); the time required for ingestion of the capsule was recorded. The patients could immediately resume normal activities and were permitted to consume clear liquids 2 h and food 4 h after ingesting the capsule. The patients or their legal guardians were asked to record the time when the capsule was excreted. Recording from the CE stopped after 7–8 h. The images obtained from the CE were analyzed by pediatric gastroenterologists using Rapid Reader software (Given Imaging).
Statistical Analyses
Comparisons of 2 groups (ingestion difficulty vs. no ingestion difficulty, or PC vs. CE) were made by using Mann-Whitney U testing. Two-sided p values <0.05 were considered to be statistically significant. Multivariate linear regression analyses were performed to identify independent factors that affected PC ingestion difficulty. The diagnostic performance of the various characteristics was evaluated by plotting receiver operating characteristic curves. The areas under the receiver operating characteristic curves (AUROCs) were used as indices of accuracy. The point on the curve that was closest to the top left corner of the graph was selected as the best cutoff value. We calculated the AUROC, optimal cutoff, sensitivity, specificity, and positive and negative predictive values for estimating PC ingestion difficulty.
Results
Patients
Sixty-one patients (40 boys; median age 12.9 years; age range 7.4–17.3 years) underwent PC examination (Table 1). The indications for capsule endoscopy are shown in Table 1. None of the patients had any neurological disorders that could have affected capsule ingestion.
Table 1.
Patient characteristics
| Number of patients | 61 |
| Boys:girls, n | 40:21 |
| Agea, years | 12.9 (7.4–17.3) |
| Heighta, cm | 150.9 (114.2–177.4) |
| Weighta, kg | 38.4 (18.2–66.7) |
| BMI percentilea, kg/m2 | 33.6 (0–88.9) |
| Indication for capsule endoscopy, n | |
| Suspected IBD | 41 |
| Obscure gastrointestinal bleeding | 6 |
| Suspected tumor | 4 |
| Confirmed Crohn's disease | 2 |
| Confirmed ulcerative colitis | 5 |
| Chronic diarrhea | 2 |
| Growth failure | 1 |
BMI, body mass index; IBD, inflammatory bowel disease. * Data are given as median values and ranges.
Feasibility of Conducting an Initial PC Examination
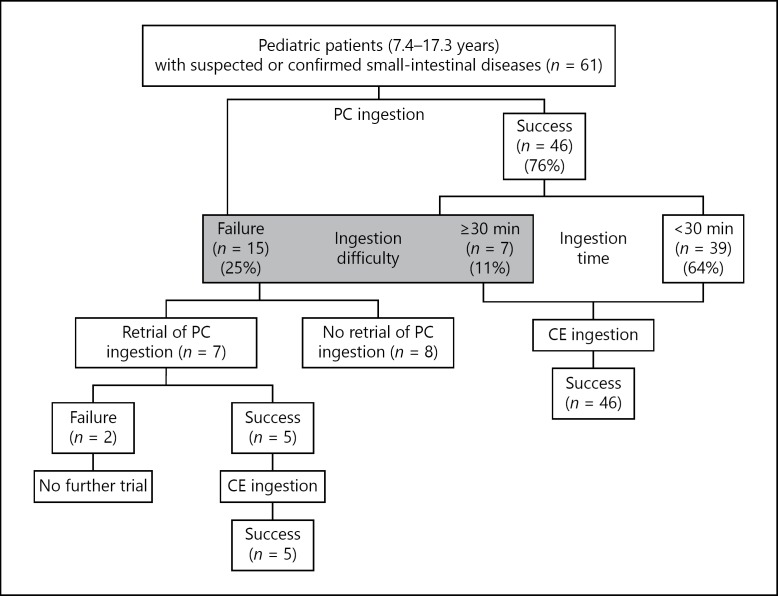
Among the 61 patients, 46 (75.4%) were able to swallow the PC (Fig. 1). The time taken to ingest the PC was less than 30 min for 39 of the 61 patients (63.9%) and 30 min or more for 7 patients (11.5%). The remaining 15 patients (24.6%) were unable to swallow the PC after at least 30 min of trying (Fig. 1). Difficulty in PC ingestion was thus noted in 22 patients (36.1%).
Fig. 1.
Flowchart of the study design and patients. Patients who could ingest the PC within 30 min were deemed to have no ingestion difficulty; those who took longer than 30 min and those who were unable to swallow the PC were considered to have ingestion difficulty. PC, patency capsule; CE, capsule endoscope.
