Abstract
At the intersection between neuroscience, microbiology, and psychiatry, the enteric microbiome has potential to become a novel paradigm for studying the psychobiological underpinnings of mental illness. Several studies provide support for the view that the enteric microbiome influences behavior through the microbiota-gut-brain axis. Moreover, recent findings are suggestive of the possibility that dysregulation of the enteric microbiota (i.e., dysbiosis) and associated bacterial translocation across the intestinal epithelium may be involved in the pathophysiology of stress-related psychiatric disorders, particularly depression. The current article reviews preliminary evidence linking the enteric microbiota and its metabolites to psychiatric illness, along with separate lines of empirical inquiry on the potential involvement of psychosocial stressors, pro-inflammatory cytokines and neuroinflammation, the HPA axis, and vagal nerve activation, respectively, in this relationship. Finally, and drawing on these independent lines of research, an integrative conceptual model is proposed in which stress-induced enteric dysbiosis and intestinal permeability confer risk for negative mental health outcomes through immunoregulatory, endocrinal, and neural pathways.
Keywords: Cytokines, HPA, Microbiome, Stress, Vagus nerve
Introduction
The last several years have seen a rapid growth of interest in the microbiome (i.e., the metagenome of the communities of microbes, including archæa, bacteria, eukaryotes, and viruses, found in different parts of the human body), doubtless facilitated in part by significant advances in high-throughput sequencing-based analytic techniques (Di Bella, Bao, Gloor, Burton, & Reid, 2013; Fraher, O’Toole, & Quigley, 2012). Indications of this increasing interest in the microbiome can be found in the launching of the Human Microbiome Project (HMP) by the National Institutes of Health (NIH) in 2007, with the goal of identifying and characterizing the human microbiome and its relation to healthy physiological functioning as well as risk for disease (The Human Microbiome Project Consortium, 2012a; Turnbaugh et al., 2007), and in the initiation of the Metagenomics of the Human Intestinal Tract (MetaHIT) project by the European Commission in 2008, with this same objective but focusing more specifically on the enteric microbiome (Qin et al., 2010).
Of the different microbial communities found in the human body, the enteric microbiota, in particular, has been the focus of much empirical attention, owing to the accumulating evidence of its importance to physical health. An estimated 39 trillion microbes populate the large intestine, yielding a ratio of bacteria to human cells of 1:1 (Bäckhed, Ley, Sonnenburg, Peterson, & Gordon, 2005; Belkaid & Hand, 2014; Gill et al., 2006; Sender, Fuchs, & Milo, 2016). Whereas a healthy enteric microbiota is generally characterized by a marked diversity in the bacterial species present (Rupnik, 2015), low biodiversity appears to be associated with gastrointestinal disorders, such as inflammatory bowel disease (Manichanh et al., 2006; The Human Microbiome Project Consortium, 2012b). Furthermore, differential enteric microbial composition has been implicated in risk for metabolic diseases, such as diabetes and obesity (Karlsson et al., 2013; Le Chatelier et al., 2013; Qin et al., 2012; Turnbaugh et al., 2009).
One of the most intriguing developments in microbiome research to date is the emerging body of empirical support for the view that the influence of the enteric microbiota extends beyond these physical health conditions to include cognitive functioning and behavior, mediated through endocrinal, neural, and immunological pathways (Collins & Bercik, 2009; Cryan & Dinan, 2012; Foster & McVey Neufeld, 2013; Kaplan, Rucklidge, Romijn, & McLeod, 2015; Mayer, Knight, Mazmanian, Cryan, & Tillisch, 2014; Rhee, Pothoulakis, & Mayer, 2009; Schmidt, 2015; Stilling, Dinan, & Cryan, 2014). Although the existence of bidirectional communication between the gut and brain has been well established, the influence of enteric microbiota on this system has gained increasing prominence in recent years, leading to an extension of the gut-brain axis to a microbiota-gut-brain axis (Collins, Surette, & Bercik, 2012; Foster & McVey Neufeld, 2013; Rhee et al., 2009).
