Abstract
This study describes the development of a microfluidic biosensor called the iterative mechanical characteristics (iMECH) analyzer which enables label-free biomechanical profiling of individual cells for distinction between metastatic and non-metastatic human mammary cell lines. Previous results have demonstrated that pulsed mechanical nanoindentation can modulate the biomechanics of cells resulting in distinctly different biomechanical responses in metastatic and non-metastatic cell lines. The iMECH analyzer aims to move this concept into a microfluidic, clinically more relevant platform. The iMECH analyzer directs a cyclic deformation regimen by pulling cells through a test channel comprised of narrow deformation channels and interspersed with wider relaxation regions which together simulate a dynamic microenvironment. The results of the iMECH analysis of human breast cell lines revealed that cyclic deformations produce a resistance in non-metastatic 184A1 and MCF10A cells as determined by a drop in their average velocity in the iterative deformation channels after each relaxation. In contrast, metastatic MDA-MB-231 and MDA-MB-468 cells exhibit a loss of resistance as measured by a velocity raise after each relaxation. These distinctive modulatory mechanical responses of normal-like non-metastatic and metastatic cancer breast cells to the pulsed indentations paradigm provide a unique bio-signature. The iMECH analyzer represents a diagnostic microchip advance for discriminating metastatic cancer at the single-cell level.
Keywords: cell mechanics, single-cell analysis, microfluidics chip, breast cancer
TOC Image
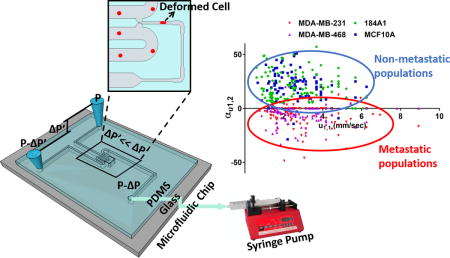
Introduction
Mechanical factors can direct cellular growth, function and homeostasis, but also cause or contribute to diseases such as cancer. The biomechanical properties of cells are modulated in response to their environmental factors and are thereby linked to physiological and pathophysiological functions.1 To date, biomechanical properties have gained broad acceptance in cell biology as a highly sensitive, label-free, non-destructive method for determining the health state of cell without the use of fluorescent, magnetic, or other cell markers. The biomechanical properties of cancer cells are constantly changing during progression of tumors to more aggressive and metastatic type2 and progression is accompanied by alterations in the levels of expression of biochemical factors and regulators of cytoskeletal organization, which impact cell migration, adherence, and invasion.3, 4 Thus, the ability to characterize the biomechanical nature of a cell could provide novel insights into how cells receive and integrate regulatory signals from the surrounding environment which can impact disease progression diagnosis and treatment. There are currently different instruments and methods available for assessing cell biomechanical properties.5, 6 There have been a handful of reports quantifying the changes take place in cellular biomechanical properties during the metastatic process.6 In general, the progression of cancer is associated with an alteration of the cell structure which in turn causes transformed cells to be softer and hence more deformable than their healthier counterparts to facilitate metastasis.7–9 The investigations have also showed that reduced surface friction of cancer cells may play another important role in further facilitating the motility.10, 11
The development of microfluidic devices for biomarker detection and point-of-care disease diagnosis has been on the rise because of their remarkable features including simplicity, low-cost, and high-speed.12–14 Among its potential uses for different disease diagnosis, prototypes to elucidate cancer cell and tumor function have gained tremendous importance.15, 16 Microfluidic-based analyzers for cell biomechanical screening and mechanophenotyping have promised a higher throughput compared to conventional tools and emerged as a clinically more relevant approach to single-cell analysis.17–20 As an important note, single-cell level analysis in cancer diagnosis is a critical need in light of the knowledge that tumor masses are comprised of a heterogeneous mixture of cells.21 Single-cell level analysis can significantly contribute to the evolution in the diagnostic and prognostic decisions to cancer patients.22, 23 Currently, several microfluidic constriction channel based analyzers have been developed for quantitative biomechanical characterizations of various cell types including tumor cells, red blood cells (RBCs), and white blood cells (WBCs). (Table 1)
Table 1.
