Abstract
Acute kidney injury (AKI) is increasingly common around the world. Because of the low availability of effective therapies and resource limitations, early preventive and therapeutic measures are essential to decrease morbidity, mortality, and cost. Timely recognition and diagnosis of AKI requires a heightened degree of suspicion in the appropriate clinical and environmental context. In low- and middle-income countries (LMICs), early detection is impaired by limited resources and low awareness. In this article, we report the consensus recommendations of the 18th Acute Dialysis Quality Initiative meeting in Hyderabad, India, on how to improve recognition of AKI. We expect these recommendations will lead to an earlier and more accurate diagnosis of AKI, and improved research to promote a better understanding of the epidemiology, etiology, and histopathology of AKI in LMICs.
Keywords: acute kidney injury, biomarkers, detection, developing countries, diagnosis, recognition, resources
The incidence of acute kidney injury (AKI) is increasing around the world.1, 2, 3, 4 The ongoing search for supporting procedures and interventions has produced improved guidelines and recommendations.5, 6 Demonstration of increasing AKI incidence has led to an emphasis on prevention or early intervention,5 but unfortunately, analytical methods that predict AKI, or preventive and therapeutic approaches to accelerate recovery or prevent progression to chronic kidney disease (CKD), are only beginning to be understood.7, 8, 9
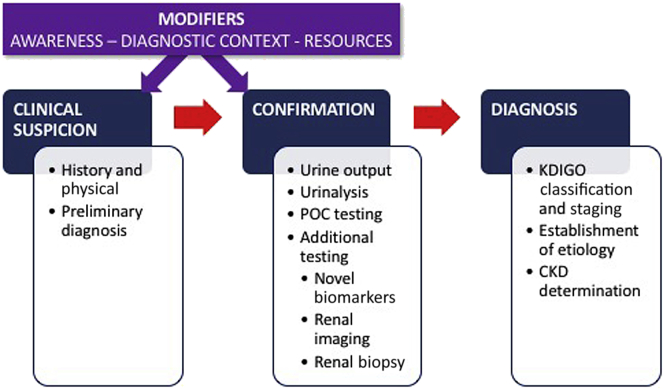
Early recognition of AKI is essential to ensure prompt and appropriate management, and to avoid progression to deadlier stages of the disease10, 11 (Figure 1). In the appropriate context, early detection requires a high degree of suspicion that AKI is occurring. Diagnosis requires a combination of a clinical history, a thorough physical examination, an accurate assessment of kidney function, appropriate imaging, and when indicated, a kidney biopsy.
Figure 1.
Acute kidney injury (AKI) recognition: the process and its modifiers. In addition to the usual AKI trajectory from clinical suspicion to confirmation to diagnosis, other factors modify the process. The degree of AKI awareness, the context in which the patient is encountered, and the available diagnostic resources may facilitate, delay, or impede the achievement of early AKI diagnosis. CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes; POC, point of care.
In low- and middle-income countries (LMICs), early detection is impaired by limited resources and poor understanding of the condition.1, 2, 9, 12, 13, 14, 15 Such limited understanding—to a large extent determined by inadequate reporting and education—limits awareness and early recognition, and delays the implementation of measures that permit early and adequate management.16
To address this goal, the steering committee of the 18th Acute Dialysis Quality Initiative (ADQI) conference dedicated a work group with the task to identify what elements affect the recognition of AKI within the limited resource constraints prevalent in LMICs. Using a modified Delphi process, this group reached consensus regarding strategies to recognize and diagnose AKI focusing on low resource countries. The group addressed the following 3 questions that served as the basis for accompanying consensus statements:
-
1.
When should AKI be suspected?
-
2.
What tests are needed when AKI is suspected?
-
3.
How do we confirm the diagnosis of AKI in patients with an initially elevated serum creatinine (Scr) level?
Methods
The ADQI process has been described previously.17, 18 Complete ADQI methodology description is available at www.adqi.org and in the editorial accompanying the ADQI 18 conference papers.19 The broad objective of ADQI is to provide expert-based statements and interpretation of current knowledge for use by clinicians according to professional judgment, and to identify clinical research priorities to address these gaps. The 18th ADQI Consensus Conference Chairs convened a diverse panel that represented relevant disciplines (i.e., adult and pediatric nephrology, critical care, and renal pathology) from several continents (e.g., Africa, Asia, North America, Latin America, and Europe) around the theme of “Management of Acute Kidney Injury in the Developing World” for a 2-1/2–day consensus conference in Hyderabad, India on September 27 to 30, 2016.
The preconference activities involved a search of the literature for evidence on the epidemiology, recognition, and management of AKI in developing countries and their differences with developed countries. A literature search was conducted using the following terms: recognition; awareness; diagnosis; point of care; and low income countries or developing countries, together with either acute kidney injury and acute renal failure in PubMed. This work group was also tasked to summarize the scope, implementation, and evaluative strategies for AKI recognition and diagnosis based on the location, resource availability, and a critical evaluation of the relevant literature. A series of phone conferences and emails that involved work group members before the meeting identified current knowledge to enable the formulation of main questions from which discussion and consensus would be developed. A formal systematic review was not conducted. During the conference, the work group developed consensus positions, and plenary sessions that involved all ADQI contributors were used to present, debate, and refine these positions. Following the meeting, this summary report was generated, revised, and approved by all members of the ADQI participants. All the participants interacted throughout the meeting in the general session, and all group deliberations were subjected to review and consensus agreement in the final versions. In addition, all participants discussed and approved the contents of this paper. The participants did not represent specific societies, but were invited because they had domain knowledge expertise. Their affiliations are provided in the Supplementary Appendix.
For the purposes of all work group discussions, we used the current Kidney Disease Improving Global Outcomes (KDIGO) definitions for AKI and stages of AKI, which defines AKI as an episode that occurred within a 7-day timeframe.5 Community-acquired AKI was defined as an episode of AKI when the initial event occurred outside of the hospital setting and where the patient was admitted to the hospital with AKI; hospital-acquired AKI was defined as an episode of AKI due to a kidney insult that occurred to hospitalized patients who developed de novo AKI during their hospital stay.15
Q1: When Should AKI Be Suspected?
Consensus Statement
-
1.
In the appropriate clinical context, AKI should be suspected in patients who present with the signs and symptoms listed in Table 1.
Table 1.
Signs and symptoms leading to suspicion of acute kidney injury in low- to middle-income countries
| In the community setting |
| History of kidney disease |
| Oliguria |
| Total body swelling |
| Hypotension |
| Dehydration |
| GI loss of volume and electrolytes |
| Dark, concentrated urine |
| Sepsis syndrome |
| Fever in the context of prevalent endemic disease |
| Exposure to potential nephrotoxins |
| Pregnancy-related complications |
| Plus, in the hospital setting, |
| Multiple organ failure |
| Nephrotoxic medication exposure |
GI, gastrointestinal.
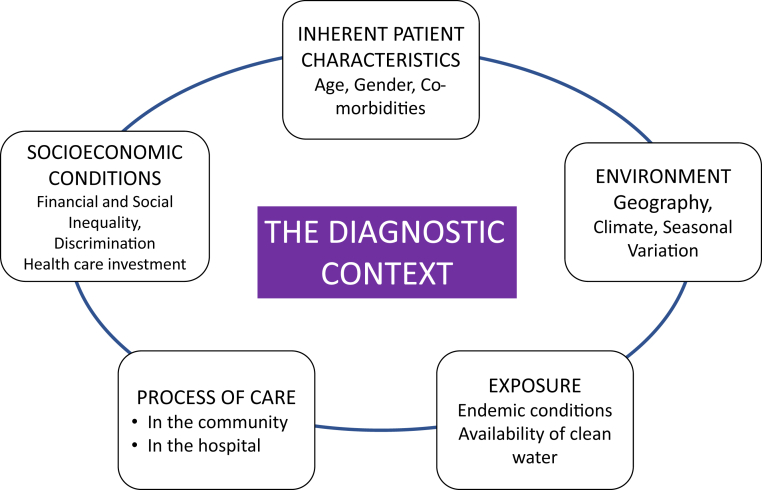
During the initial interaction of a patient with the health care system, the diagnosis of AKI is influenced by the clinical presentation and the context of the encounter11, 20 (Figure 2). Improved awareness that the presenting symptoms and signs might correspond to AKI is the first step toward timely recognition. Unfortunately, AKI is frequently not recognized or is recognized too late, at a more severe stage.21 Failure to recognize early AKI is frequently associated with disease progression that requires more aggressive therapies and support when recovery is less likely and mortality is heightened.22
Figure 2.
Main components of the acute kidney injury diagnostic context.