Just as a healthy enteric microbiota may serve an important role in adaptive brain functioning, so too may microbial dysregulation (i.e., dysbiosis) confer risk for psychiatric morbidity. Although preliminary, there is mounting evidence linking the enteric microbiome to risk for psychiatric illness, particularly depression and anxiety (Kaplan et al., 2015; Stilling et al., 2014). Additionally, several studies have found that psychosocial stressors may alter the composition of enteric microbiota in a manner that correlates with changes in the presence of cytokines (Bailey et al., 2011; Cryan & Dinan, 2012; Foster & McVey Neufeld, 2013). These proteins have separately been associated with vagus nerve activation (Collins & Bercik, 2009) and risk for certain psychiatric conditions such as depression (Dowlati et al., 2010; Mills, Scott, Wray, Cohen-Woods, & Baune, 2013). Psychosocial stressors, enteric dysbiosis, and depression have each been similarly associated with dysregulated functioning of the hypothalamic-pituitary-adrenal (HPA) axis (de Kloet, Joëls, & Holsboer, 2005; Foster & McVey Neufeld, 2013; Pariante & Lightman, 2008). Collectively, these findings are consistent with the possibility that the microbiota-gut-brain axis may be relevant to stress-related manifestations of psychopathology (e.g., depression and anxiety), and may mediate the relation between life stressors and these psychiatric disorders.
The current review highlights findings from the research literature in psychiatry, neurobiology, and microbiology relating to the enteric microbiota and its metabolites, psychosocial stressors, pro-inflammatory cytokines and neuroinflammation, the HPA axis, the vagus nerve, and psychiatric illness, with a focus on depression. Included in this discussion are several important limitations in the existing literature to be addressed in future studies. Finally, and based on these independent lines of empirical inquiry, this review proposes an integrative conceptual model of the potential role of the enteric microbiome in mental health.
The enteric microbiota and psychiatric illness
The potential clinical relevance of the microbiota-gut-brain axis to stress-related psychiatric illness has received preliminary empirical support in several studies demonstrating potential anxiogenic effects (i.e., anxiety-inducing) of infectious bacteria or parasites, as well as antidepressant and anxiolytic effects (i.e., anti-anxiety) of probiotics in rodents (see Table 1 for a summary). For example, increases in anxious behavior have been observed in rodents exposed to Citrobacter rodentium, Trichuris muris, and Campylobacter jejuni (Lyte, Varcoe, & Bailey, 1998; Stilling et al., 2014), whereas Lactobacillus spp. and Bifidobacterium spp. have been demonstrated in several rodent studies to reduce anxiety and depression-like behavior (Arseneault-Bréard et al., 2012; Bercik, Park, et al., 2011; Bravo et al., 2011; Messaoudi, Lalonde, et al., 2011). Finally, one study found that mice with enteric microbiota transplanted from donors on a high-fat diet subsequently displayed more anxious behavior than did counterparts receiving microbiota from donors on a control diet (Bruce-Keller et al., 2015). This finding is consistent with possibility that enteric dysbiosis associated with obese-type diets may in part account for the high co-occurrence between obesity and stress-related psychiatric conditions, such as depression (Needham, Epel, Adler, & Kiefe, 2010).
Table 1.
Enteric microbial influences on psychiatric symptoms and behavior.