Summary of previous works on single-cell biomechanical characterizations via microfluidic constriction channel based analyzers
| Group | Targeted Cell Lines | Quantified Parameters | Method | Key Observation |
|---|---|---|---|---|
| Erickson et al.24, 25 | MDA-MB-231 metastatic breast | Transit time | Multi-staged serial invasion channels (M.U.S.I.C.) device | The initial transit requires the longest time than the subsequent transits |
| Lim et al.26 | MCF10-A benign, MCF-7 non-metastatic breast | Entry time, elongation index, transit velocity | A constriction microchannel | MCF10-A have longer entry but not travel times than MCF-7 with similar sizes |
| Vanapalli et al.27 | Cancerous A172/1321N1, normal L0329/L036 glial | Elongation, pressure drop, speed, entry time | A microfluidic cell squeezer | Brain tumor cells take a longer time to squeeze and migrate more slowly than benign cells |
| Agah et al.28 | MDA-MB-231 metastatic breast | Entry time, travel time | A constriction with two embedded Cr/Au electrodes | Nanoparticles-treated cells are significantly stiffer than untreated cells |
| Fabry et al.29 | K562, MDA-MB-231, U2OS, HEK293T | Flow speed, cell deformation, entry time | Micro-constriction parallel arrays | A power law correlates entry time to driving pressure and cell size, and estimates cell elasticity & fluidity |
| Manalis et al.10 | MEF fibroblast, cancer (TMet, TnonMet, H1650, H1975, HCC827), L1210 leukemia | Cell size/mass, entry velocity, transit velocity | A suspended microchannel resonator (SMR) | Changing the deformability of the cell alters the entry velocity, whereas changing the surface friction alters the transit velocity |
| Liu et al.30 | Benign MCF-10A, metastatic MDA-MB-231, RPE epithelium | Cortical tension, Young’s modulus | A microfluidic pipette array (μFPA) | The device captures stiffness of single cells and mechanical gating of mechanosensitive channels |
| Iqbal et al.31 | Normal urothelial and T24 cancer bladder | Peak amplitude, translocation time | A 20 μm pore on oxide membrane | Tumor cells showed one order of magnitude shorter translocation time |
| Han et al.32 | Breast cancer MCF-7, benign MCF-10A | Cell diameter, passage time | A micro-channel with real-time controllable gap | Size-independent cell deformability cytometry causes a much smaller degree of variation in passage times |
| Jensen et al.33 | Hela cervical cancer | Cell volume, transit time | A constriction channel with electrodes | Hela cells have a faster transit time after treatment with latrunculin A and cytochalasin B |
| Sun et al.34, 35 | MC-3T3 osteoblast, MLO-Y4 osteocyte | Transit time, cell elongation, aspiration length | A constriction channel with two Ag/AgCl electrodes | Osteoblasts have a larger cell elongation and transit time compared with osteocytes |
| Sun et al.36 | Adult and Neonatal RBCs | Transit time | ˶ | Adult compared with neonatal RBCs have distinct and lower transit time |
| Han et al.37, 38 | Plasmodium falciparum infected RBCs | Transit velocity | A channel with triangular pillar array | Transit velocity of malaria infected RBCs decreases by 50% after treatment |
| Ma et al.39–44 | Malaria infected/oxidized RBCs | Cortical tension Normalized position | A funnel channel chain pipette aspiration | Malaria infected/damaged RBCs show increased cortical tension and decreased deformability |
| Chiu et al.45 | P. falciparum infected RBCs | Channel blockage | A constriction channel | Malaria decreases the deformability of infected RBCs |
| Rathod et al.46 | P. falciparum infected RBCs | MCD: Minimum cylindrical diameter | Wedge-shaped micro-channels | Malaria infected RBCs show larger MCD |
| Suresh et al.47 | Healthy RBCs | Stretch ratio, transit velocity, entrance/traversal/exit times | A pressure-control constriction | Experimental–computational analysis of the flow dynamics of RBCs |
| Kaneko et al.48 | RBCs of healthy and a diabetes patient | Dimensionless stiffness DI index | A 4.0 μm microchannel | The proposed DI can significantly highlight the differences in stiffness of the healthy and diseased cells |
| Russell et al.49 | P. vivax and P. falciparum RBCs | Normalized transit/recovery ratio | A 2.0 μm constriction | In contrast to P. falciparum-infeded, P. vivax-infected RBCs readily deform and recover a normal shape |
| Chang et al.50 | Normal RBCs and cancerous (leukemia) | Transit velocity, elongation index, shape recovery time | A PDMS microchannel under optical pressure | A 3D distribution demonstrates the differences in deformability between two RBC types |
| Stone et al.51 | Healthy RBCs, Healthy WBCs | Pressure-drop variation | A device with twin (comparator and test) channels | The device allows differentiation of cells with different mechanical properties or geometrical features |
| Theodoly et al.11, 52 | THP-1 monocytic (WBCs) leukemia | Fluorescence microscopy | A sequential constriction device | F-actin mainly contributes to cell stiffness and bleb formation |
| Theodoly et al.53 | ˶ | Loss modulus | ˶ | An apparent viscosity of single cells was estimated by numerical analysis |
| Fletcher et al.54, 55 | WBCs with sepsis and leukostasis | Transit time | Parallel capillary-like micro-channels | Transit time of blood cells correlates with symptoms of diseases |
| Kamm et al.56 | WBCs (neutrophils) | Entrance time, pseudopod projection time | A narrow PDMS capillary | Neutrophils deformation results in cytoskeletal remodeling, viscoelastic changes and pseudopod projection |
Nevertheless, most endeavors in cell biomechanical characterizations have typically utilized single-pulse transient mechanical stimulus for mechanophenotyping and cellular mechanical responses analysis.57, 58 These measurements reveal differences in the biomechanical properties of normal and cancer at the population level, but quantified parameters thus far defined cannot predict whether a given individual cell is malignant or normal. Accurate cell biomechanical characterization involves cell investigation in simulated realistic environments as living cells can sense external environmental loads and respond to them by adapting their structure.59 Some researchers have made attempts to mimic the physiological microenvironments of cells where they undergo dynamic mechanical stimuli. Particularly, cancer cells are exposed to continuous time-varying shear stresses as a result of the elevated interstitial fluid flow. These shear stresses can alter cancer cytophysiology in ways that support cancer cell invasiveness.60 Some studies have reported different responses of normal and cancer cells to such dynamic stressors even under artificial dynamic microenvironment shifts. As reported, cell stiffness of non-tumorigenic/normal cells increases in response to dynamic stress/strain. For instance, it has been shown that normal cells stiffen during tapping motions imposed by an atomic force microscopy (AFM) cantilever probe and these cells become less susceptible to deformation.61 In another study, rapid stretch of adherent normal endothelial cells resulted in a quick increase in actin lattice stiffness, and thus mechanical resistance.62 Furthermore, Mak et al. developed a microfluidic device and showed a decrease in the measured transit times of invasive MDA-MB-231 breast cells under a sequence of deformations through serial subnucleus-scaled constriction channels.24, 25 The conclusion from this work was that the MDA-MB-231 cells underwent mechanical compliance.