In LMICs, because of the common absence of access to specialized nephrology care, increased awareness of the clinical situations associated with AKI, and the implications of failing to detect it, AKI must be more understood at all levels of the health care system.23 A practical and easily accessible educational strategy focused on providers at the forefront of health care delivery is indispensable to achieve this goal. Providers must be trained to consider AKI in patients who present with certain signs and symptoms (Table 1)24 in the right clinical context. For example, in areas where infectious diseases (e.g., severe malaria, leptospirosis, or dengue) are endemic and associated with high rates of AKI,20, 25, 26, 27 a febrile patient should elicit concern for renal injury.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 Similarly, in patients with severe volume depletion due to gastrointestinal loss, volume resuscitation is central to care and to prevent renal injury—preferably before the onset of persistent oliguria.15 Management must be appropriate to the clinical condition.49, 50
The development of AKI as a maternal and neonatal complication deserves special consideration in the LMIC environment,51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 because failure to recognize renal injury frequently leads to significant consequences for both the mother and child.
Successful efforts to improve early recognition have clearly demonstrated benefit, especially by reducing some of the more dreaded consequences such as cortical necrosis.63, 64 In some areas of the world, exposure to snake venom represents a frequent cause of AKI.65, 66 Administration of herbs by traditional healers has been associated with nephrotoxicity, and must be considered when confronted with AKI of unclear etiology.12, 21, 67 Increased availability and use of over-the-counter allopathic medications (e.g., nonsteroidal anti-inflammatory drugs) significantly contribute to a rising incidence of AKI.
In LMIC, recognition of AKI in the hospital faces challenges that are akin to those seen in the developed world; hospitalized patients demonstrate a high incidence of AKI related to exposure to nephrotoxic medications, antibiotics, intravascular administration of iodinated radiocontrast, and surgical procedures.68, 69
Consensus Statement
-
2.
Evaluation for AKI should be incorporated into the diagnosis and management of specific endemic conditions associated with a high AKI risk (e.g., severe malaria, leptospirosis, dengue, and HIV).
Endemic infections contribute significantly to the burden of AKI in LMICs. Much remains to be learned about the prevalence of AKI, the clinical characteristics that predispose to the onset of AKI, and the impact of AKI on the management of patients with those infections. Thus, the HIV epidemic in Sub-Saharan Africa has contributed to the rising burden of AKI, either as a direct result of the viral infection or as an unintended consequence of antiretroviral therapy.16, 26, 70, 71, 72, 73 Other infectious diseases in LMICs have not received the same level of attention, and much remains to be understood about the nature of AKI associated with these conditions.48, 49, 74, 75
Research Recommendation
-
•
In LMICs, efforts must be directed to a better understanding of the epidemiology and management of infection-related AKI.
Q2. What Tests Are Needed When AKI Is Suspected?
Consensus Statement
-
1.
We recommend that patients suspected to have AKI should have an estimation of urinary output, a measurement of SCr levels, and a thorough urinalysis.
-
2.
Whenever possible, the performance of urine microscopy and urine biochemistry is essential to elucidate the underlying etiology and to assess severity.
-
3.
We recommend that point-of-care testing (POCT) technologies should be made available for the diagnosis of AKI in low resource settings.
-
4.
In hospitalized patients, we recommend additional testing, including renal imaging and renal biopsy, as indicated. The use of newer biomarkers of structural injury in economically constrained environments should await demonstration of efficacy.
Confirmation of AKI
The diagnosis and staging of AKI using current KDIGO definitions rests upon changes in serum creatinine and/or urinary output.5 Additional testing and urinary microscopy are necessary to identify the underlying etiology.
Urinary Output
In patients with developing AKI, urine output is a sensitive functional marker of kidney dysfunction.76, 77, 78, 79, 80 Unfortunately, oliguria may be easily confounded in its significance79 and can be difficult to record accurately, thereby limiting its reliability as a marker of AKI. In the community setting, diuresis is often unknown or inaccurately recorded, which limits its usefulness.5 In LMICs, oliguria is usually an accurate marker of AKI severity in children and neonates, and is associated with patient outcomes.39, 81, 82, 83
Urinalysis
When available, use of urine dipsticks and measurement of urinary indices such as urinary sodium,84, 85 fractional excretion of sodium,86, 87, 88 fractional excretion of urea,84, 85, 86, 89, 90, 91 urine plasma creatinine ratio,84 urine concentration (osmolality or specific gravity),84, 85, 92 and protein are useful for the initial evaluation of AKI.
The performance of basic urine microscopy,85, 91, 93, 94, 95, 96 which focuses on the presence of erythrocytes, leukocytes, eosinophils, and casts in the sediment,60, 97, 98 is invaluable to assess the initial presentation of the patient with AKI (Table 2).
Table 2.
Urine microscopy
| Reference | Test | Patients (n) |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Comments |
|---|---|---|---|---|---|---|---|
| Bagshaw86 | UMS ≥3 | 83 | 0.67 (0.39–0.86) | 0.95 (0.84–0.99) | 0.80 (0.49–0.94) | 0.91 (0.78–0.96) | The UMS was compared between septic and nonseptic AKI and correlated with NGAL, worsening AKI, RRT, and hospital mortality. UMS correlates with uNGAL, but not with pNGAL; a UMS score ≥ 3 was associated with increased odds of worsening AKI (AOR: 8.0; 95% CI: 1.03–62.5; P = 0.046). |
| Perazella93 | USS ≥2 | 267 | 0.76 | 0.86 | 100 | 44 | Using the final diagnosis as the gold standard, the ability of the urine microscopy diagnosis to distinguish ATN from prerenal AKI was fair (sensitivity 0.76; specificity 0.86; positive LR 5.75). However, the scoring system was highly predictive of the final diagnosis of ATN |
| Chawla96 | CSI | 30 | Gold standard was patients with AKI consistent with the syndrome of acute tubular necrosis. The patients with nonrenal recovery had a higher CSI compared to those patients who did recover renal function (2.55 ± 0.93 vs. 1.57 ± 0.79; P = 0.04) Limitations: small sample, lack of control for urine osmolality and pH, the number of reviewers and the variation in the reviewer’s training was relatively limited. | ||||
| Carvounis89 | Scr ≥1.1 mg/dl | 363 | 84.2 (74.4–90.7) | 77.7 (72.5–82.1) | 50.0 | 94.9 | |
| Renal epithelial cells or epithelial/granular casts | 22.4 (14.5–32.9) | 91.3 (87.5–94.0) | 40.5 | 81.6 | |||
| NGAL (ng/ml) ≥ 42.71 | 64.5 (53.3–74.3) | 64.5 (58.8–69.8) | 32.5 | 87.3 |
AKI, acute kidney injury; AOR, adjusted odds ratio; ATN, acute tubular necrosis; CI, confidence interval; CSI, Cast score index; LR, likelihood ratio; NGAL, neutrophil gelatinase-associated lipocalin; NPV, negative predictive value; PPV, positive predictive value; RRT, renal replacement therapy; UMS, urine microscopy score; USS, urinary scoring system.
We recommend that training in microscopic urine examination and availability of basic examination equipment for such testing should be promoted as a key, low-resource test for detection of AKI in LMICs.
Although the usefulness of urinary indices (Table 3) in the critically ill patient with sepsis has been questioned,86, 99 and may be confused by the use of diuretics, the combination of these tests with a thorough patient history, physical examination, and urinalysis will increase the sensitivity and specificity of AKI prediction and severity.100
Table 3.
Urine biochemistry
| Reference | Test | Patients (n) |
AUC | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Comments |
|---|---|---|---|---|---|---|---|---|
| Carvounis89 | FeU vs. FENa | 102 PR = 50 Prdiu = 27 ATN = 25 |
90 | 96 | 99 | 75 | Gold standard clinical grounds; more sensitive and specific index than FENa in differentiating between ARF due to prerenal azotemia and that due to ATN, especially if diuretics have been administered; in osmotic diuresis, the proximal tubular absorption of salt and water is impaired; thus, increased FEUN is expected despite renal hypoperfusion. A similar picture emerges in patients given a high protein diet or having excessive catabolism. | |
| Pepin90 | FeNA vs. Feur in transient AKI (prerenal) | 99 | Gold standard clinical context and whether serum creatinine level returned to baseline within 7 days. In patients without diuretic use, FENa is better able to distinguish transient from persistent AKI. In patients administered diuretics, this distinction cannot be made accurately by means of FENa. FEur cannot be used as an alternative tool because it lacks specificity. | |||||
| FENa | 0.83 ± 0.07 | 78 | 75 | 86 | 64 | |||
| FENA + dir | 0.75 ± 0.06 | 68 | 81 | 86 | 49 | |||
| FEur | 0.56 ± 0.11 | 48 | 75 | 79 | 43 | |||
| FEur + diur | 0.57 ± 0.08 | 79 | 33 | 71 | 44 | |||
| Bagshaw86 | FeU ≤35% | n = 28 | 0.54 (0.42–0.67) | 40 | 59 | Gold standard: uNGAL. In sepsis, FeNa and FEUN are not reliable markers of renal hypoperfusion. Urine biochemical profiles and microscopy do not discriminate septic and non-septic AKI. UNa, FeNa, and FeU do not reliably predict biomarker release, worsening AKI, RRT or mortality. These data imply limited utility for these measures in clinical practice in critically ill patients | ||
| FeNa <1% | n = 47 | 0.54 (0.42–0.67) | 50 | 58 |
AKI, acute kidney injury; ARF, acute renal failure; ATN, acute tubular necrosis; AUC, area under the curve; diur, diuretics; FENa, fractional excretion of sodium; FeU, fractional excretion of urea; FEUN, fractional excretion of urea nitrogen; NPV, negative predictive value; PPV, positive predictive value; RRT, renal replacement therapy; UNa, urine sodium; uNGAL, urine neutrophil gelatinase-associated lipocalin.