| Outcome | Species/microbial compound | Citation(s) |
|---|---|---|
| Rodent studies | ||
| Increased anxiety symptoms/behavior | Campylobacter jejuni , Citrobacter rodentium, Trichuris muris, high-fat microbiota | (Bruce-Keller et al., 2015; Lyte, Varcoe, & Bailey, 1998; Stilling, Dinan, & Cryan, 2014) |
| Decreased anxiety symptoms/behavior | Bifidobacterium spp., Lactobacillus spp. | (Bercik et al., 2011; Bravo et al., 2011; Messaoudi et al., 2011) |
| Decreased depressive symptoms/behavior | Bifidobacterium spp., Lactobacillus spp. | (Arseneault-Bréard et al., 2012; Bravo et al., 2011) |
| Human studies | ||
| Cross-sectional findings | ||
| Positive association with depression | Alistipes, Bacteroidales, Enterobacteriaceae | (Jiang et al., 2015; Naseribafrouei et al., 2014) |
| Negative association with depression | Faecalibacterium, Lachnospiraceae | (Jiang et al., 2015; Naseribafrouei et al., 2014) |
| Longitudinal findings | ||
| Decreased anxiety symptoms | Bifidobacterium spp., Lactobacillus spp., Lactobacillus helveticus | (Messaoudi et al., 2011; Mohammadi et al., in press; Rao et al., 2009) |
| Decreased depressive symptoms | Bifidobacterium spp., Lactobacillus spp., Lactobacillus helveticus | (Benton, Williams, & Brown, 2007; Messaoudi et al., 2011; Mohammadi et al., in press) |
| Decreased anger/hostility | Bifidobacterium longum, Lactobacillus helveticus | (Messaoudi et al., 2011) |
| Decreased cognitive reactivity to negative stimuli, mediated by reduction in rumination and aggressive thoughts | Bifadobacterium spp., Lactobacillus spp., Lactococcus lactis | (Steenbergen, Sellaro, van Hemert, Bosch, & Colzato, 2015) |
| Decreased activity in emotional and sensory brain regions in response to negative stimuli | Bifidobacterium animalis subsp. Lactis, Lactobacillus bulgaricus, Lactococcus lactis subsp. Lactis, Streptococcus thermophiles | (Tillisch et al., 2013) |
| Decreased attentional bias toward negative stimuli | Bimuno-galacto-oligosaccharides | (Schmidt et al., 2015) |
Human studies in this area have been notably rare. Two observational studies have directly examined enteric microbial profiles in relation to psychopathology in humans. In one of these cross-sectional studies, adult patients with depression were found to have higher levels of Enterobacteriaceae and Alistipes but lower levels of Faecalibacterium relative to healthy controls (Jiang et al., 2015). The second cross-sectional study noted higher concentrations of Bacteroidales but lower concentrations of Lachnospiraceae in a sample of depressed adults relative to healthy controls (Naseribafrouei et al., 2014). Furthermore, seven studies to date have evaluated the potential psychotropic effects of probiotics or prebiotics in humans. In one study, participants administered a combination of Lactobacillus helveticus and Bifidobacterium longum, when compared to a placebo control group, endorsed lower scores on measures of general psychological distress, anger-hostility, depression, and anxiety (Messaoudi, Lalonde, et al., 2011). A second study found that consuming milk with the probiotic Lactobacillus casei strain Shirota, relative to a placebo, was associated with improved mood, but only among participants with relatively low mood at baseline (Benton, Williams, & Brown, 2007). In the third study, Lactobacillus casei strain Shirota was prospectively associated with fewer anxiety symptoms among participants with chronic fatigue syndrome (Rao et al., 2009). A fourth study found a multispecies probiotic containing Lactobacillus spp., Bifadobacterium spp., and Lactococcus lactis to lead to a reduction in cognitive reactivity to dysphoric mood, mediated by a reduction in rumination and aggressive thoughts (Steenbergen, Sellaro, van Hemert, Bosch, & Colzato, 2015). A fifth found that consumption of a probiotic cocktail led to reduced activity in the insula and somatosensory cortices, and a functional network including emotional and sensory areas, in response to negative emotional stimuli assessed while subjects were in a scanner (Tillisch et al., 2013). In a more recent study, probiotic compounds that included Lactobacillus spp. and Bifidobacterium spp., when compared to conventional yogurt and placebos, were associated with lower levels of anxiety and depressive symptoms (Mohammadi et al., in press). Finally, in the one study to date assessing the effects of prebiotics, a decreased attentional bias toward negative stimuli on a behavioral task was observed in participants who ingested Bimuno-galacto-oligosaccharides when compared to those in the placebo condition (Schmidt et al., 2015), a finding of potential clinical relevance given the negative attentional bias characteristic of depression (Clark, Chamberlain, & Sahakian, 2009; Disner, Beevers, Haigh, & Beck, 2011).