Our group has recently produced solid evidence indicating that metastatic and non-metastatic single cells respond differently to nanomechanical indentations applied to them in a pulsed mode.63 According to that study, the non-metastatic MCF10A and 184A1 cells increased their resistance against deformation while metastatic cells became slightly softer when subjected to a dynamic mechanical microenvironment. Our aim here is to transition principles gained by the AFM technique63 into a suitable single-cell analysis platform on a high-throughput microfluidic chip. To this end, the microfluidic iterative mechanical characteristics (iMECH) analyzer has been developed and its ability to reveal unique information about the health status of single cells is demonstrated.
Materials and Methods
Cell Culture and Treatment
The microfluidic iMECH analyzer’s performance as a biosensor was evaluated using four breast cancer cell lines including immortalized, non-tumorigenic, non-metastatic 184A1 and MCF10A cells and tumorigenic, metastatic MDA-MB-468 and MDA-MB-231 cells (All cell lines were purchased from ATCC; American Type Culture Collection, Manassas, VA). The standard culture medium for 184A1 cell growth was MEGM kit supplemented with 5 μg/ml transferrin (Sigma Aldrich, St. Louis, MO) and 1 ng/ml cholera toxin (Lonza, Basel, Switzerland); the gentamycin-amphotericin B was omitted. MCF10A cells were grown in F12:DMEM (Lonza, Basel, Switzerland) with penicillin-streptomycin (100 Units/ml), 2.5 mM L-glutamine, 20 ng/ml epidermal growth factor (EGF), 0.1 μg/ml cholera toxin (CT), 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, and 5% horse serum. MDA-MB-468 cells were grown in L-15 supplemented with 10% fetal bovine serum (FBS), 4 mM glutamine and penicillin-streptomycin (100 Units/ml). Finally, MDA-MB-231 cells were grown in F12:DMEM with 10% FBS, 4 mM glutamine and penicillin-streptomycin (100 Units/ml). The cells were suspended in culture medium at a concentration of 5×105 cells/mL for delivery into the iMECH analyzer.
Microchip Design and Fabrication
The iMECH analyzer consists of a multi-segmented test channel which mimics a dynamic deformation paradigm and a U-shaped delivery channel (200 μm-wide and 60 μm-deep). The test channel contains three deformation channels (6 μm-wide, 12 μm-deep, 500 μm-long) separated by two relaxation regions (30μm-wide, 30μm-deep, 100μm-long). The design of the iMECH analyzer is depicted schematically in Figure 1A. To deliver single cells to the test channel, a free flow of suspended cells in culture medium is established in the delivery channel by the height difference of medium in the reservoirs at the inlet/outlet ports. Single cells are trapped and pulled into the test channel by applying a negative pressure (ΔP=−150 Pa) via a syringe pump (Harvard apparatus, Holliston, MA) at the end of the test channel. A trapping mechanism adapted from a work by Tan et al.64 based on hydrodynamic resistance at the entrance of the test channel helps the single cell trapping. As cells pass through the test channel, they undergo a series of deformation and relaxation events as they pass through deformation and relaxation regions. The information about the modulatory biomechanical properties of single cells in response to this dynamic microenvironment in the microchip is collected, resulting in the single-cell iMECH bio-signature. The velocity of individual cells in the deformation channels is measured and used as an indicator of cell biomechanics. The iMECH analyzer enables the analysis of about 1 cell/sec without sacrificing the sensitivity of the system to discriminate between the biomechanical behavior of metastatic and non-metastatic cells.
Figure 1.
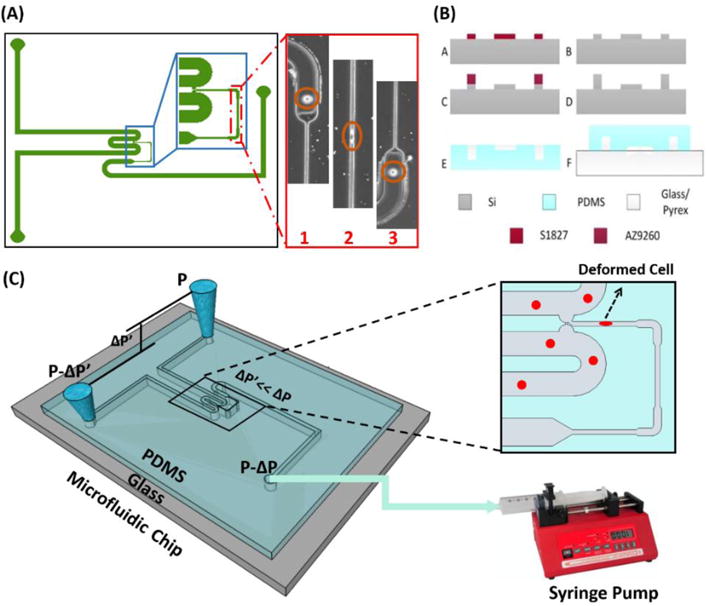
A) Illustration of design, B) fabrication process flow, and C) schematic image of setup and operation principle of the iMECH analyzer.
The iMECH analyzer was fabricated in the clean room facility. A master wafer was initially fabricated using a two-step etching process to obtain the shallow constriction channel and the deep delivery channel. Briefly, a thin photoresist (Shipley 1827) was spun coated on a silicon wafer and patterned using photolithography. A shallow etch was done using deep reactive ion etching (DRIE, Bosch, Germany) for a depth of 12 μm. The photoresist was stripped before a second round of photolithography using a thicker photoresist (AZ9260) to fabricate the delivery channel. The wafer was etched for 20 min in the DRIE to obtain an overall depth of 30 μm. Finally, the photoresist was stripped and the wafer was cleaned before a layer of saline was evaporated on the wafer to enable easy peel-off of polydimethylsiloxane (PDMS). PDMS prepolymer and curing agent were mixed at a ratio of 10:1, poured over the silicon master placed in an aluminum foil plate, degassed in a vacuum desiccators to remove air bubbles, and baked in a convection oven (15 min, 125°C). The device was allowed to cool, then peeled off from the master, diced and reservoir holes were punched through. At the end, the PDMS layer and a glass slide were concurrently treated with oxygen plasma in a plasma cleaner (Harrick Plasma, Plasma Cleaner) for 1 min and bonded together. The fabrication process of the iMECH analyzer is shown step by step in Figure 1B. A schematic of the iMECH setup and operation principles is shown in Figure 1C.