Factors of PC Ingestion Difficulty
Patients who had ingestion difficulty were significantly younger and shorter than those who did not have ingestion difficulty (Table 2). There were no significant differences between those who had ingestion difficulty and those who did not with respect to gender, weight, or body mass index percentile. To identify which independent factors affected PC ingestion difficulty, we used multiple regression analysis of age and height. Height was significantly associated with PC ingestion difficulty (coefficient = −0.0134; 95% CI −0.022 to −0.005; p = 0.002). The cutoff value of height for predicting PC ingestion difficulty was 152 cm, with 0.738 AUROC (95% CI 0.603–0.873), 81% sensitivity, 63% specificity, 55% positive predictive value, and 86% negative predictive value.
Table 2.
Comparison of patient characteristics based on PC ingestion difficulty
| PC ingestion difficulty |
p value | ||
|---|---|---|---|
| yes (n = 22) | no (n = 39) | ||
| Boys:girls, n | 15:7 | 25:14 | ns |
| Age, years | 11.7±2.2 | 13.0±1.8 | 0.04 |
| Height, cm | 143.3±14.0 | 154.6±12.5 | 0.003 |
| Weight, kg | 37.4±12.2 | 43.3±12.1 | ns |
| BMI percentile, kg/m2 | 43.3±26.5 | 32.8±28.3 | ns |
BMI, body mass index; ns, not statistically and significantly different; PC, patency capsule.
Reattempted PC Ingestion
Seven of the 15 patients who were unable to ingest the PC at the initial trial made a renewed attempt on another day (Fig. 1). Five of those 7 patients succeeded in ingesting the PC. The remaining 2 patients were again unable to ingest the PC. These results indicate that previous experience with PC ingestion improves PC ingestion on retrial.
Passage of the PC
Fifty-one patients ingested the PC during the first or repeated trial (Fig. 1). No dissolved PCs and no adverse events due to passage of the PC were noted. In 24 of the 51 patients, abdominal radiography confirmed the location of the PC in the large intestine; in 26 of the 51 patients, excretion of the PC was confirmed by the patient or his or her legal guardian. In the remaining patient, the PC was confirmed to be in the colon by colonoscopy, which was performed to evaluate a colon lesion 21 h after PC ingestion. The time taken for the PC to reach the colon, determined radiographically (29.8 ± 14.9 h, mean ± SD), did not differ significantly from the time taken for excretion of the PC (25.6 ± 10.9 h). One patient with suspected Crohn's disease, who passed the capsule at 71 h, was considered a PC test failure because of the delayed excretion (>33 h), but at his family's request and with recognition of the risks, he underwent CE for symptom assessment.
Time Taken to Ingest the CE Is Less Than That Taken to Ingest the PC
All 46 patients who swallowed the PC during the initial trial succeeded in ingesting the CE, and the time taken for its ingestion was significantly less than that taken to ingest the PC (p = 0.01; Table 3). The time taken to ingest the CE was significantly longer for patients who had difficulty ingesting the PC (50.7 ± 71.0 min) than it was for those who had no ingestion difficulty (0.4 ± 1.6 min; p < 0.001; Table 3).
Table 3.
Outcomes for the 46 patients who ingested the PC at the initial trial
| Time for PC ingestion, min | Time for CE ingestion, min | p value1 | |
|---|---|---|---|
| Total, n = 46 | 20±58 | 8±32 | 0.01 |
| PC ingestion difficulty (−), n = 39 | 2.0±4.8 | 0.4±1.6 | 0.10 |
| PC ingestion difficulty (+), n = 7 | 120.0±102.8 | 50.7±71.0 | 0.06 |
| p value2 | <0.001 | <0.001 |
PC, patency capsule; CE, capsule endoscope.
Comparison between the time needed for PC ingestion and the time needed for CE ingestion.
Comparison between the groups with and without PC ingestion difficulty. p values were calculated by using the Mann-Whitney U test.
Ease of Capsule Ingestion
The 51 patients who successfully ingested both the PC and CE were asked to assess the ease of ingesting the capsules. All of them indicated that it was easier to ingest the CE than the PC because the surface of PC was not as smooth as that of the CE. This result suggests that PC ingestion difficulty depends, in part, on the surface characteristics of the PC.
Passage and Findings of the CE
None of the 51 patients who successfully ingested the CE experienced capsule retention. CE revealed small-intestinal lesions in 11 (21.6%) patients, thereby contributing to both the selection and modification of therapy for those patients. In the patient for whom PC excretion took 71 h, no CE retention occurred despite the presence of multiple intestinal stenoses. These results indicate that successful passage of the PC without its dissolution predicts a low risk of capsule retention during subsequent CE in pediatric patients; however, a delay in the passage (>33 h) of an intact PC requires careful interpretation.
