Abstract
Background
Childhood obesity is associated with advanced bone age (BA). Previous studies suggest that androgens, oestrogens, sex hormone-binding globulin, and insulin are responsible for this phenomenon, but results are contradictory and might be biased by confounders. We aim to elucidate this matter by applying a multivariate approach.
Method
We performed a correlation analysis of BA standard deviation score (SDS) with age- and sex-specific SDS for androgens, oestrogens, and with indicators of insulin secretion derived from oral glucose tolerance testing, in a group of obese children. A multivariate analysis was performed to investigate which parameters were independently predictive of BA SDS.
Results
In this cohort (n = 101; mean age 10.9 years; mean BA 11.8 years; mean BMI SDS 3.3), BMI SDS was significantly correlated to BA SDS (r = 0.55, p < 0.001). In a regression analysis in the total cohort (B = 0.27, p < 0.001) as well as in females (B = 0.34, p = 0.042), males (B = 0.31, p = 0.006), and pubertal children (B = 0.32, p = 0.046), dehydroepiandrosterone sulphate (DHEAS) showed a positive, independent association with BA SDS. No association with indicators of insulin secretion was found.
Conclusion
BMI SDS is highly correlated to BA SDS in obese children. Increased DHEAS has a central role in advanced BA in obese children.
Keywords: Obesity, Bone age, Insulin, Androgens, Dehydroepiandrosterone
Introduction
The worldwide increase in overweight and obese children has led to significant morbidity, including type 2 diabetes, cardiovascular diseases, fatty liver disease, impaired development, and psychological problems [1]. Furthermore, children with excess weight have been reported to have accelerated sexual maturation and linear growth, often accompanied by an advanced bone age (BA) and a decreased pubertal growth spurt compared to normal-weight children [2, 3, 4]. The mechanism driving this BA advancement, however, has remained unclear.
Various alterations in hormone levels have been proposed to be responsible for this phenomenon, such as androgens [5, 6, 7, 8], oestrogens [5, 6, 7, 9], and sex hormone-binding globulin (SHBG) [7]. Furthermore, 2 recent studies indicated that increasing insulin resistance and insulin secretion are associated with BA advancement [10, 11]. These studies, however, vary widely in study design and outcome parameters investigated, and led to contradictory results. For example, some studies evaluated the difference between BA and chronological age (CA) [8, 10], whereas others assessed the ratio between these parameters [11], while an age-adjusted indicator would theoretically be superior. Furthermore, some studies included prepubertal children only [6, 10], while others also included pubertal children [5, 7]. Additionally, most studies reported on androgen and oestrogen levels as absolute levels, although these vary significantly with age and pubertal staging, making age an important potential confounder in association studies. Finally, various factors are expected to be mutually dependent.
Therefore, we investigated the multivariate relationship between BA standard deviation score (SDS) for age and sex versus age- and sex-adjusted serum concentrations of serum androgens, oestradiol (E2), SHBG (expressed as SDS), and indicators of insulin secretion in a cohort of prepubertal and pubertal obese children.
Methods
Study Cohort
Obese children visiting our obesity clinic between January 2012 and July 2015, in whom BA assessment and an oral glucose tolerance test (OGTT) were performed, were included in this retrospective cohort study. The OGTT, BA assessment, and endocrine measurements were, at that time, part of an extensive diagnostic package which we performed as standard care for all obese children. The aims of this diagnostic approach were (a) early detection of glucose metabolism abnormalities and other complications of obesity such as polycystic ovary syndrome and (b) detection of endocrine or genetic causes of obesity. Exclusion criteria for this study were endocrine disorders (e.g., hypothyroidism), syndromes known to affect insulin sensitivity or increased skeletal maturation BA (e.g., Bardet-Biedl syndrome or overgrowth syndromes), medication affecting insulin sensitivity or skeletal maturation (e.g., metformin or methylphenidate), missing fasting insulin or unreliable OGTT data (e.g., due to vomiting or problems with i.v. catheter), and missing BA SDS (e.g., outside age reference range of BoneXpert). In this cohort, patients with marked hyperphagia and early-onset obesity (onset of obesity <5 years of age) were tested for genetic causes of obesity by means of a genetic panel developed at the University Medical Center in Utrecht. It tests for 53 genes known to cause monogenic obesity. Patients with genetic defects indicating monogenic obesity were included in this study, since there is no reason to assume that their BA is affected in any other way than in other obese children. The results for these patients are shown with specific symbols in the Figures. The study was approved by the Medical Ethics Committee of the Leiden University Medical Center and conducted within the terms of the Declaration of Helsinki. Since all participants received standard of care only, subject consent was waived.
Anthropometric Data and Definitions
At the first visit, height and weight were measured using a stadiometer and calibrated scale, respectively. Obesity and BMI SDS were determined using the International Obesity Taskforce criteria [12]. Height SDS was determined based on the Dutch nationwide growth study performed in 2009 [13]. Modified Tanner staging [14] was performed to determine pubertal stage (Tanner stage >G1 in males or >B1 in females were scored as pubertal).
BA Evaluation
We used BoneXpert to determine BA and BA SDS on a radiograph of the left hand [15]. BoneXpert is a fully automated system based on an extensive database, which determines the Greulich and Pyle BA by analyzing 15 bones of the left hand and wrist [15, 16]. BoneXpert is validated to determine BA and associated SDS in males aged 2.5–17 years and in females aged 2–15 years for different ethnicities [15, 16]. We used Caucasian as standard reference, since our cohort was largely Caucasian and the non-Caucasian participants were of North African and Middle Eastern descent, for which BoneXpert does not provide ethnicity-specific SDS. The radiographs were made on the date of the first visit or during the visit for OGTT.