Data Acquisition and Analysis
For data acquisition, the device was mounted onto the Zeiss Axio Observer inverted microscope (Carl Zeiss, Jena, Germany). The video images of the cells passing through the test channel were recorded via Motion Xtra NX4-S3 high speed camera (IDT, Tallahassee, FL) with 5 GB memory and Motion Studio software at 500 frames per second. To examine and validate the reproducibility and consistency of the results, at least three separate tests were conducted for each cell population with at least n=100 cells from each sample. Within the same cell population, there was no more than 5% variation between the average measured parameters of any two reported test results. P-values between the different populations were calculated using two independent samples t-tests (α = 0.05). Results are presented as arithmetic mean ± standard error of the mean (SEM) using GraphPad Prism software.
Results and discussion
Cell Mechanical Characteristics in Micro-channels
The cell movement in the deformation channel is driven by a pressure gradient applied across two sides of the deformation channels. The two-dimensional plot of the pressure gradient in the microfluidic channels is simulated in COMSOL Multiphysics and is shown in Figure 2A. It can be seen that the pressure gradients across the narrow deformation channels are equal and about ΔP=−40 Pa. It implies that the cells are experiencing the same applied pulling pressure across each deformation channel. Importantly, 4 μm beads passed through the test channel showed equal traveling times in all three deformation channels. It indicates that the deformation channels were fabricated with an identical size and cross section and the beads experience an equal pressure gradient across them. Cell velocity in the deformation channels was measured visually and by a developed MATLAB code using the obtained image videos. A cell’s movement in the deformation channel can be separated into two phases as shown previously;48 during phase I, the cell moves with a non-constant velocity when undergoing a heavy deformation within the narrow deformation channel and during phase II, the cell velocity remains constant when reaching a steady state. Figure 2B shows a schematic profile of location versus time for a cell in a deformation channel.
Figure 2.
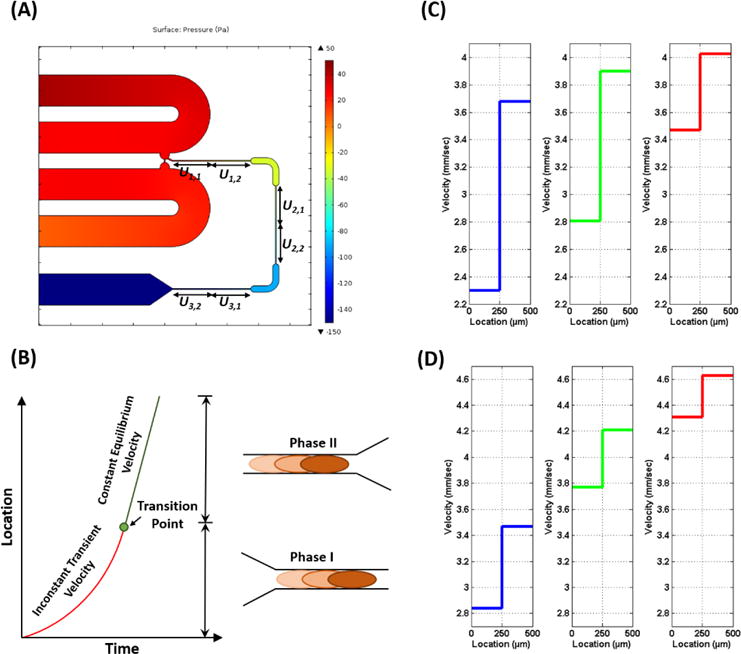
A) The plot of pressure gradient in the microfluidic channels. B) The plot of the location versus time for a single cell traveling through a deformation channel. The average transient velocity and equilibrium velocity in the iterative deformation channels for a typical (C) non-metastatic cell (MCF10A) and (D) metastatic cancer cell (MDA-MB-231) cell.
Analysis of the cell movements in the deformation channels revealed that the transition point between phase I (non-constant velocity) and phase II (constant velocity) always occurred in the first-half of the 500 μm deformation channels. For our analysis, we divided the deformation channels into two halves: the transient and equilibrium sections. The corresponding average velocities of transient and equilibrium sections (ui,1 and ui,2, respectively) of the deformation channels (i=1, 2, 3) are depicted in Figure 2A. The average transient and equilibrium velocities of the iterative deformation channels for a typical non-metastatic and metastatic cell are depicted in Figure 2C and 2D, respectively. The average velocities for the cells subjected to iterative mechanical deformations through the deformation channels revealed that the non-metastatic cells exhibit a velocity drop after each relaxation region once they enter to the next deformation channel as shown in Figure 2C. The drop in the average transient velocity of the non-metastatic cells after a recovery period afforded by passage through a wider channel interspersed between each deformation channel can be due to the effect of mechanical resistance in their structural characteristics in response to iterative deformations. However, this trend is reversed in the metastatic cells. After each relaxation region, the average transient velocity of the metastatic cells rises or remains constant as cells proceed to enter subsequent deformation channels (Figure 2D). It implies that the metastatic cells averagely exhibit a mechanical compliance with iterative deformations in their structure.