Despite the preliminary support across these studies for the potential role of the enteric microbiome in the development of anxiety and depression, all seven probiotic and prebiotic studies featured psychiatrically healthy participants, significantly constraining the clinical generalizability of their findings. Indeed, four studies explicitly excluded individuals with psychiatric illness (Benton et al., 2007; Schmidt et al., 2015; Steenbergen et al., 2015; Tillisch et al., 2013), and another excluded individuals with elevated scores on a measure of depression and anxiety (Messaoudi, Lalonde, et al., 2011). Thus, there is a clear need for research directly evaluating the relation between enteric microbiota and clinically significant psychopathology. Furthermore, these studies all featured adult samples, and thus are limited in their generalizability to adolescents. This is particularly important, given distinct differences in enteric microbiome composition across the lifespan (Biagi et al., 2010; Claesson et al., 2011, 2012), with greater inter-individual variation among children than adults (Yatsunenko et al., 2012), not to mention significant age-of-onset differences in terms of presentation, course, and risk factors for certain psychiatric disorders (Hill, Pickles, Rollinson, Davies, & Byatt, 2004; Jaffee et al., 2002; Kaufman, Martin, King, & Charney, 2001). Additionally, although it appears that the enteric microbiota develops throughout childhood and adolescence before stabilizing and becoming more diversified in adulthood (Rea, Dinan, & Cryan, in press), it is unclear how these developmental considerations may relate to the development of risk for psychiatric outcomes. For example, risk for and gender differences in prevalence of depression begin to increase dramatically in early adolescence (Hankin & Abramson, 2001; Hasin, Goodwin, Stinson, & Grant, 2005). The degree to which hormonal influences in early puberty may contribute to these trends indirectly by influencing the enteric microbiota remains to be empirically examined (Rea et al., in press). Indeed, even normative changes in the enteric microbiota in adolescence remains poorly characterized, as most studies on developmental differences have focused on early infancy and old age (McVey Neufeld, Luczynski, Dinan, & Cryan, 2016; McVey Neufeld, Luczynski, Seira Oriach, Dinan, & Cryan, in press). Future research in this area is a necessary first step in establishing a reference point for understanding aberrations in the enteric microbiota in relation to adolescent psychopathology.
Microbial metabolites
An interesting possibility yet to be explored in the clinical literature is whether microbial metabolites may be a means through which the enteric microbiota influences risk for mental illness, given findings that several of these metabolites may possess neuroactive qualities (Mayer et al., 2014; Russell, Hoyles, Flint, & Dumas, 2013; Wall et al., 2014). Microbial metabolites have been known to influence the blood-brain barrier, with germ-free mice, for example, found to exhibit greater long-term blood-brain barrier permeability than mice with normal gut flora (Braniste et al., 2014). Complex carbohydrates are metabolized by enteric microbes into short-chain fatty acids (SCFAs), including butyrate, which tightens the junctions between cells in the blood-brain barrier, thereby reducing its permeability (Sampson & Mazmanian, 2015; Smith, 2015). These SCFAs are capable of crossing the blood-brain barrier (Sampson & Mazmanian, 2015). Butyrate also may relate to mental health by affecting brain-derived neurotrophic factor (BDNF) expression in the hippocampus (Dinan, Stilling, Stanton, & Cryan, 2015), which is noteworthy given the finding that BDNF relates to hippocampal hyperactivation in response to emotional stimuli in anxious and depressed adolescents (Lau et al., 2010). Additionally, the metabolite propionic acid has been associated with anxiety symptoms in humans (Collins et al., 2012).