OGTT Procedure
OGTT was performed after an overnight fast with a minimum of 10 h. A standardized dose of oral glucose of 1.75 g/kg, with a maximum of 75 g, was administered at the beginning of the test. An intravenous catheter was used to collect the blood samples at t = 0, 30, 60, and 120 min. These samples were analyzed for insulin and glucose concentrations. An extra sample to measure concentrations of E2, testosterone (T), androstenedione (Adione), dehydroepiandrosterone sulphate (DHEAS), and SHBG was obtained at t = 0.
Laboratory Measurements
Blood samples were analyzed in the clinical laboratory of the Leiden University Medical Center (LUMC, The Netherlands). Immulite 2000 XPi (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) immunoassays were used to determine the serum concentrations of insulin (mU/L), SHBG (nmol/L), and DHEAS (μmol/L). T (nmol/L) was analyzed by immunoassay (ECLIA) on a Roche Modular E170 immunoanalyzer, and Adione (nmol/L) was analyzed using a radioimmunoassay of Beckman Coulter (formerly DSL, Woerden, The Netherlands). Glucose was analyzed in serum using a hexokinase method on a Roche Modular P800 chemistry analyzer. Two different but compatible methods, the automated ECLIA assay of Roche and the Orion ultrasensitive radioimmunoassay, were used to measure the E2 levels (pmol/L). Concentrations of the Orion radioimmunoassay method were converted to ECLIA by a conversion factor. Due to the retrospective nature of this study, using data obtained in standard care, no mass spectrometry measurements were available for E2 and T. The Roche testosterone (generation 2) and oestradiol (generation 2) assays are state-of-the-art immunoassays with limits of detection of 0.09 nmol/L and 18.4 pmol/L, respectively. Both assays have been standardized against international reference methods (ID-GCMS). The Orion ultrasensitive radioimmunoassay for E2 had a similar limit of detection with excellent correlation in comparison with the Roche assay.
Concentrations of measured outcomes under the detection limit were defined as the mean between the lower detection limit of the test and zero. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: T0 glucose (mmol/L) × T0 insulin (mU/L)/22.5 [17]. We calculated the area under the curve for insulin levels during the OGTT using the trapezoid method.
Conversion of Serum Steroid Levels to SDS
In order to estimate the possible influence of serum SHBG, E2, and steroid levels on BA advancement, we converted patients’ serum levels to SDS, based on published reference values using the same assays. Since the age distribution is skewed, for each age interval separate SD values were calculated above and below the mean. Values for +1 SD and −1 SD were estimated by dividing the difference between P97.5 and P50 and the difference between P50 and P2.5 by 1.96, respectively, as previously described [18]. For DHEAS and SHBG we used the age- and sex-specific centiles provided by Elmlinger et al. [19].
Reference data for T and E2 were derived from the Caliper database [20]. For these parameters, we calculated SDS only for children ≥9.0 years of age, since the reference values for children <9 years of age were largely below the detection limit. For children aged ≥9.0 years with plasma concentrations below the detection limit we imputed the data by dividing the lower detection limit by 2. We used the data from the Caliper database to directly calculate 1 SDS and −1 SDS in different age groups.
For DHEAS, SHBG, T, and E2 smoothed-fit lines of the −1 SD, P50, and 1 SD data points were created, providing an equation to calculate age- and sex-adjusted SDS for these hormones: (serum concentration − age-specific P50)/(age-specific −1 or +1 SD); SDS(X) = ([X]-P50)/SD. There were no reference data applicable for our assay for serum insulin and Adione, so these concentrations in our patients could not be expressed as SDS. The results of smooth-fitting and plots of SDS in our cohort are summarized in the supplementary Figures (SHBG SDS, online suppl. Fig. 1; DHEAS SDS, online suppl. Fig. 2; T SDS, online suppl. Fig. 3; E2 SDS, online suppl. Fig. 4; see www.karger.com/doi/10.1159/000467393).
Statistical Analysis
All analyses were performed using IBM 23.0 SPSS Statistics. We performed analyses for the total cohort as well as for subgroups based on sex and puberty. Normality was tested using the Kolmogorov-Smirnov and the Shapiro-Wilk tests (p > 0.05 was considered normally distributed). We report on mean and SD and median with interquartile range for Gaussian and non-Gaussian distributed data, respectively. For the various SDS we investigated whether they significantly differed from zero using one-sample t tests or a one-sample Kolmogorov-Smirnov test. Correlation analyses were performed using Pearson and Spearman correlations depending on normality.
First, we performed correlation analyses exploring the possible effect of age and BMI SDS on the various parameters possibly influencing BA. We then investigated the correlation of these parameters with BA SDS. In both analyses, we report on significant correlations (p < 0.05).
As a last step, we investigated which parameters were independently associated with BA SDS using backward regression analyses, using the pairwise exclusion option in SPSS. In a model with BA SDS as the dependent variable we entered age, sex, DHEAS SDS, SHBG SDS, fasting insulin, HOMA-IR, and area under the curve for insulin measurements during OGTT and investigated independent relationships to BA SDS in the total cohort and subgroups split on sex and puberty. E2 SDS and T SDS were only entered in the model for the pubertal subgroup, since they were unavailable for most prepubertal subjects. We tested the assumptions of each model by checking the independence of the residuals (Durbin-Watson test), inspecting their homogeneity (inspection of the scatterplot), and testing their normality (Kolmogorov-Smirnov test >0.05)
Results
Study Cohort Characteristics
Out of the 184 children who visited the Willem-Alexander Children's Hospital, 101 children met the inclusion criteria for this study. Figure 1 summarizes the reasons for exclusion of the remaining subjects.