Cell iMECH Bio-Signature
The average transient and equilibrium velocity measurements of breast cell type populations are presented in Figure 3A. MDA-MB-231 and MDA-MB-468 metastatic cells showed 5% and 4% increases in the average velocities from u1,2 to u2,1 and also 4% and 4% increases from u2,2 to u3,1, respectively. Therefore, these data are suggestive that the representative metastatic cells slightly lose their mechanical resistance to travel through narrow channels in response to iterative deformations by increasing in their transient velocities after the first and second deformation channels. In contrast, the representative non-metastatic cells responded by decreasing in their transient velocities after each deformation channels. the average velocity of 184A1 and MCF10A cells decreased by ~21% and ~20% from u1,2 to u2,1 and ~8% and ~6% from u2,2 to u3,1, respectively. These data imply that non-metastatic cell behavior is the reverse of that seen in the metastatic cells and that these cells show a rise in their mechanical resistance to travel through the deformation channel.
Figure 3.
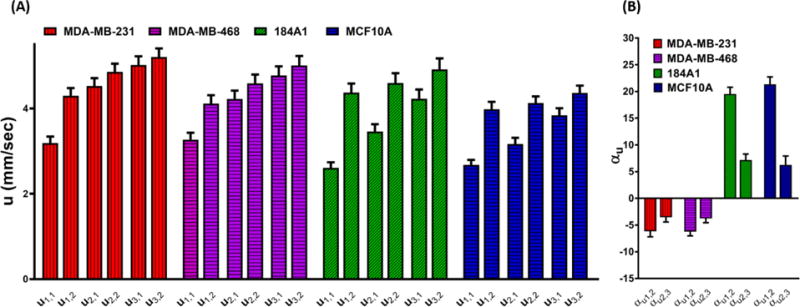
A) Depiction of changes in the average transient (ui,1) and equilibrium (ui,2) velocities in the iterative deformation channels (i=1, 2, 3) for the four breast cell lines. B) Column bars of the calculated relative percentage change of velocities between each two successive deformation channels (αu1,2 and αu2,3) for the selected breast cell lines. The results are shown as mean ± standard error of mean (SEM) for at least n=100 cells of each cell line.
Here, we define the relative percentage changes of velocity (αu) for individual cells as the average equilibrium velocity in one deformation channel compared to the average transient velocity in the next deformation channel (αu i,i+1 = (ui,2-ui+1,1)/ui,2) for i=1, 2). The relative percentage change of velocity is used as an index for examining the modulation in the mechanical resistance of cells. The αu between each two successive deformation channels for the four cell types was calculated and is depicted by the column bars in Figures 3B.
In accordance with the results in Figure 3A, the average values of αu for both non-metastatic cell lines were positive at each two successive deformation channels (Figure 3B), substantiating the idea that these cell lines exhibit a continuous mechanical resistance response against deformations. Importantly, the average values of αu for the two non-metastatic cell lines appreciably reveal decreasing trends from αu1,2 to αu2,3. These data indicate that the mechanical resistance trend in the non-metastatic cells drops sharply after the initial deformation. Moreover, the average αu values for metastatic cell lines (Figure 3B) are both negative. It is also remarkable that the average αu1,2 is larger than the average αu2,3 in the both metastatic cells, suggesting that the greatest degree of cell mechanical compliance occurred at the initial deformation.
We also examined the αu values of individual cells; only 6% and 4% of all tested MCF10A and 184A1 cells, respectively, whose u1,1 velocity were relatively close to the average velocity of the metastatic cells, did not show a positive number in αu1,2. In addition, 71% and 67% of MCF10A and 184A1 cells showed positive values for αu2,3, respectively. There are non-metastatic cells that have positive values for both αu1,2 and αu2,3 parameters which can be labeled with some confidence as sub-populations with a persistent mechanical resistance. Accordingly, 67% and 65% of MCF10A and 184A1 cells fall into the persistent resistance sub-populations. Therefore, the majority of non-metastatic cells exhibited some degree of mechanical resistance in response to the iterative deformations.
At the single-cell level of the metastatic cells, 91% and 93% of MDA-MB-231 and MDA-MB-468 cells, have negative/zero values for their αu1,2, respectively. Also, 84% and 85% of MDA-MB-231 and MDA-MB-468 cells have negative/zero values for their αu2,3, respectively. There is a sub-population of 78% of MDA-MB-231 and 80% of MDA-MB-468 cells that exhibit a persistent mechanical compliance as measured by negative/zero values for both αu1,2 and αu2,3. More importantly, only 4% and 3% of MDA-MB-231 and MDA-MB-468 cells were observed to exhibit a persistent positive value in both of their αu1,2 and αu2,3, respectively. Therefore, the populations of non-metastatic and metastatic cells can begin to be identified by the trend of their modulatory mechanical behaviors in response to an iterative deformations paradigm.
According to the results in Figure 3B, the relative percentage change of velocity between two initial successive channels (αu1,2) provides a more distinct and sensitive index for differentiation between non-metastatic and metastatic cells. Thus, only two successive deformation channels in this design might be enough to discriminate between metastatic and non-metastatic cells with single-cell resolution. Together, these distinct modulatory responses of metastatic and non-metastatic cells to dynamic mechanical stimuli provided a proof-of-principle for the significance of our proposed iMECH bio-signature.
Single-Cell Level Identification using iMECH Bio-Signature
Figure 4A shows a scatter plot of every individual cell’s average transient velocity versus equilibrium velocity corresponding to the first deformation channel (u1,1, u1,2). It is equivalent to the results extracted from a single-pulse mechanical stimulus, typically utilized for cell biomechanical characterizations. Figure 4A clearly illustrates inadequacy of using a single-pulse mechanical deformation to differentiate non-metastatic and metastatic cells at single-cell level due to a significant overlap in their measured u1,1 and u1,2. Therefore, a single microfluidic constriction channel typically used for measuring difference in the biomechanical properties of cells, although effective at population-level, cannot differentiate malignancy or normalcy between cell types at the single-cell level. Our hypothesis that non-metastatic and metastatic cells show overall opposite trends in adjusting their mechanical resistance under an iterative deformations paradigm suggested that incorporation of the relative change of velocity (αu) for the individual cells through consecutive deformation channels rather than their absolute velocity provides a more effective biomarker to label single cells.