Serum Creatinine
Despite limitations in the use of serum creatinine as a marker of renal function, changes in SCr and/or urine output form the basis of all AKI diagnostic criteria. SCr is a frequently inaccurate biomarker due to the need for a baseline and/or historical value to provide context101, 102, 103, 104 and the limitations of a delayed diagnosis.105, 106, 107, 108 Serum creatinine concentrations are affected by age, sex, and muscle mass109; they can change in response to certain drugs and are unreliable in patients with liver dysfunction or fluid overload. Serum levels take 24 to 36 hours to rise after a definite insult.110, 111, 112, 113 In addition, although changes in creatinine concentration remain central to the diagnosis of AKI, differences in individual body composition that result in differences in creatinine production and volume of distribution across populations, as well as variations in dietary composition, have largely been ignored,102 and may be different from current estimates originated in the developed world.
Until recently, the most common assay for measurement creatinine was the alkaline picrate (Jaffé) assay. However, chromogens other than creatinine interfere with the assay, giving rise to errors in up to 20% in subjects with a normal glomerular filtration rate (GFR). Modern assays do not detect noncreatinine chromogens and yield lower levels of creatinine. The lack of standardization to adjust for this interference affects the ability to estimate kidney function based on SCr concentration by different laboratories, especially at higher levels of estimated GFR. Standardization will reduce but not completely eliminate this error.114
Blood and Saliva Urea Nitrogen
Serum urea and blood urea nitrogen (BUN) levels must be carefully interpreted as markers of kidney function in view of the numerous non-GFR factors that influence their blood concentrations. Levels of urea and/or BUN depend on protein intake, endogenous urea production, and tubular reabsorption. Reduced kidney perfusion in the setting of volume depletion enhances reabsorption of urea, which may lead to an elevation of BUN disproportionate to the concomitant decrease in GFR. Conversely, decreased protein intake or underlying liver disease can prevent the expected rise in BUN, whereas increased urea production (gastrointestinal bleeding, hypercatabolic status) or impaired protein anabolism (corticosteroid administration) can increase BUN in the absence of increased urea reabsorption.84, 115 Because of multiple confounding, the use of BUN as an isolated marker of kidney injury may be unreliable. Additional POCT tools such as saliva urea nitrogen have been recently proposed and may be effective to screen patients with elevated urea nitrogen levels when blood tests may be unavailable or unaffordable.116
Serum Cystatin C
Currently, cystatin C is not being widely used. The absence of a relationship with body composition makes this marker an interesting alternative, but its value is limited by changes in concentration in response to inflammation, lung disease, and cigarette smoking.117
Point-of-Care Testing
POCT for creatinine measurements occurs close to the patient instead of in a central laboratory (Table 4). It can be performed by nonlaboratory trained individuals, thus eliminating delays in testing and reporting of results.118 Although POCT is a particularly attractive option in remote and low resource environments, it requires the implementation of a quality assurance program that ensures accurate and reliable results. Several POCTs for Scr are available in the market across the world116, 118, 119, 120, 121, 122, 123, 124 and can be classified into blood gas analyzers and nonblood gas analyzers. They also vary with respect to the types of samples that can be processed—whole blood, plasma, or serum. Other specific requirements include a power source, availability of deionized water, specific consumables (which sometimes require refrigeration), space, and requirements for calibration and disposal as a biohazard waste. As a result, most POCTs for SCr are not yet cost-effective and must be further tested for their usefulness in the detection of AKI.119 The failure of most of POCT creatinine devices to be in full alignment with isotope dilution mass spectrometry equivalent standards is another limitation.118, 125 Definitive studies to determine the best practices to incorporate POCTs in low-resource health care settings are needed.
Table 4.
Issues that must be considered when selecting a point-of-care test
| Ease of use |
| Accuracy |
| Low error rate (imprecision + bias) |
| Consumable need: strips, cassettes, cartridges, rotor system, etc. |
| Portability (handheld vs. bench top); different models may be appropriate for field vs. hospital settings |
| Power source (battery vs. mains) |
| Scalability |
| Processing time |
| Sample source and volume |
| Connectivity (e.g., Bluetooth integration) |
| Ability for integration into electronic decision support systems |
| Possibility to do >1 test |
| Cost of the device and consumables |
Novel Biomarkers
As discussed, SCr as the current gold standard remains a flawed marker of renal dysfunction. Newer biomarkers are being developed, but even in high-income countries their use is yet to become a standard of care; their application in the developing world is even more challenging.126
Because of their simplicity of use and limited requirement for technological support, dipsticks are one of the most widely used tools to assess renal injury. Although traditional dipsticks allow the assessment of renal injury by primarily testing glomerular integrity (albuminuria and/or proteinuria), newer devices have more recently been modified as markers of renal dysfunction by estimating elevated BUN using saliva, or novel blood or urine markers of tubular injury such as kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin.123, 127 Recently, newer biomarkers in dipstick format have been made commercially available.112
AKI etiologies in low-resource rural areas, where volume depletion, infection, and nephrotoxic agents are leading causes of AKI,12, 26 are usually different from those seen in the developed world.12 Such differences pose a challenge in our understanding of how potential novel biomarkers can be deployed. The ideal biomarker would facilitate the distinction between AKI due to volume depletion and AKI due to intrinsic kidney injury, and must be able to distinguish transient elevations in SCr from persistent changes consistent with injury. Such markers should allow early detection of the most likely cause of AKI, facilitate a diagnosis in the absence of historical information on baseline renal function, and support early therapeutic intervention.12, 127 Unfortunately, novel AKI biomarkers remain poorly studied in clinical conditions commonly associated with AKI in LMICs; such limitations raise questions about their potential usefulness and practical implementation in those areas.128
Newer AKI Definitions, Staging Criteria, and Recent Uncertainties
Although newer AKI definitions and staging criteria such as KDIGO; acute kidney injury network (AKIN); and risk, injury, failure, loss, and end-stage kidney disease (RIFLE)5, 129, 130, 131 are appropriate to define AKI epidemiology and to design clinical trials, questions have been raised on their clinical application to the individual patient.111, 112 The classification of AKI and its various stages has been validated in multiple hospitalized populations by demonstrating a strong association with short- and long-term outcomes,13, 132 but significant problems in the usefulness of this classification persist.110 Because they rely on renal function changes, current AKI definitions only permit a relatively late diagnosis hours or days after the risk of injury or when the actual lesion began. As discussed previously, efforts to achieve an earlier diagnosis have led to the development of biomarkers of injury and are currently in progress.133 It is expected that newer biomarkers may detect kidney damage before the SCr and GFR become abnormal, but it is unclear how accurately those biomarkers will measure kidney damage instead of the severity of disease.134, 135, 136, 137, 138, 139
Because of current uncertainties on the correlation among AKI definitions, biomarker data, and histopathology,140 better availability of histopathologic data in LMICs provides a unique opportunity to probe such correlation, and begins to close the gap between our understanding of actual human histopathology, the pathogenesis of AKI, and our current, strictly functional KDIGO, AKIN, and RIFLE definitions.5, 129, 130, 131
Histopathology in AKI
A better understanding of the histopathology and pathogenesis of AKI is indispensable to continue to unveil the process of kidney injury,141 and by developing bench-to-bedside processes, to foster a better understanding on how to avoid and how to treat kidney injury.142
During the evaluation of patients with renal injury, a diagnosis based on histopathology remains important because it not only provides insight into the injury pattern, but often guides patient management. Multiple causes of AKI require histopathological diagnosis, but unfortunately, the number of biopsies and publications on the histopathology of AKI is declining.110, 143 Concerns about procedural complications, including the risk of bleeding and the perception that AKI is commonly the result of acute tubular necrosis, appear to contribute to the reluctance to perform biopsies in the acute setting, despite evidence to the contrary.144, 145, 146, 147, 148
Kidney biopsies are indicated when: (i) The clinical presentation suggests that biopsy findings will likely lead to important therapeutic changes, an improved probability of recovery, and avoidance of further injury; (ii) when the magnitude of benefit is assessed to be greater than the risk of the procedure; and (iii) when the temporal course of the disease and delayed recovery dictates the need for further ascertainment of histopathologic diagnosis and prognosis. Multiple old and new studies have reviewed the indications and attested to the safety and usefulness of percutaneous kidney biopsies in the management of kidney disease.149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161
Currently, kidney biopsies in patients with AKI are more common in LMICs than in high-income countries; thus, there is a greater appreciation of the relative incidence of multiple etiologies and the value of a renal biopsy to guide management.1, 15, 20, 21, 162 Although results from biopsy series are likely confounded by indication bias, those studies suggest that the role of a renal biopsy must be reconsidered in the diagnosis and management of AKI of unclear etiology, such as: unexplained AKI; acute interstitial nephritis60, 163, 164; acute or chronic glomerulonephritis, or rapidly progressive glomerulonephritis165; interstitial or tubular injury due to drug toxicity, or exposure to traditional herbal remedies21, 166, 167, 168, 169, 170; thrombotic microangiopathies171; or leptospirosis.172, 173, 174, 175, 176
Because of current uncertainties on the relationship among AKI definitions, biomarker data and renal histopathology, and their effects on treatment and prognosis,140 we strongly recommend that kidney biopsies be considered in patients with AKI, whenever appropriate and feasible.