Fig. 1.
Flowchart with reasons of exclusion.
Baseline characteristics are presented in Table 1. The cohort had a mean age of 10.9 years and a mean BA of 11.8 years, resulting in a mean BA SDS of 1.2; 57% of the children were pubertal, and 47% were female. The mean height SDS was 0.6 and the mean BMI SDS 3.3. Mean BA SDS and DHEAS SDS were increased (both p < 0.001), while T SDS and SHBG SDS were decreased compared to age references (p = 0.032 and 0.003, respectively).
Table 1.
Baseline characteristics
| Total cohort |
Subgroups by sex |
Subgroups by puberty |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (n = 101) | n | female (n = 47) | n | male (n = 54) | n | prepubertal (n = 43) | n | pubertal (n = 57) | |
| Age, years | 101 | 10.9 (3.1) | 47 | 10.3 (2.9) | 54 | 11.4 (3.1) | 43 | 8.4 (1.9) | 57 | 12.8 (2.3) |
| BA, years | 101 | 11.8 (2.1) | 47 | 10.9 (2.9) | 54 | 12.6 (2.9) | 43 | 9.6 (2.0) | 57 | 13.5 (2.5) |
| BA SDS | 101 | 1.2 (1.1)* | 47 | 0.8 (1.2)* | 54 | 1.4 (1.0)* | 43 | 1.6 (1.1)* | 57 | 0.8 (1.1)* |
| Caucasiana | 101 | 64 (63.4) | 47 | 27 (57.4) | 54 | 37 (68.5) | 43 | 19 (44.2) | 57 | 44 (77.2) |
| Height SDS | 101 | 0.6 (1.0)* | 47 | 0.4 (1.0)* | 54 | 0.7 (1.1)* | 43 | 0.8 (1.0)* | 57 | 0.4 (1.0)* |
| BMI SDS | 101 | 3.4 (0.6)* | 47 | 3.1 (0.5)* | 54 | 3.6 (0.7)* | 43 | 3.6 (0.7)* | 57 | 3.2 (0.5)* |
| Fasting insulin, mU/Lb | 101 | 12 (6/18) | 47 | 11 (6/19) | 54 | 12 (7/16) | 43 | 7 (4/11) | 57 | 16 (10/26) |
| HOMA-IRb | 101 | 2.1 (1.3/3.7) | 47 | 2.1 (1.3/3.9) | 54 | 2.3 (1.3/3.5) | 43 | 1.3 (0.8/2.0) | 57 | 2.4 (2.1/4.8) |
| AUC insulin, mU/Lb | 94 | 305 (202/468) | 43 | 267 (186/454) | 51 | 309 (210/482) | 40 | 211 (159/397) | 53 | 362 (238/544) |
| Oestradiol SDS | 50 | 0.0 (1.4) | 21 | −0.2 (1.1) | 29 | 0.1 (1.5) | − | 39 | 0.0 (1.3) | |
| Testosterone SDSb | 64 | −0.6 (–1.2/0.3)* | 28 | −0.7 (–2.7/1.6) | 36 | −0.6 (–1.1/0.0)* | − | 51 | −0.7 (–1.3/0.4)* | |
| DHEAS SDSb | 96 | 0.4 (0.0/1.0)* | 45 | 0.3 (–0.8/0.7) | 51 | 0.4 (0.1/1.0)* | 41 | 0.4 (–0.3/1.1) | 54 | 0.3 (0.0/0.9)* |
| SHBG SDSb | 96 | −1.9 (–2.4/–1.1)* | 46 | −1.9 (–1.2/–2.2)* | 50 | −1.9 (–2.4/–1.1)* | 40 | −1.5 (–2.2/–0.7)* | 55 | −2.0 (–2.4/–1.4)* |
| Androstenedione, nmol/Lb | 100 | 2.25 (1.30/3.60) | 47 | 2.40 (1.20/4.10) | 53 | 2.20 (1.30/3.30) | 42 | 1.25 (0.78/2.23) | 57 | 3.00 (2.15/4.50) |
Data are expressed as mean (standard deviation) unless otherwise stated. Data on oestradiol SDS and testosterone SDS are on the age group of ≥9 years. SDS, standard deviation score; BA, bone age; BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; AUC, area under the curve; DHEAS, dehydroepiandrosterone sulphate; SHBG, sex hormone-binding globulin.
n (%).
Median (interquartile range).
SDS, p < 0.05.
In 5 subjects of the final cohort, a genetic mutation was found. Two male subjects showed a heterozygous MC4R mutation (Cys293Tyr en Ile251Leu) and 1 male subject showed a heterozygous mutation in WDPCP (Leu379Ser). Furthermore, 1 female subject showed a heterozygous mutation in BBS7 (Gln365Leu), while another female subject was found to have 2 heterozygous variants in CEP290 (Ile1059fs) and MKKS (Ala242Ser).