Figure 4.
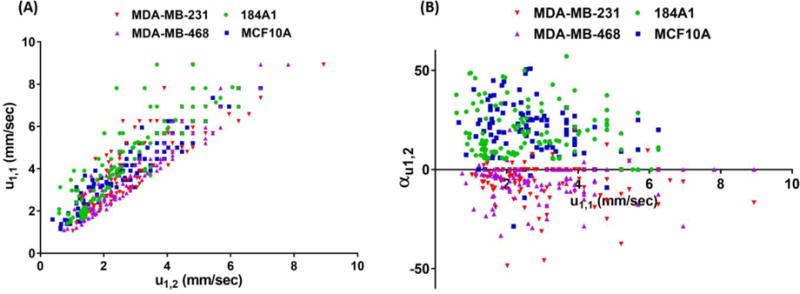
A) Scatter plot of the first deformation channel’s absolute transient (u1,1) and equilibrium (u1,2) velocities of breast cell lines. B) Scatter plot of the relative percentage change of velocity (αu1,2) vs. the first deformation channel’s absolute transient velocity (u1,1) provides a new bio-signature for single-cell level identification.
The scatter plot of the relative percentage change of velocity between two initial deformation channels (αu1,2) vs. the first deformation channel’s absolute transient velocity (u1,1) for the individual tested cells are depicted in Figure 4B. These data again show that a new mechanical descriptor of a non-metastatic cell can be defined as having positive values for αu1,2, and for a metastatic cell is defined as having one negative/zero value for αu1,2. Using these definitions, about 95% of the non-metastatic (normal-like) cells (94% of MCF10A cells and 96% of 184A1 cells) and about 92% of the metastatic cancer cells (91% of MDA-MB-231 cells and 93% of MDA-MB-468 cells) and overall, about 93% of all tested cells are classified as non-metastatic or metastatic.
In summary, dynamic behaviors of subcellular fibrous proteins and cytoskeleton network remodeling induced by periodic stretch or compression were previously reported.65–70 Here, we defined a unique bio-signature by evaluating and quantifying this dynamic biomechanical characteristic of individual cells for distinguishing between non-metastatic (normal-like) and metastatic cancer cells with a high confidence level.
iMECH Bio-Signatures of Mixed Cell Line Populations
In another set of experiments, the iMECH analyzer was used for testing different mixtures of metastatic MDA-MB-231 and non-metastatic MCF10A cells with ascending ratio. The percentage of tested cells from each mixture that are identified as metastatic cells using the iMECH bio-signature (αu1,2) are reported in Table 2. For each test and reported percentage, three replicates were performed. As expected, as the ratio of metastatic MDA-MB-231 to non-metastatic MCF10A cells in the mixture increased, the percentage of tested cells in the mixture recognized as potential metastatic cells based on iMECH bio-signature gradually increased. As a note, when there were no MDA-MB-231 cells in the “mixture”, about 7% were identified as metastatic; this is a measure of the false positive rate of this indicator. When the mixture contained 100% MDA-MB-231 cells, 95% of cells were identified as metastatic, indicating that the false negative rate for predicting the presence of a metastatic cells is about 5%.
Table 2.
Percentage of tested single cells identified as metastatic cells via iMECH bio-signature (αu1,2) in mixtures of MDA-MB-231 and MCF10A cells with ascending ratio.
| MDA-MB-231:MCF10A mixture ratio | % of cells predicted metastasis | % of cells identified metastasis via iMECH bio-signature |
|---|---|---|
| 0:1 | 0% | 7% |
| 1:10 | 10% | 15% |
| 1:1 | 50% | 59% |
| 1:0.1 | 90% | 87% |
| 1:0 | 100% | 95% |
Conclusion
A biosensing microfluidic chip was developed in this work to provide iterative mechanical characteristics (iMECH) of human breast cells in response to a dynamic microenvironment. The described iMECH microfluidic device, the first of its kind, provides a low-cost yet high-throughput for single-cell level metastatic detection. In summary, as cells pass through successive deformation/relaxation regions, the resistance of metastatic cells decreased while that of the non-metastatic cells increased after each relaxation period. Hence, two populations can be differentiated from one another based upon their dynamic behavior even if the two populations produce the same velocity in the first deformation channel. The described iMECH microchip makes the modulatory biomechanical behavior of cells a readily available label-free biomarker to be able to identify metastatic cells from non-metastatic cells. In the future, the iMECH analyzer may provide instrumentation enabling novel ways for detecting metastatic cells in clinical samples and also drug screening.
Acknowledgments
This research is supported by the National Science Foundation (NSF) under award number CBET-1403304 and by the National Institute of Health (NIH) under award number 1R21CA210126-01. We acknowledge the Micro & Nano Fabrication Laboratory at Virginia Tech for equipment support.