We further recommend that in LMIC settings, basic training be provided to local pathologists on renal histopathology, understanding that even the limited information provided by light microscopy may provide invaluable guidance in patient management. Training of members of the health care team in simple imaging, including ultrasonography, when feasible, is also desirable.
Research Recommendation
-
•
We recommend the development, validation, and standardization of POCT to facilitate the diagnosis of AKI in the community.
Q3: How Do We Confirm the diagnosis of AKI in Patients With an Initially Elevated SCr Level?
Consensus Statement
-
1.
We recommend that patients with an isolated (single) elevated creatinine or oliguria be considered to have AKI until proven otherwise, to ensure rapid implementation of effective treatment measures.
Concerns that the initially elevated SCr may be due to CKD may unnecessarily delay the initiation of urgent therapeutic measures. We strongly recommend that patients with apparently acute, severe dysfunction be emergently treated as if they had AKI, until proven otherwise (see the following).
Consensus Statements
-
2.
We recommend that the presence of CKD be evaluated using clinical history, urinalysis, renal imaging, and biopsy when indicated.
-
3.We recommend that the diagnosis of AKI should be confirmed by repeat assessment of renal function at no later than 7 days.
-
a.We recommend that the frequency of repeat assessment of renal function be guided by the clinical context and response to intervention.
-
a.
Differentiation Between AKI and CKD
When a patient without historical information presents in the community center with clinical features and/or an elevated creatinine consistent with a diagnosis of kidney injury, distinguishing isolated AKI from AKI superimposed on CKD or baseline CKD can be challenging. We believe this distinction should not be immediately relevant to the initial management, which should focus on the amelioration of the urgent metabolic and/or volume imbalances and on the correction of all known precipitating factors (Table 5).
Table 5.
Factors that can cause worsening renal function in a patient with preexisting kidney disease
| Systemic infection |
| Infection of the urinary tract |
| Volume deficit |
| Urinary tract obstruction |
| Uncontrolled hypertension |
| Unrecognized renovascular disease |
| Drug-induced (hemodynamic, interstitial nephritis) |
We suggest that all patients without a known history of renal disease who present with a first episode of kidney injury must be presumed to have potentially reversible AKI, until proven otherwise. Moreover, even when the presence of CKD is demonstrated, modifiable factors that could have led to potentially reversible acute deterioration of renal function should be identified and corrected. This distinction becomes very relevant in certain regions of the world, where decisions are made on resource allocation in countries where public health care systems only offer support for the dialytic management of potentially reversible AKI, but frequently deny dialysis if renal failure is irreversible.
In patients presenting with kidney failure, all attempts should be made to explore whether previous measures of kidney function are available. This information can be part of previous encounters in the health care system, such as during pregnancy; presurgical screening; evaluation during an unrelated illness; or as part of medical screening before employment, insurance, or during school, corporate, or community health checks. In the fragmented LMIC health care systems, records are often unavailable, so when consulting, patients should be encouraged to bring all records of previous encounters with the health care system, which is a common practice in LMICs.
Certain symptoms, signs, and laboratory or imaging findings (Table 6) can increase the suspicion of preexisting kidney disease, but should not be used to exclude the presence of coexisting AKI.
Table 6.
Features that indicate the presence of preexisting kidney disease in a patient presenting with kidney injury
| History of long-standing nocturia |
| History of edema, hematuria or renal stones |
| History of long-term intake of painkillers, herbal medicines, over-the-counter drugs |
| History of recurrent dehydration |
| Family history of kidney disease |
| Urinalysis showing broad casts |
| Musculoskeletal manifestations: growth retardation, rickets, or proximal myopathy |
| Anemia out of proportion to the duration of symptoms in the absence of another cause |
| Elevated phosphate and/or PTH levels |
| Characteristic imaging abnormalities (e.g., renal cysts or obstruction) |
| Small and/or highly echogenic kidneys on ultrasound |
PTH, parathyroid hormone.
In high-income countries, the first 48 hours of the SCr trajectory of patients hospitalized with initially elevated SCr has been used to evaluate the rate of AKI development and to assess whether kidney injury is transient or persistent.177 In this approach, the attainment of peak SCr after the initial creatinine elevation is considered an indication of persistent AKI. In LMICs, when community patients reach hospitals with established AKI such time-course information is usually not available. In those situations, excluding the possibility of the preexisting presence of CKD on a clinical basis may not be possible. Diagnosis may require either a kidney biopsy or be made retrospectively, when kidney function fails to improve despite appropriate supportive therapy.
Limitations
The recommendations in this paper should not be limited to LMICs, but extended to all areas where nephrology resources are not widely available due to a variety of reasons, including cultural, geographic, or religious limitations. The World Bank country economic classification does not necessarily reflect either the health care structure or health care investment of each country. Many countries included in the LMIC category offer universal health care coverage, whereas some subpopulations in high-income countries may not have access to primary care, such as refugees, minorities, aboriginal peoples, or persons without health care coverage. Efforts should be directed toward a more granular analysis of the impact of health care investment and delivery on the recognition and management of AKI. Current limitations in the understanding of the epidemiology of AKI in LMICs are only beginning to be understood; continuously improving information will be necessary to enable the development of more accurate recommendations.
Conclusions
Measures to increase AKI awareness and recognition are essential to improve the treatment and prognosis of AKI in all regions of the world. To ensure a prompt to potentially reversible AKI, once a preliminary diagnosis is obtained by the demonstration of an elevated SCr, patients must be managed as if they had AKI until proven otherwise. Whenever possible, we recommend the pursuit of a diagnostic strategy geared toward the identification of the etiology of AKI to guide therapeutic options. This is particularly important in LMICs, where various endemic infections and toxicities often underlie renal damage. AKI is potentially treatable and reversible, and treatment is often specific to the underlying condition.
To enhance AKI recognition, it is necessary to promote a better understanding of this epidemiological association of AKI with highly prevalent conditions, including endemic diseases, and to promote widespread education on AKI at all levels and to all members of the health care system.
Disclosure
All the authors declared no competing interests.
Author Contributions
JC, SM, GG, VJ, SS, SG, RC, and RM all participated in the consensus-building process and drafting of this paper. RM, RC, and AB provided a critical review of this paper.
Acknowledgment
Supported through the UAB-UCSD O’Brien Center NIH-NIDDK Grant DK079337.
Footnotes
Appendix S1. ADQI participants’ names and affiliations.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
ADQI participants’ names and affiliations.