Indirect support for the potential influences of microbial by-products on mental health also comes from studies documenting the involvement of enteric microbial metabolites in the production of neurotransmitters, including serotonin. First, germ-free mice exhibit reduced levels of circulatory serotonin, which can be remedied with spore-forming microbes that metabolizes SCFAs (e.g., Clostridium spp.), and contrastingly, mice with natural microbiota experience a reduction in serotonin when exposed to antibiotics (Collins et al., 2012; Smith, 2015; Yano et al., 2015). Also implicated in serotonin production are Candida, Enterococcus, Escherichia, and Streptococcus spp. (Dinan et al., 2015), possibly through microbial SCFAs (Sampson & Mazmanian, 2015). Moreover, the probiotic Bifidobacterium infantis has been observed to affect concentrations of serotonin indirectly by influencing levels of kynurenine, which in turn metabolizes tryptophan, a precursor to serotonin (Dinan et al., 2015). Although serotonin meets with resistance at the blood-brain barrier, tryptophan is not so hindered. It is therefore possible that microbial-derived tryptophan may influence mood and behavior through the synthesis of serotonin after crossing this barrier (Sampson & Mazmanian, 2015). These findings are particularly notable because approximately 90% of peripheral serotonin in humans originates in the digestive tract (Berger, Gray, & Roth, 2009), and given the considerable interest in the role of serotonin in depression and its treatment (Andrews, Bharwani, Lee, Fox, & Thomson, 2015). Furthermore, certain species of bacteria have been found to produce other neurotransmitters, including Lactobacillus and Bifidobacterium in the case of gamma-aminobutyric acid (GABA), Eschericha, Bacillus, and Saccharomyces for norepinephrine, and Bacillus in the case of dopamine (De Palma, Collins, Bercik, & Verdu, 2014; Dinan et al., 2015), dyregulation of which has been hypothesized to be associated with psychiatric illnesses such as depression (Croarkin, Levinson, & Daskalakis, 2011; Pizzagalli, 2014; Southwick, Vythilingam, & Charney, 2005). Additional research is needed to demonstrate that microbiota-influenced alterations in these neurotransmitters have a clinically significant impact on mental health functioning in humans.
Psychosocial stressors
Of the risk factors to have been implicated in the etiology of several forms of mental illness, one of the most studied is psychosocial stressors. Indeed, stress exposure and diathesis-stress models (i.e., the view that life stress interacts with pre-existing diatheses to increase risk for psychopathology) are core components of several theories of mental illness, including depression (Beck, 1987) and post-traumatic stress disorder (Elwood, Hahn, Olatunji, & Williams, 2009). Empirical support for psychosocial stressors as a risk factor for these manifestations of psychiatric illness has been found (Hammen, 2005; Jones & Barlow, 1990; Klauke, Deckert, Reif, Pauli, & Domschke, 2010; Liu & Miller, 2014). For example, distal psychosocial stressors, in the form of adverse childhood experiences, have been found to heighten long-term risk for depression (Harkness, Bruce, & Lumley, 2006; McLaughlin et al., 2010), and proximal ones occurring in the one to three months prior to depressive onset appear to be of particular etiologically relevance to this disorder (Hammen, 2005; Harkness et al., 2006).
Recent evidence has lent preliminary support for the view that psychosocial stressors may similarly have a role in enteric dysbiosis. In rats, early life stressors have been observed to lead to alterations in the enteric microbiome that persist into adulthood (Barouei, Moussavi, & Hodgson, 2012; O’Mahony et al., 2009). Stressors experienced in adulthood similarly appear to disturb the composition of the enteric microbiota (Bailey et al., 2011). Lactobacillus spp., in particular, have been consistently found to decrease in response to stress (Galley & Bailey, 2014). In primate models, exposure to early life stressors is associated with decrease in fecal bifidobacteria and lactobacilli (Bailey & Coe, 1999; Galley & Bailey, 2014). It is also interesting to note that Alistipes, found in one of the two aforementioned studies of depressed patients to be positively correlated with depressive symptom severity (Jiang et al., 2015), has also been observed to increase appreciably in mice following exposure to stress (Bangsgaard Bendtsen et al., 2012). Research in this area has been largely limited to animal models, however, and human studies of psychosocial stressors in relation to enteric microbiota are needed. Indeed, only one study has been conducted with humans, finding a reduction in lactobacilli concentrations in undergraduates during exams (Knowles, Nelson, & Palombo, 2008).