Correlation between Outcome Parameters and Age or BMI SDS
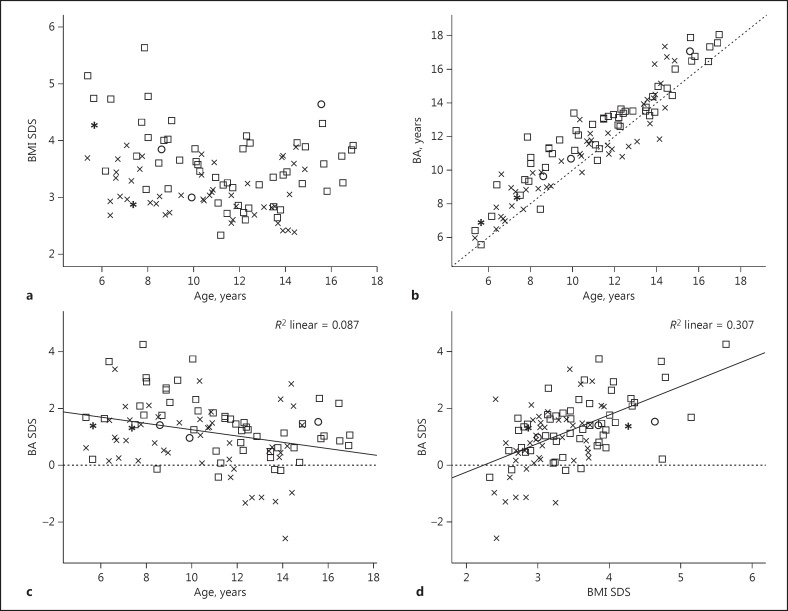
The correlation analysis of the outcome parameters with age and BMI SDS are presented in Table 2 and scatterplots are shown in Figure 2. The data are presented as Pearson correlation or Spearman ρ, where applicable. BMI SDS was negatively correlated with age in prepubertal children and positively in pubertal children, showing a U-shape over the whole age range (Fig. 2a).
Table 2.
Correlations between outcome parameters and age or BMI SDS
| BMI SDSa | Fasting insulin | HOMA-IR | AUC insulin | Oestradiol SDSa | Testosterone SDS | DHEAS SDS | SHBG SDS | |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| Total cohort | −0.22* | 0.65*** | 0.65*** | 0.48*** | 0.19 | −0.17 | 0.05 | −0.12 |
| Female | −0.26 | 0.68*** | 0.69*** | 0.58*** | 0.29 | −0.09 | 0.00 | 0.02 |
| Male | −0.35** | 0.63*** | 0.62*** | 0.40** | 0.15 | −0.35* | 0.03 | −0.20 |
| Prepubertal | −0.36* | 0.59*** | 0.61*** | 0.53*** | – | – | 0.35* | −0.38* |
| Pubertal | 0.28* | 0.33* | 0.30* | 0.24 | 0.28 | −0.10 | −0.03 | 0.19 |
| BMI SDS | ||||||||
| Total cohort | – | −0.08 | −0.10 | 0.11 | 0.19 | 0.14 | 0.20 | −0.17 |
| Female | – | −0.11 | 0.11 | 0.06 | 0.28 | 0.36 | 0.22 | −0.15 |
| Male | – | −0.13 | −0.15 | 0.08 | 0.13 | −0.16 | 0.07 | −0.14 |
| Prepubertal | – | −0.19 | −0.25 | −0.05 | – | – | 0.13 | −0.08 |
| Pubertal | – | 0.28* | 0.28* | 0.42** | 0.23 | 0.07 | 0.27 | −0.39** |
Correlations are shown as Spearman ρ unless otherwise stated. Correlations for oestradiol SDS and testosterone SDS are calculated on the age group of ≥9 years. BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; AUC insulin, area under the curve of insulin during oral glucose tolerance test; SDS, standard deviation score; DHEAS, dehydroepiandrosterone sulphate; SHBG, sex hormone-binding globulin.
Pearson correlation.
p < 0.05
p < 0.01
p < 0.001.
Fig. 2.
a Association between BMI SDS and age (years). b Association between BA (BA) (years) and chronological age (years). c Association between BA SDS and age. d Association between BA SDS and BMI SDS. SDS, standard deviation score; BMI, body mass index; BA, bone age; R2, coefficient of deviation. Squares represent males, x's represent females, circles represent males with monogenetic obesity, and asterisks represent females with monogenetic obesity.
The insulin parameters in the total cohort as well as in subgroups according to sex and puberty showed a positive correlation with age. T SDS showed a significant negative correlation with age (ρ = −0.35) in males. In the prepubertal subgroup, SHBG SDS negatively correlated with age (ρ = −0.38), in contrast to a positive correlation with age (ρ = 0.35) for DHEAS SDS.
Fasting insulin (ρ = 0.28), HOMA-IR (ρ = 0.28), and area under the curve for insulin measurements during OGTT (ρ = 0.42) showed a significant positive correlation with BMI SDS in the pubertal subgroup. BMI SDS showed a trend toward a positive correlation with DHEAS SDS in the whole cohort and the pubertal subgroup. In contrast, there was a trend toward a negative correlation with SHBG SDS in the whole cohort, which reached statistical significance in the pubertal subgroup.
Correlation of BA SDS with Clinical and Biochemical Parameters
Figure 2b shows that in the great majority of patients BA is advanced. As shown in Table 3 and Figure 2c, BA SDS is relatively more advanced in young children: there was a significant negative correlation between BA SDS and age in the total cohort (r = −0.29) as well as in subgroups split on sex (female, r = −0.31; male, r = −0.41). BMI SDS showed a strongly significant correlation with BA SDS in the total cohort (ρ = 0.55) (Fig. 2d) as well as in the female (ρ = 0.49), male (ρ = 0.55), prepubertal (ρ = 0.52), and pubertal (ρ = 0.51) subgroups.