Notes and References
- 1.Hoffman BD, Crocker JC. Annu Rev Biomed Eng. 2009;11:259–288. doi: 10.1146/annurev.bioeng.10.061807.160511. [DOI] [PubMed] [Google Scholar]
- 2.Lambrechts A, Van Troys M, Ampe C. Int J Biochem Cell Biol. 2004;36:1890–1909. doi: 10.1016/j.biocel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Wirtz D, Konstantopoulos K, Searson PC. Nat Rev Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Weaver VM. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Carlo D. J Lab Autom. 2012;17:32–42. doi: 10.1177/2211068211431630. [DOI] [PubMed] [Google Scholar]
- 6.Suresh S. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward KA, Li WI, Zimmer S, et al. Biorheology. 1991;28:301–313. doi: 10.3233/bir-1991-283-419. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan V, Mythreye K, OBrien ET, Berchuck A, Blobe GC, Superfine R. Cancer Res. 2011;71:5075–5080. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W, Mezencev R, Kim B, Wang L, McDonald J, Sulchek T. PLoS One. 2012;7:e46609. doi: 10.1371/journal.pone.0046609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byun S, Son S, Amodei D, Cermak N, Shaw J, Kang JH, Hecht VC, Winslow MM, Jacks T, Mallick P, Manalis SR. Proc Natl Acad Sci USA. 2013;110:7580–7758. doi: 10.1073/pnas.1218806110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabriele S, Benoliel AM, Bongrand P, Theodoly O. Biophys J. 2009;96:4308–4318. doi: 10.1016/j.bpj.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanjay ST, Fu G, Dou M, Xu F, Liu R, Qi H, Li X. Analyst. 2015;140:7062–7081. doi: 10.1039/c5an00780a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valizadeh A, Yari Khosroushahi A. Anal Methods. 2015;7:8524–8533. [Google Scholar]
- 14.Zhang W, Guo S, Carvalho WSP, Jiang Y, Serpe MJ. Anal Methods. 2016;8:7847–7867. [Google Scholar]
- 15.Pappas D. Analyst. 2016;141:525–535. doi: 10.1039/c5an01778e. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JZ, Nagrath S. Biomed Microdevices. 2013;15:595–609. doi: 10.1007/s10544-012-9734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue C, Wang J, Zhao Y, Chen D, Yue W, Chen J. Micromachines. 2015;6:1794–1804. [Google Scholar]
- 18.Zheng Y, Nguyen J, Wei Y, Sun Y. Lab Chip. 2013;13:2464–2483. doi: 10.1039/c3lc50355k. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Gonzalez VH, Gallo-Villanueva RC, Camacho-Leon S, Gomez-Quinones JI, Rodriguez-Delgado JM, Martinez-Chapa SO. IET Nanobiotechnology. 2016;10:263–275. doi: 10.1049/iet-nbt.2015.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhuri PK, Ebrahimi Warkiani M, Jing T, Kenry, Lim CT. Analyst. 2016;141:504–524. doi: 10.1039/c5an00382b. [DOI] [PubMed] [Google Scholar]
- 21.Meacham CE, Morrison SJ. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen XX, Bai F. Cancer Biol Med. 2015;12:184–192. doi: 10.7497/j.issn.2095-3941.2015.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navin NE. Cancer Res. 2016;76:1305–1312. [Google Scholar]
- 24.Mak M, Erickson D. Integr Biol. 2013;5:1374–1384. doi: 10.1039/c3ib40128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mak M, Reinhart-King CA, Erickson D. Lab Chip. 2013;13:340–348. doi: 10.1039/c2lc41117b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou HW, Li QS, Lee GYH, Kumar AP, Ong CN, Lim CT. Biomed Microdevices. 2009;11:557–564. doi: 10.1007/s10544-008-9262-8. [DOI] [PubMed] [Google Scholar]
- 27.Khan ZS, Vanapallia SA. Biomicrofluidics. 2013;7:011806. doi: 10.1063/1.4774310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babahosseini H, Srinivasaraghavan V, Zhao Z, Gillam F, Childress E, Strobl JS, Santos WL, Zhang C, Agah M. Lab Chip. 2016;16:188–198. doi: 10.1039/c5lc01201e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange JR, Steinwachs J, Kolb T, Lautscham LA, Harder I, Whyte G, Fabry B. Biophys J. 2015;109:26–34. doi: 10.1016/j.bpj.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LM, Liu AP. Lab Chip. 2015;15:264–273. doi: 10.1039/c4lc01218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilyas A, Asghar W, Ahmed S, Lotan Y, Hsieh JT, Kim YT, Iqbal SM. Anal Methods. 2014;6:7166–7174. [Google Scholar]
- 32.Guan G, Chen PCY, Peng WK, Bhagat AA, Ong CJ, Han J. J Micromech Microeng. 2012;22:105037. [Google Scholar]
- 33.Adamo A, Sharei A, Adamo L, Lee BK, Mao S, Jensen KF. Anal Chem. 2012;84:6438–6443. doi: 10.1021/ac300264v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Zheng Y, Tan Q, Zhang YL, Li J, Geddie WR, Jewett MAS, Sun Y. Biomicrofluidics. 2011;5:14113. doi: 10.1063/1.3571530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Zheng Y, Tan Q, Shojaei-Baghini E, Zhang YL, Li J, Prasad P, You L, Wu XY, Sun Y. Lab Chip. 2011;11:3174–3181. doi: 10.1039/c1lc20473d. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y, Shojaei-Baghini E, Azad A, Wang C, Sun Y. Lab Chip. 2012;12:2560–2567. doi: 10.1039/c2lc21210b. [DOI] [PubMed] [Google Scholar]