References
- 1.Mehta R.L., Cerda J., Burdmann E.A. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 2.Cerda J., Lameire N., Eggers P. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881–886. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 3.Hsu R.K., McCulloch C.E., Dudley R.A. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24:37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susantitaphong P., Cruz D.N., Cerda J. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KDIGO KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;Suppl 2:1–138. [Google Scholar]
- 6.Ronco C. Italian AKI guidelines: The best of the KDIGO and ADQI results. Blood Purif. 2015;40:i–iii. doi: 10.1159/000439261. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz A., Sanchez-Nino M.D., Izquierdo M.C. Translational value of animal models of kidney failure. Eur J Pharmacol. 2015;759:205–220. doi: 10.1016/j.ejphar.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Liu K.D. Therapeutic strategies for clinical trials targeting renal recovery. Nephron Clin Pract. 2014;127:113–116. doi: 10.1159/000363703. [DOI] [PubMed] [Google Scholar]
- 9.Cerda J., Liu K.D., Cruz D., on behalf of the American Society of Nephrology Acute Kidney Injury Advisory Group Promoting kidney function recovery in patients with acute kidney injury requiring renal replacement therapy. Clin J Am Soc Nephrol. 2015;10:1859–1867. doi: 10.2215/CJN.01170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lameire N.H., Bagga A., Cruz D. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 11.Lewington A.J., Cerda J., Mehta R.L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerda J., Bagga A., Kher V. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–153. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 13.Susantitaphong P., Cruz D.N., Cerda J., for the Acute Kidney Injury Advisory Group of the American Society of Nephrology Worldwide incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lameire N, Van Biesen W, Vanholder R. Epidemiology of acute kidney injury in children worldwide, including developing countries [e-pub ahead of print] Pediatr Nephrol. http://dx.doi.org/10.1007/s00467-016-3433-2. Accessed May 29, 2017. [DOI] [PubMed]
- 15.Mehta R.L., Burdmann E.A., Cerda J. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 16.Swanepoel C.R., Wearne N., Okpechi I.G. Nephrology in Africa–not yet uhuru. Nat Rev Nephrol. 2013;9:610–622. doi: 10.1038/nrneph.2013.168. [DOI] [PubMed] [Google Scholar]
- 17.Ronco C., Kellum J.A., Mehta R. Acute dialysis quality initiative (ADQI) Nephrol Dial Transplant. 2001;16:155–1558. doi: 10.1093/ndt/16.8.1555. [DOI] [PubMed] [Google Scholar]
- 18.Kellum J.A., Mehta R.L., Angus D.C. The first international consensus conference on continuous renal replacement therapy. Kidney Int. 2002;62:1855–1863. doi: 10.1046/j.1523-1755.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 19.Mehta R., Bagga A., Patibandla R. Detection and management of AKI in the developing world: the 18th Acute Disease Quality Initiative (ADQI) International Consensus Conference. Kidney Int Rep. 2017;2:515–518. [Google Scholar]
- 20.Naicker S., Aboud O., Gharbi M.B. Epidemiology of acute kidney injury in Africa. Semin Nephrol. 2008;28:348–353. doi: 10.1016/j.semnephrol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Olowu W.A., Niang A., Osafo C. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Global Health. 2016;4:e242–e250. doi: 10.1016/S2214-109X(15)00322-8. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein S.L., Chawla L., Ronco C. Renal recovery. Crit Care. 2014;18:301. doi: 10.1186/cc13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sitprija V. Nephrology in South East Asia: fact and concept. Kidney Int Suppl. 2003:S128–S130. doi: 10.1046/j.1523-1755.63.s83.27.x. [DOI] [PubMed] [Google Scholar]
- 24.Himmelfarb J., Joannidis M., Molitoris B. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:962–967. doi: 10.2215/CJN.04971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha V., Parameswaran S. Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol. 2013;9:278–290. doi: 10.1038/nrneph.2013.36. [DOI] [PubMed] [Google Scholar]
- 26.Swanepoel C.R., Wearne N., Duffield M.S. The evolution of our knowledge of HIV-associated kidney disease in Africa. Am J Kidney Dis. 2012;60:668–678. doi: 10.1053/j.ajkd.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 27.Repizo L.P., Malheiros D.M., Yu L. Biopsy proven acute tubular necrosis due to rhabdomyolysis in a dengue fever patient: a case report and review of literature. Rev Inst Med Trop Sao Paulo. 2014;56:85–88. doi: 10.1590/S0036-46652014000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Mendalawi M.D. Acute renal failure associated with malaria in children. Saudi J Kidney Dis Transpl. 2013;24:1255. doi: 10.4103/1319-2442.121298. [DOI] [PubMed] [Google Scholar]
- 29.Aloni M.N., Nsibu C.N., Meeko-Mimaniye M. Acute renal failure in Congolese children: a tertiary institution experience. Acta Paediatr. 2012;101:e514–e518. doi: 10.1111/j.1651-2227.2012.02827.x. [DOI] [PubMed] [Google Scholar]
- 30.Barsoum R.S. Malarial acute renal failure. J Am Soc Nephrol. 2000;11:2147–2154. doi: 10.1681/ASN.V11112147. [DOI] [PubMed] [Google Scholar]
- 31.Basu G., Chrispal A., Boorugu H. Acute kidney injury in tropical acute febrile illness in a tertiary care centre–RIFLE criteria validation. Nephrol Dial Transplant. 2011;26:524–531. doi: 10.1093/ndt/gfq477. [DOI] [PubMed] [Google Scholar]
- 32.Bouth D.M., Giboda M. Malaria in Kampuchea: clinical course of falciparum malaria in Chemin de Fer Hospital, Phnom Penh. Folia Parasitol. 1987;34:11–18. [PubMed] [Google Scholar]
- 33.Conroy A.L., Hawkes M., Elphinstone R.E. Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis. 2016;3:ofw046. doi: 10.1093/ofid/ofw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das B.S. Renal failure in malaria. J Vector Borne Dis. 2008;45:83–97. [PubMed] [Google Scholar]
- 35.Esezobor C.I., Ladapo T.A., Lesi F.E. Clinical profile and hospital outcome of children with severe acute kidney injury in a developing country. J Trop Pediatr. 2015;61:54–60. doi: 10.1093/tropej/fmu066. [DOI] [PubMed] [Google Scholar]
- 36.Esezobor C.I., Ladapo T.A., Osinaike B. Paediatric acute kidney injury in a tertiary hospital in Nigeria: prevalence, causes and mortality rate. PloS One. 2012;7:e51229. doi: 10.1371/journal.pone.0051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S., Sengar G.S., Meti P.K. Acute kidney injury in Pediatric Intensive Care Unit:Incidence, risk factors, and outcome. Indian J Critical Care Med. 2016;20:526–529. doi: 10.4103/0972-5229.190368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imani P.D., Odiit A., Hingorani S.R. Acute kidney injury and its association with in-hospital mortality among children with acute infections. Pediatr Nephrol. 2013;28:2199–2206. doi: 10.1007/s00467-013-2544-2. [DOI] [PubMed] [Google Scholar]
- 39.Kapoor K., Gupta S. Malarial acute kidney injury in a paediatric intensive care unit. Trop Doctor. 2012;42:203–205. doi: 10.1258/td.2012.120196. [DOI] [PubMed] [Google Scholar]
- 40.Kaul A., Sharma R.K., Tripathi R. Spectrum of community-acquired acute kidney injury in India: a retrospective study. Saudi J Kidney Dis Transpl. 2012;23:619–628. [PubMed] [Google Scholar]
- 41.Krishnamurthy S., Mondal N., Narayanan P. Incidence and etiology of acute kidney injury in southern India. Indian J Pediatr. 2013;80:183–189. doi: 10.1007/s12098-012-0791-z. [DOI] [PubMed] [Google Scholar]
- 42.Krishnamurthy S., Narayanan P., Prabha S. Clinical profile of acute kidney injury in a pediatric intensive care unit from Southern India: a prospective observational study. Indian J Crit Care Med. 2013;17:207–213. doi: 10.4103/0972-5229.118412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra S.K., Dietz K., Mohanty S. Influence of acute renal failure in patients with cerebral malaria - a hospital-based study from India. Trop Doctor. 2007;37:103–104. doi: 10.1177/004947550703700216. [DOI] [PubMed] [Google Scholar]
- 44.Prakash J., Singh T.B., Ghosh B. Changing epidemiology of community-acquired acute kidney injury in developing countries: analysis of 2405 cases in 26 years from eastern India. Clin Kidney J. 2013;6:150–155. doi: 10.1093/ckj/sfs178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saravu K., Rishikesh K., Parikh C.R. Risk factors and outcomes stratified by severity of acute kidney injury in malaria. PloS One. 2014;9:e90419. doi: 10.1371/journal.pone.0090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S.K., Manandhar D.N., Khanal B. Malarial nephropathy in a tertiary care setup–an observational study. Nepal Med Coll J. 2011;13:123–127. [PubMed] [Google Scholar]