Table 3.
Correlation between BA SDS and clinical and biochemical variables
| Total cohort | Sex |
Puberty |
|||
|---|---|---|---|---|---|
| female | male | prepubertal | pubertal | ||
| Age (years)a | −0.29** | −0.31* | −0.41** | −0.15 | −0.07 |
| BMI SDSa | 0.55*** | 0.49*** | 0.55*** | 0.52*** | 0.51*** |
| Fasting insulin | −0.14 | −0.22 | −0.15 | 0.02 | 0.09 |
| HOMA-IR | −0.14 | −0.21 | −0.14 | −0.03 | 0.12 |
| AUC insulin | 0.07 | −0.06 | 0.13 | 0.13 | 0.22 |
| Oestradiol SDSa | 0.14 | 0.13 | 0.13 | − | 0.10 |
| Testosterone SDS | 0.24 | 0.44* | −0.06 | − | 0.18 |
| DHEAS SDS | 0.29** | 0.18 | 0.33* | 0.32* | 0.28* |
| SHBG SDS | −0.17 | −0.10 | −0.22 | −0.24 | −0.31* |
Correlations are expressed as Spearman ρ (p value) unless otherwise stated. Correlations for oestradiol SDS and testosterone SDS are calculated on the age group of ≥9 years. SDS, standard deviation score; BA, bone age; BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; AUC, area under the curve; DHEAS, dehydroepiandrosterone sulphate; SHBG, sex hormone-binding globulin.
Pearson correlation (p value).
p < 0.05
p < 0.01
p < 0.001.
Correlations between BA SDS and biochemical variables are presented in Table 3. In females, T SDS showed a positive correlation with BA SDS (ρ = 0.44). In the total cohort, as well as in the male and both puberty subgroups, DHEAS SDS showed a positive correlation with BA SDS. SHBG SDS was negatively associated with BA SDS, particularly in pubertal children (ρ = −0.31). The insulin parameters and E2 SDS did not show significant correlations with BA SDS in the total cohort or in any subgroup.
Regression Analysis for BA SDS
The results of backward regression analysis are summarized in Table 4. In the total cohort, backward regression analysis resulted in a model, including sex, DHEAS SDS, and age, explaining 27% of the total variance in BA SDS (overall fit of the regression model F = 10.55, p < 0.001). In the female subgroup we found a model explaining 21% of the variance in BA SDS including DHEAS SDS, SHBG SDS, and age (F = 3.33, p = 0.030). In contrast, in males the model did not include SHBG SDS, but only contained DHEAS SDS and age (F = 9.50, p < 0.001), explaining 30% of the variance. For the subgroups split on puberty, regression analysis showed a model explaining 31% of the variance in BA SDS, including sex, SHBG SDS, and age in prepubertal subjects (F = 4.88, p = 0.006), and a model explaining 11% of the variance only including DHEAS SDS (F = 4.27, p = 0.046) in pubertal subjects.
Table 4.
Backward regression analysis of BA SDS
| Coefficient | 95% CI | R2 | p value | |
|---|---|---|---|---|
| Total cohort (n = 88) | ||||
| Constant | 2.17 | 1.39/2.96 | <0.001 | |
| Male sex | 0.62 | 0.18/1.06 | 0.006 | |
| DHEAS SDS | 0.27 | 0.09/0.44 | <0.001 | |
| Age | −0.13 | −0.20/–0.06 | 0.036 | |
| Model | 0.27 | <0.001 | ||
| Female (n = 52) | ||||
| Constant | 2.68 | 1.25/4.11 | 0.001 | |
| DHEAS SDS | 0.34 | 0.01/0.66 | 0.042 | |
| SHBG SDS | 0.29 | −0.04/0.62 | 0.086 | |
| Age | −0.14 | −0.26/–0.02 | 0.024 | |
| Model | 0.21 | 0.030 | ||
| Male (n = 46) | ||||
| Constant | 2.81 | 1.80/3.81 | <0.001 | |
| DHEAS SDS | 0.31 | 0.09/0.52 | 0.006 | |
| Age | −0.14 | −0.22/–0.05 | 0.002 | |
| Model | 0.30 | <0.001 | ||
| Prepubertal (n = 36) | ||||
| Constant | 2.26 | 1.17/4.02 | 0.001 | |
| Male sex | 0.98 | 0.30/1.67 | 0.006 | |
| SHBG SDS | −0.41 | −0.76/–0.09 | 0.013 | |
| Age | −0.27 | −0.46/–0.07 | 0.009 | |
| Model | 0.31 | 0.006 | ||
| Pubertal (n = 37) | ||||
| Constant | 0.70 | 0.32/1.08 | 0.001 | |
| DHEAS SDS | 0.43 | 0.01/0.85 | 0.046 | |
| Model | 0.11 | 0.046 | ||
Variables included in all models: age, fasting insulin, HOMA-IR, AUC insulin, DHEAS SDS, SHBG SDS. In the total cohort and pubertal subgroups, sex was added as an independent variable. In the pubertal subgroup only, oestradiol SDS and testosterone SDS were added as independent variables. BA, bone age; HOMA-IR, homeostatic model assessment of insulin resistance; SHBG, sex hormone-binding globulin; DHEAS, dehydroepiandrosterone sulphate; SDS, standard deviation score.