- 37.Huang S, Undisz A, Diez-Silva M, Bow H, Dao M, Han J. Integr Biol. 2013;5:414–422. doi: 10.1039/c2ib20161e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bow H, Pivkin IV, Diez-Silva M, Goldfless SJ, Dao M, Niles JC, Suresh S, Han J. Lab Chip. 2011;11:1065–1073. doi: 10.1039/c0lc00472c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Q, Reiling SJ, Rohrbach P, Ma H. Lab Chip. 2012;12:1143–1150. doi: 10.1039/c2lc20857a. [DOI] [PubMed] [Google Scholar]
- 40.Guo Q, Park S, Ma H. Lab Chip. 2012;12:2687–2695. doi: 10.1039/c2lc40205j. [DOI] [PubMed] [Google Scholar]
- 41.Kwan JM, Guo Q, Kyluik-Price DL, Ma H, Scott MD. Am J Hematol. 2013;88:682–689. doi: 10.1002/ajh.23476. [DOI] [PubMed] [Google Scholar]
- 42.Guo Quan, Duffy Simon P, Matthews Kerryn, Santoso Aline T, Scott Mark D, Ma Hongshen. J Biomech. 2014;47:1767–1776. doi: 10.1016/j.jbiomech.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 43.Santoso AT, Deng X, Lee JH, Matthews K, Duffy SP, Islamzada E, McFaul SM, Myrand-Lapierre ME, Ma H. Lab Chip. 2015;15:4451–4460. doi: 10.1039/c5lc00945f. [DOI] [PubMed] [Google Scholar]
- 44.Myrand-Lapierre ME, Deng X, Ang RR, Matthews K, Santoso AT, Ma H. Lab Chip. 2015;15:159–167. doi: 10.1039/c4lc01100g. [DOI] [PubMed] [Google Scholar]
- 45.Shelby JP, White J, Ganesan K, Rathod PK, Chiu DT. Proc Natl Acad Sci USA. 2003;100:14618–14622. doi: 10.1073/pnas.2433968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herricks T, Antia M, Rathod PK. Cell Microbiol. 2009;11:1340–1353. doi: 10.1111/j.1462-5822.2009.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinn DJ, Pivkin I, Wong SY, Chiam KH, Dao M, Karniadakia GE, Suresh S. Ann Biomed Eng. 2011;39:1041–1050. doi: 10.1007/s10439-010-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai CHD, Sakuma S, Arai F, Kaneko M. IEEE Trans Biomed Eng. 2014;61:1187–1195. doi: 10.1109/TBME.2013.2296624. [DOI] [PubMed] [Google Scholar]
- 49.Handayani S, Chiu DT, Tjitra E, Kuo JS, Lampah D, Kenangalem E, Renia L, Snounou G, Price RN, Anstey NM, Russell B. J Infect Dis. 2009;199:445–450. doi: 10.1086/596048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee WG, Bang H, Yun H, Lee J, Park J, Kim JK, Chung S, Cho K, Chung C, Han DC, Chang JK. Lab Chip. 2007;7:516–519. doi: 10.1039/b614912j. [DOI] [PubMed] [Google Scholar]
- 51.Abkarian M, Faivre M, Stone HA. Proc Natl Acad Sci USA. 2006;103:538–542. doi: 10.1073/pnas.0507171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabriele S, Versaevel M, Preira P, Theodoly O. Lab Chip. 2010;10:1459–1467. doi: 10.1039/c002257h. [DOI] [PubMed] [Google Scholar]
- 53.Preira P, Valignat MP, Bico J, Theodoly O. Biomicrofluidics. 2013;7:024111. doi: 10.1063/1.4802272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam WA, Rosenbluth MJ, Fletcher DA. Blood. 2007;109:3505–3508. doi: 10.1182/blood-2006-08-043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenbluth MJ, Lam WA, Fletcher DA. Lab Chip. 2008;8:1062–1070. doi: 10.1039/b802931h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yap B, Kamm RD. J Appl Physiol. 2005;98:1930–1939. doi: 10.1152/japplphysiol.01226.2004. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman BD, Grashoff C, Schwartz MA. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babahosseini H, Strobl JS, Agah M. Nanomedicine. 2015;10:2635–2638. doi: 10.2217/nnm.15.104. [DOI] [PubMed] [Google Scholar]
- 59.Bao G, Suresh S. Nature Materials. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- 60.Munson JM, Shieh AC. Cancer Manag Res. 2014;6:317–328. doi: 10.2147/CMAR.S65444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Putman CAJ, Van der Werf KO, De Grooth BG, Van Hulst NF, Greve J. Biophys J. 1994;67:1749–1753. doi: 10.1016/S0006-3495(94)80649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pourati J, Maniotis A, Spiegel D, Schaffer JL, Butler JP, Fredberg JJ, Ingber DE, Stamenovic D, Wang N. Am J Physiol. 1998;274:C1283–1289. doi: 10.1152/ajpcell.1998.274.5.C1283. [DOI] [PubMed] [Google Scholar]
- 63.Babahosseini H, Strobl SS, Agah M. Nanotechnology. 2015;26:354004. doi: 10.1088/0957-4484/26/35/354004. [DOI] [PubMed] [Google Scholar]
- 64.Tan WH, Takeuchi S. Proc Natl Acad Sci U S A. 2007;104:1146–1151. doi: 10.1073/pnas.0606625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu HJ, Lee CF, Kaunas R. PLoS ONE. 2009;4:e4853. doi: 10.1371/journal.pone.0004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu B, Qu MJ, Qin KR, Li H, Li ZK, Shen BR, Jiang ZL. Biophys J. 2008;94:1497–1507. doi: 10.1529/biophysj.106.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Icard-Arcizet D, Cardoso O, Richert A, Henon S. Biophys J. 2008;94:2906–2913. doi: 10.1529/biophysj.107.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng L, Fairbank NJ, Fabry B, Smith PG, Maksym GN. Am J Physiol Cell Physiol. 2004;287:C440–448. doi: 10.1152/ajpcell.00374.2003. [DOI] [PubMed] [Google Scholar]
- 69.Wolff L, Fernandez P, Kroy K. PLoS ONE. 2012;7:e40063. doi: 10.1371/journal.pone.0040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaudhuri O, Parekh SH, Fletcher DA. Nature. 2007;445:295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]