Discussion
Difficulty ingesting capsules is a recognized problem among small children younger than 5 or 6 years of age; however, our study revealed that capsule ingestion is not guaranteed even in school-aged children. Our findings suggest that several factors contribute to the difficulty or inability of school-aged children to swallow PCs, including the patient's body size and lack of experience with oral capsule ingestion. This difficulty becomes a key issue when PCs and CEs are indicated for pediatric patients with suspected small-intestinal diseases. Our study results indicate that successful ingestion of the capsules depends largely on the child's height: children whose height was less than 152 cm had a higher risk of PC ingestion difficulty than did taller children. In the process of PC ingestion, the transportation of PC from oral cavity to the esophagus through the oropharynx is necessary. Thus, size and motility of oral cavity to the esophagus are considered important factors for PC ingestion in growing children. A previous study demonstrated that vocal cord length is correlated with body height [17]; thus, our results that body height is shorter in the PC ingestion difficulty group than in patients without PC ingestion difficulty indicate that the small size of the pharynx may cause the PC ingestion difficulty. It is difficult for pediatric gastroenterologists to choose between endoscopic placement or an ingestion trial of a PC or CE in school-aged children, but our results indicate that a child's height should be part of the assessment of his or her potential for PC ingestion difficulty prior to CE ingestion.
Another factor thought to contribute to PC ingestion difficulty is lack of experience with oral drug ingestion and swallowing capsules the size of a PC. To overcome this lack of experience, the so-called “jelly bean test” has proven useful prior to administering oral medications [18], although tracheal aspiration of both jelly beans and CEs has been reported in children and elderly patients [13, 19]. Therefore, although jelly beans may be useful in preparing for PC ingestion, careful attention must be paid to the risk of tracheal aspiration.
In addition to a patient's height and lack of experience ingesting capsules, the patients' judgment that it was easier to ingest the CE than the PC suggests that the rough surface of the PC compared to that of the CE may also contribute to ingestion difficulty. This factor could be solved by the manufacturer in the future.
The PC and CE are of the same size, so successful ingestion of the PC promises successful ingestion of the CE. Therefore, our results suggest that an initial PC ingestion trial is effective not only for evaluating the patient's intestinal patency but also for facilitating the subsequent ingestion of the CE, thus helping to avoid CE failure. In addition, our study indicates that patients who experience difficulty ingesting the PC should be monitored for difficulty ingesting the CE. Additional reasons to conduct an initial ingestion trial with a PC are the cost and the battery of the CE. The CE is an expensive disposable device that costs approximately USD 830 (¥94,200); therefore, it is desirable to avoid CE ingestion failure. In contrast, the cost of a PC is approximately USD 53 (¥6,000); therefore, having patients try ingesting a PC first and proceeding with the CE only if the PC trial is successful is one of the most noteworthy and attractive advantages of this noninvasive technique. In addition, because the battery in the CE has limited capacity, it is preferable to have the patient ingest the capsule as rapidly as possible to facilitate taking capsule images in the digestive tract for as long as possible. If the initial ingestion trial continues for a long time due to difficulty swallowing the capsule, the trial becomes stressful for the patient. A later retrial of PC ingestion may be effective for patients who initially fail to ingest the PC because they become familiar with the PC ingestion process.
One possible complication of capsule endoscopy is capsule retention. In our study, 1 patient, for whom excretion of the PC took 71 h, did not experience CE retention despite multiple intestinal stenoses; therefore, delayed passage (>33 h) of an intact PC requires careful interpretation.
In conclusion, the PC is a useful noninvasive tool for evaluating intestinal patency and facilitating the performance of the CE examination, but attention needs to be paid to the possibility of PC ingestion difficulty, particularly in children shorter than 152 cm who lack experience with capsule ingestion. On the basis of our results, we recommend that physicians estimate PC ingestion ability by considering the child's height prior to performing a PC ingestion trial. In that way, the physician can explain the risk of PC ingestion difficulty to the patient or legal guardian before obtaining informed consent to perform the CE examination. In the case of a patient at risk for PC ingestion difficulty, the physician can explain the alternative method, endoscopic placement of the PC, and may prepare for it prior to the PC examination.
Statement of Ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of our institutional research committee and with the principles of the Declaration of Helsinki and were approved by our institutional Ethics Committee (approved number: 3416).