Discussion
The results of this study show that the mechanisms driving BA advancement in obese children are complex. In multiple regression analyses we have shown that DHEAS levels positively associate with BA SDS and SHBG levels negatively. However, results are variable across subgroups according to sex and pubertal status. Furthermore, we were able to explain only a limited percentage of the variance in BA SDS (with a maximum of 31% in prepubertal children), indicating that some factors driving BA advancement were not included in this analysis.
As expected, in this cohort of obese children, mean height SDS was above average for the population, and BA was advanced compared to CA. Furthermore, BA SDS and BMI SDS were strongly correlated. This is in line with studies reporting advanced linear growth and skeletal maturation in children with excess weight [2, 3, 4, 21]. In addition, we have confirmed previous studies [3, 7, 22] showing that obese children have high DHEAS levels compared to a reference population. Our observation that DHEAS SDS is associated with BA SDS, especially in pubertal children, independent of various confounders, is in accordance with the results of a study by Sopher et al. [6], which showed that, in a group of obese children, the highest tertile of the ratio between BA and CA was associated with high DHEAS levels. These authors posed that high DHEAS levels indicate high levels of androgens, leading to increased levels of E2 by peripheral conversion, which in turn leads to advanced bone maturation. The absence of an association between E2 and BA SDS in our cohort might be caused by the fact that our E2 assay lacks sensitivity in the lower ranges. Consistent with this explanation is a study by Klein et al. [9] showing that E2 levels correlated with BA in obese and lean children when using a more sensitive assay. Alternatively, it has been suggested that the production of E2 takes place at the tissue level [6], so that no rise in circulating E2 levels can be detected, thereby explaining the lack of association between E2 and BA SDS in our cohort. Furthermore, our findings are in line with the work of DeSalvo et al. [23] who showed that, in a cohort of children with premature adrenarche, the subgroup of children with BA advancement >2 years had higher BMI and higher DHEAS levels than the subgroup of children with BA advancement <1 year. This might suggest an overlap between the pathophysiological mechanisms leading to BA advancement in patients with premature adrenarche and patients with obesity [23].
Although the pathophysiological mechanism remains uncertain, the results of our study show an independent association between DHEAS SDS and BA SDS in the total cohort as well as in males, females, and pubertal children, indicating a central role for DHEAS in the BA advancement found in obese children. The scientific implications of the results of our study are that insulin is an unlikely cause of bone advancement in obese children, while DHEAS secretion can now be viewed as at least one of the intermediary factors. A possible clinical implication of our findings could be that it would be useful to measure DHEAS in obese children with substantial BA advancement and/or increased statural growth. If available, it would also be useful to measure serum E2 with an ultrasensitive assay. In case of high concentrations, these could be accepted as causes of the clinical phenotype, so that the clinician can consider abstaining from further diagnostic workup of the patient.
Our finding of decreased plasma SHBG levels in obese children compared to reference intervals, based on lean children, is in accordance with previous reports [3, 7, 23] and has been reported to be caused by hyperinsulinaemia, related to insulin resistance and low-grade inflammation [24]. Using sensitive E2 assays, it was also shown that obese adolescents have increased E2 levels, combined with decreased SHBG levels, possibly resulting in high levels of free E2 [23], which in turn might lead to increased bone maturation [24].
In addition to the generally decreased SHBG in obese children, we found a negative association in the regression analysis of SHBG SDS with BA SDS in prepubertal children. Decreased SHBG is associated with the increase of adrenal androgens during puberty [25], which in turn can stimulate bone maturation by locally increasing oestrogen levels via expression of aromatase [26]. In contrast, a trend toward a positive association between SHBG SDS and BA SDS was found in regression analysis in the female subgroup, possibly reflecting increased gonadal oestrogen production during puberty, stimulating SHBG in girls. This association, however, did not reach significance (p = 0.086), possibly because it is obscured by a lack of assay sensitivity or the combination of the results of 2 oestrogen immunoassays.
It is of interest that we did not find an association between any of the insulin parameters with BA advancement, neither in correlation analyses, nor in regression analyses. In the literature, contradictory results on the association between hyperinsulinaemia and advanced BA have been reported. No association between insulin resistance and the ratio between BA and CA was found in prepubertal children in a study by Sopher et al. [6], whereas Klein et al. [5] found an association between insulin levels and the top tertile of this ratio in a cohort aged 3–18 years. Furthermore, Pinhas-Hamiel et al. [11] showed that overweight children aged 4–13 years with a fasting insulin >30 mU/L had a 6.8-fold increased risk of falling into the top tertile of the ratio between BA and CA, independent of the degree of obesity. Lee et al. [10] investigated the relation between insulin resistance and BA in prepubertal obese children and found an independent, positive correlation between HOMA-IR and the difference between BA and CA using multiple regression analysis. None of these 3 studies, however, corrected for the possible confounding effects of androgens and oestrogens, which might bias these results, and the outcome parameter of BA advancement was not adjusted for age and gender. Furthermore, there was considerable variability in ethnicity between studies, which might in part explain the differences in outcome. In addition, the positive association between insulin secretion and age could lead to bias, too. Another possible explanation for the lack of association between BA SDS and insulin parameters in this cohort might be that a large number of the subjects in this cohort is already insulin resistant. Possibly, the effects of insulin on BA are more pronounced in children in the early stages of developing insulin resistance.