- 47.Shukla V.S., Singh R.G., Rathore S.S. Outcome of malaria-associated acute kidney injury: a prospective study from a single center. Ren Fail. 2013;35:801–805. doi: 10.3109/0886022X.2013.800808. [DOI] [PubMed] [Google Scholar]
- 48.Thaha M., Pranawa, Yogiantoro M. Acute renal failure in a patient with severe malaria and dengue shock syndrome. Clin Nephrol. 2008;70:427–430. doi: 10.5414/cnp70427. [DOI] [PubMed] [Google Scholar]
- 49.Maitland K., Kiguli S., Opoka R.O. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 50.Molyneux E.M., Maitland K. Intravenous fluids–getting the balance right. N Engl J Med. 2005;353:941–944. doi: 10.1056/NEJMe058135. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal R.S., Mishra V.V., Jasani A.F. Acute renal failure in pregnancy: our experience. Saudi J Kidney Dis Transpl. 2014;25:450–455. doi: 10.4103/1319-2442.128621. [DOI] [PubMed] [Google Scholar]
- 52.Alaro D., Bashir A., Musoke R. Prevalence and outcomes of acute kidney injury in term neonates with perinatal asphyxia. African Health Sci. 2014;14:682–688. doi: 10.4314/ahs.v14i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Askenazi D.J., Griffin R., McGwin G. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009;24:991–997. doi: 10.1007/s00467-009-1133-x. [DOI] [PubMed] [Google Scholar]
- 54.Bouaziz M., Chaari A., Turki O. Acute renal failure and pregnancy: a seventeen-year experience of a Tunisian intensive care unit. Ren Fail. 2013;35:1210–1215. doi: 10.3109/0886022X.2013.819767. [DOI] [PubMed] [Google Scholar]
- 55.Dambal A., Lakshmi K.S., Gorikhan G. Obstetric acute kidney injury: a three year experience at a medical college hospital in north karnataka, India. J Clin Diagn Res. 2015;9 doi: 10.7860/JCDR/2015/12897.5634. Oc01-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eswarappa M., Madhyastha P.R., Puri S. Postpartum acute kidney injury: a review of 99 cases. Ren Fail. 2016;38:889–893. doi: 10.3109/0886022X.2016.1164015. [DOI] [PubMed] [Google Scholar]
- 57.Kamal E.M., Behery M.M., Sayed G.A. RIFLE classification and mortality in obstetric patients admitted to the intensive care unit with acute kidney injury: a 3-year prospective study. Reprod Sci. 2014;21:1281–1287. doi: 10.1177/1933719114525277. [DOI] [PubMed] [Google Scholar]
- 58.Prakash J., Niwas S.S., Parekh A. Acute kidney injury in late pregnancy in developing countries. Ren Fail. 2010;32:309–313. doi: 10.3109/08860221003606265. [DOI] [PubMed] [Google Scholar]
- 59.Prakash J., Pant P., Prakash S. Changing picture of acute kidney injury in pregnancy: study of 259 cases over a period of 33 years. Indian J Nephrol. 2016;26:262–267. doi: 10.4103/0971-4065.161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perazella M.A. Diagnosing drug-induced AIN in the hospitalized patient: a challenge for the clinician. Clin Nephrol. 2014;81:381–388. doi: 10.5414/CN108301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perazella M.A., Markowitz G.S. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. 2010;6:461–470. doi: 10.1038/nrneph.2010.71. [DOI] [PubMed] [Google Scholar]
- 62.Xu X., Nie S., Liu Z. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10:1510–1518. doi: 10.2215/CJN.02140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chugh K.S., Jha V., Sakhuja V. Acute renal cortical necrosis–a study of 113 patients. Ren Fail. 1994;16:37–47. doi: 10.3109/08860229409044846. [DOI] [PubMed] [Google Scholar]
- 64.Prakash J., Pant P., Singh A.K. Renal cortical necrosis is a disappearing entity in obstetric acute kidney injury in developing countries: our three decade of experience from India. Ren Fail. 2015;37:1185–1189. doi: 10.3109/0886022X.2015.1062340. [DOI] [PubMed] [Google Scholar]
- 65.Kanjanabuch T., Sitprija V. Snakebite nephrotoxicity in Asia. Semin Nephrol. 2008;28:363–372. doi: 10.1016/j.semnephrol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Pinho F.M., Yu L., Burdmann E.A. Snakebite-induced acute kidney injury in Latin America. Semin Nephrol. 2008;28:354–362. doi: 10.1016/j.semnephrol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Luyckx V.A., Steenkamp V., Stewart M.J. Acute renal failure associated with the use of traditional folk remedies in South Africa. Ren Fail. 2005;27:35–43. [PubMed] [Google Scholar]
- 68.Mehta R.L., Awdishu L., Davenport A. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88:226–234. doi: 10.1038/ki.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schetz M., Dasta J., Goldstein S. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11:555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- 70.Arendse C., Okpechi I., Swanepoel C. Acute dialysis in HIV-positive patients in Cape Town, South Africa. Nephrology (Carlton) 2011;16:39–44. doi: 10.1111/j.1440-1797.2010.01358.x. [DOI] [PubMed] [Google Scholar]
- 71.Arendse C.G., Wearne N., Okpechi I.G. The acute, the chronic and the news of HIV-related renal disease in Africa. Kidney Int. 2010;78:239–245. doi: 10.1038/ki.2010.155. [DOI] [PubMed] [Google Scholar]
- 72.Herlitz L.C., Mohan S., Stokes M.B. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78:1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 73.Agarwala R., Mohan S., Herlitz L.C. The case: 41-year-old HIV patient with proteinuria and progressive renal dysfunction. Tenofovir toxicity. Kidney Int. 2010;77:475–476. doi: 10.1038/ki.2009.486. [DOI] [PubMed] [Google Scholar]
- 74.Hodgson S.H., Angus B.J. Malaria: fluid therapy in severe disease. BMJ Clin Evid. 2016:2016. [PMC free article] [PubMed] [Google Scholar]
- 75.Mendez A., Gonzalez G. Dengue haemorrhagic fever in children:ten years of clinical experience [Spanish] Biomedica. 2003;23:180–193. [PubMed] [Google Scholar]
- 76.Macedo E.M., Malhotra R., Bouchard E. Episodes and duration of oliguria affect outcomes in critically-ill patients. Kidney Int. 2011;80:760–767. doi: 10.1038/ki.2011.150. [DOI] [PubMed] [Google Scholar]
- 77.Lehner G.F., Forni L.G., Joannidis M. Oliguria and biomarkers of acute kidney injury: star struck lovers or strangers in the night? Nephron. 2016;134:183–190. doi: 10.1159/000447979. [DOI] [PubMed] [Google Scholar]
- 78.Schetz M., Hoste E. Understanding oliguria in the critically ill [epub ahead of print] Intensive Care Med. 2016 Sept 12 doi: 10.1007/s00134-016-4537-7. [DOI] [PubMed] [Google Scholar]
- 79.Schortgen F., Schetz M. Does this critically ill patient with oliguria need more fluids, a vasopressor, or neither? [epub ahead of print] Intensive Care Med. 2017 Mar 14 doi: 10.1007/s00134-017-4744-x. http://dx.doi.org/10.1007/s00134-017-4744-x [DOI] [PubMed] [Google Scholar]
- 80.van der Zee E.N., Egal M., Gommers D. Targeting urine output and 30-day mortality in goal-directed therapy: a systematic review with meta-analysis and meta-regression. BMC Anesthesiol. 2017;17:22. doi: 10.1186/s12871-017-0316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Badawy A.A., Makar S., Abdel-Razek A.R. Incidence and risk factors of acute kidney injury among the critically ill neonates. Saudi J Kidney Dis Transpl. 2015;26:549–555. doi: 10.4103/1319-2442.157362. [DOI] [PubMed] [Google Scholar]
- 82.Garisto C., Favia I., Ricci Z. Acute kidney injury in the pediatric population. Contrib Nephrol. 2010;165:345–356. doi: 10.1159/000313776. [DOI] [PubMed] [Google Scholar]
- 83.Shalaby M., Khathlan N., Safder O. Outcome of acute kidney injury in pediatric patients admitted to the intensive care unit. Clin Nephrol. 2014;82:379–386. doi: 10.5414/cn108348. [DOI] [PubMed] [Google Scholar]
- 84.Esson M.L., Schrier R.W. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137:744–752. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 85.Lameire N., Van Biesen W., Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 86.Bagshaw S.M., Langenberg C., Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48:695–705. doi: 10.1053/j.ajkd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 87.Langenberg C., Wan L., Bagshaw S.M. Urinary biochemistry in experimental septic acute renal failure. Nephrol Dialysis Transpl. 2006;21:3389–3397. doi: 10.1093/ndt/gfl541. [DOI] [PubMed] [Google Scholar]
- 88.Nanji A.J. Increased fractional excretion of sodium in prerenal azotemia:need for careful interpretation. Clin Chem. 1981;27:1314–1315. [PubMed] [Google Scholar]
- 89.Carvounis C.P., Nisar S., Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–2229. doi: 10.1046/j.1523-1755.2002.00683.x. [DOI] [PubMed] [Google Scholar]
- 90.Pepin M.N., Bouchard J., Legault L. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50:566–573. doi: 10.1053/j.ajkd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Bagshaw S.M., Haase M., Haase-Fielitz A. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol Dial Transplant. 2012;27:582–588. doi: 10.1093/ndt/gfr331. [DOI] [PubMed] [Google Scholar]
- 92.Fogazzi GB. Urianalysis. In: Floege J, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology, 4th ed. vol. 1. St. Louis, MO: Elsevier Saunders; 2010, pp. 39–55.