Written informed consent was obtained from each patient and/or each child's legal guardian.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Fritscher-Ravens A, Scherbakov P, Bufler P, Torroni F, Ruuska T, Nuutinen H, Thomson M, Tabbers M, Milla P. The feasibility of wireless capsule endoscopy in detecting small intestinal pathology in children under the age of 8 years: a multicentre European study. Gut. 2009;58:1467–1472. doi: 10.1136/gut.2009.177774. [DOI] [PubMed] [Google Scholar]
- 2.Nuutinen H, Kolho KL, Salminen P, Rintala R, Koskenpato J, Koivusalo A, Sipponen T, Färkkilä M. Capsule endoscopy in pediatric patients: technique and results in our first 100 consecutive children. Scand J Gastroenterol. 2011;46:1138–1143. doi: 10.3109/00365521.2011.584900. [DOI] [PubMed] [Google Scholar]
- 3.Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643–653. doi: 10.1053/j.gastro.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 4.Tokuhara D, Watanabe K, Okano Y, Tada A, Yamato K, Mochizuki T, Takaya J, Yamano T, Arakawa T. Wireless capsule endoscopy in pediatric patients: the first series from Japan. J Gastroenterol. 2010;45:683–691. doi: 10.1007/s00535-010-0209-5. [DOI] [PubMed] [Google Scholar]
- 5.Barkin JS, Friedman S. Wireless capsule endoscopy requiring surgical intervention. The world's experience. Am J Gastroenterol. 2002;97:S298. [Google Scholar]
- 6.Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol. 2006;101:2218–2222. doi: 10.1111/j.1572-0241.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 7.Sears DM, Avots-Avotins A, Culp K, Gavin MW. Frequency and clinical outcome of capsule retention during capsule endoscopy for GI bleeding of obscure origin. Gastrointest Endosc. 2004;60:822–827. doi: 10.1016/s0016-5107(04)02019-x. [DOI] [PubMed] [Google Scholar]
- 8.Cave D, Legnani P, de Franchis R, Lewis BS, ICCE ICCE consensus for capsule retention. Endoscopy. 2005;37:1065–1067. doi: 10.1055/s-2005-870264. [DOI] [PubMed] [Google Scholar]
- 9.Atay O, Mahajan L, Kay M, Mohr F, Kaplan B, Wyllie R. Risk of capsule endoscope retention in pediatric patients: a large single-center experience and review of the literature. J Pediatr Gastroenterol Nutr. 2009;49:196–201. doi: 10.1097/MPG.0b013e3181926b01. [DOI] [PubMed] [Google Scholar]
- 10.Postgate AJ, Burling D, Gupta A, Fitzpatrick A, Fraser C. Reliability and limitations of the given patency capsule in patients at risk of capsule retention: a 3-year technical review. Dig Dis Sci. 2008;53:2732–2738. doi: 10.1007/s10620-008-0210-5. [DOI] [PubMed] [Google Scholar]
- 11.Delvaux M, Ben Soussan E, Laurent V, Lerebours E, Gay G. Clinical evaluation of the use of the M2A patency capsule system before a capsule endoscopy procedure, in patients with known or suspected intestinal stenosis. Endoscopy. 2005;37:801–807. doi: 10.1055/s-2005-870241. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SA, Gralnek IM, Ephrath H, Stallworth A, Wakhisi T. The use of a patency capsule in pediatric Crohn's disease: a prospective evaluation. Dig Dis Sci. 2011;56:860–865. doi: 10.1007/s10620-010-1330-2. [DOI] [PubMed] [Google Scholar]
- 13.Lucendo AJ, González-Castillo S, Fernández-Fuente M, De Rezende LC. Tracheal aspiration of a capsule endoscope: a new case report and literature compilation of an increasingly reported complication. Dig Dis Sci. 2011;56:2758–2762. doi: 10.1007/s10620-011-1666-2. [DOI] [PubMed] [Google Scholar]
- 14.Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, de Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. 2005;62:712–716. doi: 10.1016/j.gie.2005.05.002. quiz 752, 754. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M, Hirooka Y, Yamamura T, Miyahara R, Watanabe O, Ando T, Ohmiya N, Goto H. Clinical usefulness of novel tag-less Agile patency capsule prior to capsule endoscopy for patients with suspected small bowel stenosis. Dig Endosc. 2015;27:61–66. doi: 10.1111/den.12306. [DOI] [PubMed] [Google Scholar]
- 16.Gay G, Delvaux M, Laurent V, Reibel N, Regent D, Grosdidier G, Roche JF. Temporary intestinal occlusion induced by a “patency capsule” in a patient with Crohn's disease. Endoscopy. 2005;37:174–177. doi: 10.1055/s-2004-826195. [DOI] [PubMed] [Google Scholar]
- 17.Fitch WT, Giedd J. Morphology and development of the human vocal tract: a study using magnetic resonance imaging. J Acoust Soc Am. 1999;106((3 pt 1)):1511–1522. doi: 10.1121/1.427148. [DOI] [PubMed] [Google Scholar]
- 18.Sockolow RE, Solomon AB. The jelly bean test: a novel technique to help children swallow medications. in Pediatric Drug Development: Concepts and Applications. 2013:583–587. [Google Scholar]
- 19.Higuchi O, Adachi Y, Ichimaru T, Asai M, Kawasaki K. Foreign body aspiration in children: a nationwide survey in Japan. Int J Pediatr Otorhinolaryngol. 2009;73:659–661. doi: 10.1016/j.ijporl.2008.12.026. [DOI] [PubMed] [Google Scholar]