Pro-inflammatory cytokines and neuroinflammation
Enteric dysbiosis has been associated with increased intestinal permeability. Normally, the mucosal and tight junction barrier, as well as mesenteric lymph nodes (MLNs), effectively maintain separation of enterobacteria from the interstitium (Macpherson & Uhr, 2004). Several studies with rats, however, have documented increased intestinal permeability after exposure to restraint stress (Bailey et al., 2011; Saunders, Hanssen, & Perdue, 1997; Saunders, Kosecka, McKay, & Perdue, 1994). This stress-induced alteration in the permeability of the epithelial barrier, in turn, facilitates translocation of gram-negative bacteria and bacterial antigens across the intestinal mucosa into the bloodstream and MLNs (Foster & McVey Neufeld, 2013; Söderholm & Perdue, 2001). Moreover, this dysfunction of the epithelial barrier may be evident shortly after stress exposure. Increased bacterial antigen translocation across the intestinal epithelium in rats, for example, has been demonstrated to occur as soon as two hours after administration of restraint stress (Kiliaan et al., 1998). Interestingly, it appears that this stress-induced increase in epithelial permeability may be addressed prophylactically in rodent models through the administration of the probiotic Lactibacillus farciminis (Ait-Belgnaoui et al., 2012), Bifidobacteria (Savignac, Kiely, Dinan, & Cryan, 2014), as well as a combination of Lactobacillus helveticus and Lactobacillus rhamnosus (Zareie et al., 2006).
The migration of bacteria and bacterial antigens across the intestinal mucosal lining, in turn, appears to elicit a heightened pro-inflammatory immune response (Ait-Belgnaoui et al., 2012; Bailey et al., 2010, 2011; Galley & Bailey, 2014; Gareau, Silva, & Perdue, 2008; Maslanik et al., 2012). In particular, gram-negative enterobacteria contain the endotoxin lipopolysaccharide (LPS) within their bacterial walls and vesicles, and the presence of LPS in translocated bacterial outer membrane is detected by CD14-Toll-like receptor-4 on CD14 cells (neutrophils, macrophages, and dendritic cells; Maes, Kubera, Leunis, & Berk, 2012), stimulating the production of circulating pro-inflammatory cytokines (e.g. interleukin-1α [IL-1α], IL-1β, IL-6, and tumor necrosis factor-α [TNF-α]) through the activation of cell signalling networks (e.g., nuclear factor κB [NF-κB] and mitogen-activated protein kinase [MAPK]). In contrast, administration of probiotic compounds including bifidobacteria and lactobacilli have been noted to reduce pro-inflammatory responses (e.g., IL-1α, IL-6, and TNF-α) and increase anti-inflammatory activity (e.g., IL-4 and IL-10) in rodents (Messaoudi, Violle, et al., 2011). Similarly, microbial metabolites, SCFAs, particularly butyrate, appear to have a role in reducing this inflammation (Arpaia & Rudensky, 2014; Maslowski et al., 2009).
Importantly, bacterial translocation, and LPS in particular, does not simply induce a peripheral inflammatory response, but rather may also regulate neuroinflammatory processes (Mills et al., 2013). Of particular relevance in this context, microglia, a type of immune cells accounting for approximately 5–12% of brain cells, have a central role in neuroinflammation, being involved in the release of cytokines in the brain (Rea et al., in press). Recent evidence has emerged to indicate that the enteric microbiota has a prominent role in the maturation and immunological functioning of microglia. Germ-free mice, for example, exhibit widespread malformations in microglia and associated impairments in immune responsiveness. These defects appear to be remedied, at least in part, by the introduction of a complex microbiota, or even SCFAs in the absence of a complex microbiota (Erny et al., 2015). The manner through which the enteric microbiota is involved in regulating neuroinflammation may be through the migration of microbial by-products (e.g., SCFAs) and proinflammatory cytokines across the blood-brain barrier (Rea et al., in press). Furthermore, TNF-α, in particular, appears to activate microglia, which draw inflammatory monocytes from the peripheral immune system into the brain (D’Mello, Le, & Swain, 2009).