The finding of independent effects of sex in the multiple regression analysis is remarkable. It suggests that male and female subjects are differentially affected by increased BMI in their advanced bone maturation. This is in agreement with the findings of Crocker et al. [27] who have recently shown that pubertal development is differentially affected in obese male and female subjects. They showed that, in female subjects, progressive Tanner staging correlated with advanced BA, while in boys BA advancement was independent of testicular development. Furthermore, insulin resistance correlated positively with breast development in girls, while it was negatively correlated with testicular size in boys [27]. This underlines the sexual dimorphism in the way obesity affects maturation.
As shown in our regression models, the maximum percentage of variance explained by a model was 31%, suggesting that factors not included in this study might contribute to BA advancement. It has been suggested that leptin [28] and IGF-1 [8] might contribute to BA advancement in obesity, although recent work by Sopher et al. [6] showed no association between these parameters and BA advancement. Future studies in larger cohorts should include these parameters to clarify the role these factors play in this matter.
A major strength of our study is the use of an automated method for BA assessment, which results in a reduced inter-subject and an absent inter-observer variance [29]. The use of BoneXpert also enabled the calculation of a reliable BA SDS from a representative population reference. Furthermore, where possible, we used age- and sex-specific SDS to investigate the relationship between hormone levels and BA SDS, thereby correcting for variance in these hormones caused by age and sex. Furthermore, we corrected for multiple confounders using regression analysis, which makes a causal relationship between the observed factors associated with advanced BA more plausible.
A limitation of our study is the fact that BoneXpert only supports the BA assessment of boys between 2.5 and 17 years and girls between 2.0 and 15 years of age [15, 16]. However, older adolescents have usually reached near-adult height by this age, and we pose that therefore they are a clinically less relevant study group. Secondly, BoneXpert contains reference data for SDS of Caucasian, Asian, Hispanic, or Afro-Americans [16] but not for children of Turkish or Moroccan background. Therefore, we used Caucasian references as the standard for all children. The majority of the cohort, however, is Caucasian. A third limitation is that the assay for E2 has a limited sensitivity, which might obscure its association with BA SDS in prepubertal children. Finally, due to the large number of potential confounders included in the regression models, our sample size was too small to investigate sex effects separately in the prepubertal and pubertal age group. In addition, the small sample size in some subgroups (e.g., prepubertal), may have led to false negative results in the multiple regression analysis. Future research should therefore include larger cohorts, allowing for adjusting for multiple confounders in the regression analysis. Furthermore, longitudinal designs could help to gain additional insights into the mechanisms driving accelerated bone maturation in obesity. In addition, future studies would benefit from age- and sex-specific SDS for Adione and insulin and should include leptin and IGF-1.
In conclusion, using multiple regression analysis, we have shown that increased DHEAS levels, reflecting adrenal androgen production, play a central role in BA advancement in obese children and adolescents and that decreased SHBG levels may further contribute to this phenomenon, though this finding needs further investigation.
Disclosure Statement
There are no conflicts of interest and no sources of funding relevant for this article.
Acknowledgments
We thank Dr. N. van Geloven for advice on the statistical analysis. The authors express their gratitude to Prof. Dr. H.A. Delemarre-van de Waal for her efforts invested in setting up the obesity outpatient clinic in the Willem-Alexander Children's Hospital and for facilitating the set-up of this study. Prof. Delemarre-van de Waal deceased on February 13, 2014. Furthermore, we thank Dr. A. Felius for his support in the clinical part of this study.
References
- 1.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4:187–192. doi: 10.4103/2249-4863.154628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leonibus C, Marcovecchio ML, Chiavaroli V, de Giorgis T, Chiarelli F, Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatr Obes. 2014;9:292–299. doi: 10.1111/j.2047-6310.2013.00176.x. [DOI] [PubMed] [Google Scholar]
- 3.Denzer C, Weibel A, Muche R, Karges B, Sorgo W, Wabitsch M. Pubertal development in obese children and adolescents. Int J Obes. 2007;31:1509–1519. doi: 10.1038/sj.ijo.0803691. [DOI] [PubMed] [Google Scholar]
- 4.He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001;49:244–251. doi: 10.1203/00006450-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Klein KO, Newfield RS, Hassink SG. Bone maturation along the spectrum from normal weight to obesity: a complex interplay of sex, growth factors and weight gain. J Pediatr Endocrinol Metab. 2015;29:311–318. doi: 10.1515/jpem-2015-0234. [DOI] [PubMed] [Google Scholar]