- 93.Perazella M.A. The urine sediment as a biomarker of kidney disease. Am J Kidney Dis. 2015;66:748–755. doi: 10.1053/j.ajkd.2015.02.342. [DOI] [PubMed] [Google Scholar]
- 94.Mohapatra M.K., Behera A.K., Karua P.C. Urinary bile casts in bile cast nephropathy secondary to severe falciparum malaria. Clin Kidney J. 2016;9:644–648. doi: 10.1093/ckj/sfw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klahr S., Miller S.B. Acute oliguria. N Engl J Med. 1998;338:671–675. doi: 10.1056/NEJM199803053381007. [DOI] [PubMed] [Google Scholar]
- 96.Chawla L.S., Dommu A., Berger A. Urinary sediment cast scoring index for acute kidney injury: a pilot study. Nephron Clin Pract. 2008;110:c145–c150. doi: 10.1159/000166605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muriithi A.K., Nasr S.H., Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8:1857–1862. doi: 10.2215/CJN.01330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perazella M.A., Bomback A.S. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8:1841–1843. doi: 10.2215/CJN.08620813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bagshaw S.M., Bennett M., Devarajan P. Urine biochemistry in septic and non-septic acute kidney injury: a prospective observational study. J Crit Care. 2013;28:371–378. doi: 10.1016/j.jcrc.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schönermarck U., Kehl K., Samtleben W. Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis. 2008;51:870–871. doi: 10.1053/j.ajkd.2008.01.032. author reply 871. [DOI] [PubMed] [Google Scholar]
- 101.Schetz M., Darmon M. Measuring acute kidney injury around the world: are we using the right thermometer (and adequately)? Intensive Care Med. 2015;41:1857–1859. doi: 10.1007/s00134-015-3972-1. [DOI] [PubMed] [Google Scholar]
- 102.Waikar S.S., Bonventre J.V. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Libório A.B., Macedo E., Bayas de Queiroz R.E. Kidney disease improving global outcomes or creatinine kinetics criteria in acute kidney injury: a proof of concept study. Nephrol Dialysis Transpl. 2013;28:2779–2787. doi: 10.1093/ndt/gft375. [DOI] [PubMed] [Google Scholar]
- 104.Lin J., Fernandez H., Shashaty M.G.S. False-positive rate of AKI using consensus creatinine–based criteria. Clin J Am Soc Nephrol. 2015;10:1723–1731. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dennen P., Parikh C. Biomarkers of acute kidney injury: can we replace serum creatinine? Clin Nephrol. 2007;68:269–278. doi: 10.5414/cnp68269. [DOI] [PubMed] [Google Scholar]
- 106.Coresh J., Astor B.C., McQuillan G. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 107.Fuller N.J., Elia M. Factors influencing the production of creatinine: implications for the determination and interpretation of urinary creatinine and creatine in man. Clin Chim Acta. 1988;175:199–210. doi: 10.1016/0009-8981(88)90096-4. [DOI] [PubMed] [Google Scholar]
- 108.Baron J.M., Cheng X.S., Bazari H. Enhanced creatinine and estimated glomerular filtration rate reporting to facilitate detection of acute kidney injury. Am J Clin Pathol. 2015;143:42–49. doi: 10.1309/AJCP05XBCQPHTLGQ. [DOI] [PubMed] [Google Scholar]
- 109.Cerda J., Cerda M., Kilcullen P. In severe acute kidney injury, a higher serum creatinine is paradoxically associated with better patient survival. Nephrol Dial Transplant. 2007;22:2781–2784. doi: 10.1093/ndt/gfm395. [DOI] [PubMed] [Google Scholar]
- 110.Ostermann M. Diagnosis of acute kidney injury: Kidney Disease Improving Global Outcomes criteria and beyond. Curr Opin Crit Care. 2014;20:581–587. doi: 10.1097/MCC.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 111.Palevsky P.M., Liu K.D., Brophy P.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 112.Vijayan A., Faubel S., Askenazi D.J. Clinical Use of the urine biomarker [TIMP-2] x [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68:19–28. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Macedo E., Bouchard J., Soroko S.H. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stevens LA, Shastry S, Levey AS. Assessment of Renal Function. In: Floege J, Johnson RJ, Feehally, eds. Comprehensive Clinical Nephrology, 4th ed, vol. 1. St. Louis, MO: Elsevier Saunders; 2010, pp. 31–38.
- 115.Yang L, Bonventre JV. Diagnosis and clinical evaluation of acute kidney injury. In: Floege J, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology, 4th ed., vol. 1. St. Louis, MO: Elsevier Saunders, 2010, pp. 821–829.
- 116.Calice-Silva V., Vieira M.A., Raimann J.G. Saliva urea nitrogen dipstick - a novel bedside diagnostic tool for acute kidney injury. Clin Nephrol. 2014;82:358–366. doi: 10.5414/CN108370. [DOI] [PubMed] [Google Scholar]
- 117.Okura T., Jotoku M., Irita J. Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol. 2010;14:584–588. doi: 10.1007/s10157-010-0334-8. [DOI] [PubMed] [Google Scholar]
- 118.Shephard M.D. Point-of-care testing and creatinine measurement. Clin Biochem Rev. 2011;32:109–114. [PMC free article] [PubMed] [Google Scholar]
- 119.Calzavacca P., Tee A., Licari E. Point-of-care measurement of serum creatinine in the intensive care unit. Ren Fail. 2012;34:13–18. doi: 10.3109/0886022X.2011.623558. [DOI] [PubMed] [Google Scholar]
- 120.Cao D., Maynard S., Mitchell A.M. Point of care testing provides an accurate measurement of creatinine, anion gap, and osmolal gap in ex-vivo whole blood samples with nitromethane. Clin Toxicol (Philadelphia) 2014;52:611–617. doi: 10.3109/15563650.2014.918628. [DOI] [PubMed] [Google Scholar]
- 121.Haneder S., Gutfleisch A., Meier C. Evaluation of a handheld creatinine measurement device for real-time determination of serum creatinine in radiology departments. World J Radiol. 2012;4:328–334. doi: 10.4329/wjr.v4.i7.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Minnings K., Kerns E., Fiore M. Chronic kidney disease prevalence in Rivas, Nicaragua: use of a field device for creatinine measurement. Clin Biochem. 2015;48:456–458. doi: 10.1016/j.clinbiochem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 123.Raimann J.G., Calice-Silva V., Thijssen S. Saliva urea nitrogen continuously reflects blood urea nitrogen after acute kidney injury diagnosis and management: longitudinal observational data from a collaborative, international, prospective, multicenter study. Blood Purif. 2016;42:64–72. doi: 10.1159/000445041. [DOI] [PubMed] [Google Scholar]
- 124.You J.S., Chung Y.E., Park J.W. The usefulness of rapid point-of-care creatinine testing for the prevention of contrast-induced nephropathy in the emergency department. Emerg Med J. 2013;30:555–558. doi: 10.1136/emermed-2012-201285. [DOI] [PubMed] [Google Scholar]
- 125.Burtonwood C., Pearson S., Bennett W. National Health Service; London: 2010. Point of Care Tests for the Measurement of Blood Creatinine. CEP 10042. Available at: http://www.calxis.com/common/comm_down.php?file=/upload/pds/12994949206E2B.pdf&org=CEP10042%5B1%5D.pdf. Accessed September 27, 2016. [Google Scholar]
- 126.Lunyera J., Kilonzo K., Lewington A. Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis. 2016;67:834–840. doi: 10.1053/j.ajkd.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 127.Vaidya V.S., Ford G.M., Waikar S.S. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohamed F., Endre Z.H., Buckley N.A. Role of biomarkers of nephrotoxic acute kidney injury in deliberate poisoning and envenomation in less developed countries. Br J Clin Pharmacol. 2015;80:3–19. doi: 10.1111/bcp.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mehta R.L., Kellum J.A., Shah S.V. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Molitoris B.A., Levin A., Warnock D.G. Improving outcomes of acute kidney injury: report of an initiative. Nat Clin Pract Nephrol. 2007;3:439–442. doi: 10.1038/ncpneph0551. [DOI] [PubMed] [Google Scholar]
- 131.Kellum J.A., Bellomo R., Ronco C. Classification of acute kidney injury using RIFLE: what's the purpose? Crit Care Med. 2007;35:1983–1984. doi: 10.1097/01.CCM.0000277518.67114.F8. [DOI] [PubMed] [Google Scholar]