- 6.Sopher AB, Jean AM, Zwany SK, Winston DM, Pomeranz CB, Bell JJ, McMahon DJ, Hassoun A, Fennoy I, Oberfield SE. Bone age advancement in prepubertal children with obesity and premature adrenarche: possible potentiating factors. Obesity. 2011;19:1259–1264. doi: 10.1038/oby.2010.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandewalle S, Taes Y, Fiers T, Van Helvoirt M, Debode P, Herregods N, Ernst C, van Caenegem E, Roggen I, Verhelle F, De Schepper J, Kaufman JM. Sex steroids in relation to sexual and skeletal maturation in obese male adolescents. J Clin Endocrinol Metab. 2014;99:2977–2985. doi: 10.1210/jc.2014-1452. [DOI] [PubMed] [Google Scholar]
- 8.Reinehr T, de Sousa G, Wabitsch M. Relationships of IGF-I and androgens to skeletal maturation in obese children and adolescents. J Pediatr Endocrinol Metab. 2006;19:1133–1140. doi: 10.1515/jpem.2006.19.9.1133. [DOI] [PubMed] [Google Scholar]
- 9.Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab. 1998;83:3469–3475. doi: 10.1210/jcem.83.10.5204. [DOI] [PubMed] [Google Scholar]
- 10.Lee HS, Shim YS, Jeong HR, Kwon EB, Hwang JS. The Association between bone age advancement and insulin resistance in prepubertal obese children. Exp Clin Endocrinol Diabetes. 2015;123:604–607. doi: 10.1055/s-0035-1559795. [DOI] [PubMed] [Google Scholar]
- 11.Pinhas-Hamiel O, Benary D, Mazor-Aronovich K, Ben-Ami M, Levy-Shraga Y, Boyko V, Modan-Moses D, Lerner-Geva L. Advanced bone age and hyperinsulinemia in overweight and obese children. Endocr Pract. 2014;20:62–67. doi: 10.4158/EP13193.OR. [DOI] [PubMed] [Google Scholar]
- 12.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schonbeck Y, Talma H, van Dommelen P, Bakker B, Buitendijk SE, HiraSing RA, van Buuren S. Increase in prevalence of overweight in Dutch children and adolescents: a comparison of nationwide growth studies in 1980, 1997 and 2009. PLoS One. 2011;6:e27608. doi: 10.1371/journal.pone.0027608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 15.Thodberg HH, Kreiborg S, Juul A, Pedersen KD. The BoneXpert method for automated determination of skeletal maturity. IEEE Trans Med Imaging. 2009;28:52–66. doi: 10.1109/TMI.2008.926067. [DOI] [PubMed] [Google Scholar]
- 16.Thodberg HH, Savendahl L. Validation and reference values of automated bone age determination for four ethnicities. Acad Radiol. 2010;17:1425–1432. doi: 10.1016/j.acra.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Gerver WJM, de Bruin R. Paediatric Morphometrics. ed 2. Groningen: Wetenschappelijke uitgeverij Bunge; 2001. [Google Scholar]
- 19.Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med. 2002;40:1151–1160. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- 20.Konforte D, Shea JL, Kyriakopoulou L, Colantonio D, Cohen AH, Shaw J, Bailey D, Chan MK, Armbruster D, Adeli K. Complex biological pattern of fertility hormones in children and adolescents: a study of healthy children from the CALIPER cohort and establishment of pediatric reference intervals. Clin Chem. 2013;59:1215–1227. doi: 10.1373/clinchem.2013.204123. [DOI] [PubMed] [Google Scholar]
- 21.Quattrin T, Liu E, Shaw N, Shine B, Chiang E. Obese children who are referred to the pediatric endocrinologist: characteristics and outcome. Pediatrics. 2005;115:348–351. doi: 10.1542/peds.2004-1452. [DOI] [PubMed] [Google Scholar]
- 22.l'Allemand D, Schmidt S, Rousson V, Brabant G, Gasser T, Gruters A. Associations between body mass, leptin, IGF-I and circulating adrenal androgens in children with obesity and premature adrenarche. Eur J Endocrinol. 2002;146:537–543. doi: 10.1530/eje.0.1460537. [DOI] [PubMed] [Google Scholar]
- 23.DeSalvo DJ, Mehra R, Vaidyanathan P, Kaplowitz PB. In children with premature adrenarche, bone age advancement by 2 or more years is common and generally benign. J Pediatr Endocrinol Metab. 2013;26:215–221. doi: 10.1515/jpem-2012-0283. [DOI] [PubMed] [Google Scholar]
- 24.Vandewalle S, Taes Y, van Helvoirt M, Debode P, Herregods N, Ernst C, Roef G, van Caenegem E, Roggen I, Verhelle F, Kaufman JM, de Schepper J. Bone size and bone strength are increased in obese male adolescents. J Clin Endocrinol Metab. 2013;98:3019–3028. doi: 10.1210/jc.2012-3914. [DOI] [PubMed] [Google Scholar]
- 25.Garces C, Oya I, Lasuncion MA, Lopez-Simon L, Cano B, de Oya M. Sex hormone-binding globulin and lipid profile in pubertal children. Metabolism. 2010;59:166–171. doi: 10.1016/j.metabol.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Oz OK, Millsaps R, Welch R, Birch J, Zerwekh JE. Expression of aromatase in the human growth plate. J Mol Endocrinol. 2001;27:249–253. doi: 10.1677/jme.0.0270249. [DOI] [PubMed] [Google Scholar]
- 27.Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, Shawker TH, Hubbard VS, Yanovski JA. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. 2014;99:E1519–E1529. doi: 10.1210/jc.2014-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maor G, Silbermann M, von der Mark K, Heingard D, Laron Z. Insulin enhances the growth of cartilage in organ and tissue cultures of mouse neonatal mandibular condyle. Calcif Tissue Int. 1993;52:291–299. doi: 10.1007/BF00296654. [DOI] [PubMed] [Google Scholar]
- 29.Martin DD, Wit JM, Hochberg Z, Savendahl L, van Rijn RR, Fricke O, Cameron N, Caliebe J, Hertel T, Kiepe D, Albertsson-Wikland K, Thodberg HH, Binder G, Ranke MB. The use of bone age in clinical practice – part 1. Horm Res Paediatr. 2011;76:1–9. doi: 10.1159/000329372. [DOI] [PubMed] [Google Scholar]