- 132.Ricci Z., Cruz D., Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 133.Murray P.T., Mehta R.L., Shaw A. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative Consensus Conference. Kidney Int. 2014;85:513–521. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cruz D.N., Bagshaw S.M., Maisel A. Use of biomarkers to assess prognosis and guide management of patients with acute kidney injury. Contrib Nephrol. 2013;182:45–64. doi: 10.1159/000349965. [DOI] [PubMed] [Google Scholar]
- 135.Endre Z.H., Pickering J.W., Walker R.J. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79:1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lameire N.H., Vanholder R.C., Van Biesen W.A. How to use biomarkers efficiently in acute kidney injury. Kidney Int. 2011;79:1047–1050. doi: 10.1038/ki.2011.21. [DOI] [PubMed] [Google Scholar]
- 137.Mehta R.L. Biomarker explorations in acute kidney injury: the journey continues. Kidney Int. 2011;80:332–334. doi: 10.1038/ki.2011.181. [DOI] [PubMed] [Google Scholar]
- 138.Moore E., Bellomo R. Novel biomarkers of acute kidney injury: ready for clinical application? Curr Opin Crit Care. 2010;16:523–525. doi: 10.1097/MCC.0b013e32834008ea. [DOI] [PubMed] [Google Scholar]
- 139.Wheeler D.S., Devarajan P., Ma Q. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chu R., Li C., Wang S. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol. 2014;9:1175–1182. doi: 10.2215/CJN.06150613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zuk A., Bonventre J.V. Acute kidney injury. Ann Rev Med. 2016;67:293–307. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Skrypnyk N.I., Siskind L.J., Faubel S. Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am J Physiol Renal Physiol. 2016;310:F972–F984. doi: 10.1152/ajprenal.00552.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lameire N., Van Massenhove J., Van Biesen W. What is the difference between prerenal and renal acute kidney injury? Acta Clin Belgica. 2012;67:309–314. doi: 10.2143/ACB.67.5.2062681. [DOI] [PubMed] [Google Scholar]
- 144.Bagshaw S.M., Uchino S., Bellomo R. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 145.Bellomo R., Wan L., Langenberg C. Septic acute kidney injury: the glomerular arterioles. Contrib Nephrol. 2011;174:98–107. doi: 10.1159/000329246. [DOI] [PubMed] [Google Scholar]
- 146.Bellomo R., Wan L., Langenberg C. Septic acute kidney injury: new concepts. Nephron Exp Nephrol. 2008;109:e95–e100. doi: 10.1159/000142933. [DOI] [PubMed] [Google Scholar]
- 147.Doi K., Leelahavanichkul A., Yuen P.S. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Langenberg C., Bagshaw S.M., May C.N. The histopathology of septic acute kidney injury: a systematic review. Crit Care. 2008;12:R38. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bandari J., Fuller T.W., Turner Іі R.M., D'Agostino L.A. Renal biopsy for medical renal disease: indications and contraindications. Can J Urol. 2016;23:8121–8126. [PubMed] [Google Scholar]
- 150.Fiorentino M., Bolignano D., Tesar V. Renal biopsy in 2015–from epidemiology to evidence-based indications. Am J Nephrol. 2016;43:1–19. doi: 10.1159/000444026. [DOI] [PubMed] [Google Scholar]
- 151.Hogan J.J., Mocanu M., Berns J.S. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11:354–362. doi: 10.2215/CJN.05750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kilcoyne A., Gervais D.A. Kidney, ureter, and bladder biopsy. Tech Vasc Interv Radiol. 2016;19:237–244. doi: 10.1053/j.tvir.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 153.Madaio M.P. Renal biopsy. Kidney Int. 1990;38:529–543. doi: 10.1038/ki.1990.236. [DOI] [PubMed] [Google Scholar]
- 154.Moroni G., Depetri F., Ponticelli C. Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmunity. 2016;74:27–40. doi: 10.1016/j.jaut.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 155.Okpechi I.G., Ameh O.I., Bello A.K. Epidemiology of histologically proven glomerulonephritis in Africa: a systematic review and meta-analysis. PloS One. 2016;11:e0152203. doi: 10.1371/journal.pone.0152203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Visconti L., Cernaro V., Ricciardi C.A. Renal biopsy: still a landmark for the nephrologist. World J Nephrol. 2016;5:321–327. doi: 10.5527/wjn.v5.i4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fuiano G., Mazza G., Comi N. Current indications for renal biopsy: a questionnaire-based survey. Am J Kidney Dis. 2000;35:448–457. doi: 10.1016/s0272-6386(00)70197-1. [DOI] [PubMed] [Google Scholar]
- 158.Iseki K., Miyasato F., Uehara H. Outcome study of renal biopsy patients in Okinawa. Japan. Kidney Int. 2004;66:914–919. doi: 10.1111/j.1523-1755.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 159.Richards N.T., Darby S., Howie A.J. Knowledge of renal histology alters patient management in over 40% of cases. Nephrol Dial Transplant. 1994;9:1255–1259. [PubMed] [Google Scholar]
- 160.Cohen A.H., Nast C.C., Adler S.G. Clinical utility of kidney biopsies in the diagnosis and management of renal disease. Am J Nephrol. 1989;9:309–315. doi: 10.1159/000167986. [DOI] [PubMed] [Google Scholar]
- 161.Kitterer D., Gurzing K., Segerer S. Diagnostic impact of percutaneous renal biopsy. Clin Nephrol. 2015;84:311–322. doi: 10.5414/CN108591. [DOI] [PubMed] [Google Scholar]
- 162.Bouchard J., Acharya A., Cerda J. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324–1331. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raghavan R., Eknoyan G. Acute interstitial nephritis - a reappraisal and update. Clin Nephrol. 2014;82:149–162. doi: 10.5414/CN108386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gonzalez E., Gutierrez E., Galeano C. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73:940–946. doi: 10.1038/sj.ki.5002776. [DOI] [PubMed] [Google Scholar]
- 165.Couser W.G. Rapidly progressive glomerulonephritis: classification, pathogenetic mechanisms, and therapy. Am J Kidney Dis. 1988;11:449–464. doi: 10.1016/s0272-6386(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 166.Uwaezuoke S. Pediatric acute kidney injury in the developing world: how realizable is the goal of the Zero-Preventable-Deaths by 2025 initiative? Int J Nephrol Kidney Fail. 2015;2.1:1–4. [Google Scholar]
- 167.Isnard Bagnis C., Deray G., Baumelou A. Herbs and the kidney. Am J Kidney Dis. 2004;44:1–11. doi: 10.1053/j.ajkd.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 168.Sitprija V. Overview of tropical nephrology. Semin Nephrol. 2003;23:3–11. doi: 10.1053/snep.2003.50000. [DOI] [PubMed] [Google Scholar]
- 169.Luyckx V.A. Nephrotoxicity of alternative medicine practice. Adv Chronic Kidney Dis. 2012;19:129–141. doi: 10.1053/j.ackd.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 170.Luyckx V.A., Naicker S. Acute kidney injury associated with the use of traditional medicines. Nat Clin Pract Nephrol. 2008;4:664–671. doi: 10.1038/ncpneph0970. [DOI] [PubMed] [Google Scholar]
- 171.Hofer J., Giner T., Safouh H. Diagnosis and treatment of the hemolytic uremic syndrome disease spectrum in developing regions. Semin Thromb Hemostasis. 2014;40:478–486. doi: 10.1055/s-0034-1376154. [DOI] [PubMed] [Google Scholar]
- 172.Liborio A.B., Braz M.B., Seguro A.C. Endothelial glycocalyx damage is associated with leptospirosis acute kidney injury. Am J Trop Med Hygiene. 2015;92:611–616. doi: 10.4269/ajtmh.14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seguro A.C., Andrade L. Pathophysiology of leptospirosis. Shock. 2013;39 Suppl 1:17–23. doi: 10.1097/SHK.0b013e31828fae49. [DOI] [PubMed] [Google Scholar]
- 174.Abdulkader R.C., Silva M.V. The kidney in leptospirosis. Pediatr Nephrol. 2008;23:2111–2120. doi: 10.1007/s00467-008-0811-4. [DOI] [PubMed] [Google Scholar]
- 175.Langston C.E., Heuter K.J. Leptospirosis. A re-emerging zoonotic disease. Veterin Clin N Am Small Animal Pract. 2003;33:791–807. doi: 10.1016/s0195-5616(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 176.Visith S., Kearkiat P. Nephropathy in leptospirosis. J Postgrad Med. 2005;51:184–188. [PubMed] [Google Scholar]
- 177.Warnock D.G., Powell T.C., Siew E.D. Serum creatinine trajectories for community- versus hospital-acquired acute kidney injury. Nephron. 2016;134:177–182. doi: 10.1159/000447757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ADQI participants’ names and affiliations.