It is worth noting too that several studies in humans have also documented a link between stress exposure and pro-inflammatory cytokines. Much of the focus in this area has been on interpersonal stress, which, relative to other forms of stress, is particularly predictive of depression and anxiety (Hammen, 2005; Heimberg, Brozovich, & Rapee, 2010; Kendler, Gardner, & Prescott, 2002). Specifically, interpersonal stress has been prospectively associated with an elevated Il-6 response to a microbial challenge in the form of LPS (Miller, Rohleder, & Cole, 2009). Furthermore, in a sample of adolescent girls at risk for developing depression, interpersonal events involving rejection by others were prospectively linked with greater NF-κB mRNA activity (Murphy, Slavich, Rohleder, & Miller, 2013). Additional support of an association between psychosocial stressors and cytokine in humans comes from studies involving experimental induction of interpersonal stress. That is, exposure to a laboratory social stressor results in an increase in circulating pro-inflammatory cytokines, particularly IL-1β, IL-6, and TNF-α (Kemeny, 2009; Steptoe, Hamer, & Chida, 2007). Indeed, one study found LPS-stimulated production of TNF-α to increase subsequent to experiencing a social-evaluative stressor (Dickerson, Gable, Irwin, Aziz, & Kemeny, 2009). In addition to increasing concentrations of pro-inflammatory cytokines, psychosocial stressors in humans have been associated with reductions in anti-inflammatory cytokines (e.g., IL-10; Raison, Capuron, & Miller, 2006).
Pro-inflammatory cytokines, in turn, have been implicated in the pathophysiology of stress-related psychiatric disorders such as depression. In a meta-analysis, IL-6 and TNF-α concentrations were consistently elevated in depressed relative to non-depressed individuals (Dowlati et al., 2010). Suggestive of a causal relation, exposure to IL-1β and TNF-α appear to produce depressotypic behavior in rodents (Dantzer, O’Connor, Freund, Johnson, & Kelley,2008). Moreover, individuals administered IL-2 and interferon-α have been noted to be at greater risk for clinically elevated symptoms of depression (Raison et al., 2006). Documenting a link between psychosocial stressors, pro-inflammatory cytokines, and depression, a recent study found that among adolescents with a history of childhood adversity, elevated IL-6 concentrations predicted the subsequent occurrence of depression (Miller & Cole, 2012). Importantly, this relation was not observed in adolescents with no prior history of childhood adversity.
Of note, evidence of epithelial permeability and bacterial translocation has also been found for depression, with elevated serum immunoglobulin A (IgA) and immunoglobulin M (IgM) mediated immune responses to the LPS of several gram-negative enterobacteria in depressed individuals relative to controls (Maes, Kubera, & Leunis, 2008; Maes et al., 2012). What remains to be directly evaluated, however, is whether this greater intestinal permeability and associated increase in circulating pro-inflammatory cytokines are induced by exposure to psychosocial stressors in humans, and leads to subsequently heightened risk for stress-related psychiatric illnesses such as depression, through pro-inflammatory mediational pathways.
HPA axis
There is accumulating evidence from separate lines of research consistent with the possibility that neuroendocrinological dysregulation may also be involved in this potential pathway linking psychosocial stressors, the enteric microbiome, pro-inflammatory cytokines, and mental illness. First, that response to psychosocial stressors is mediated by the HPA system has been well established (de Kloet et al., 2005; Gunnar & Quevedo, 2007). This appears to be particularly true for interpersonal and uncontrollable stressors (Dickerson & Kemeny, 2004), such as those that are especially relevant to the etiology of several stress-related psychiatric conditions (e.g., depression; Hammen, 2005; Heimberg et al., 2010; Kendler et al., 2002; Mazure, 1998). Exposure to psychosocial stressors stimulates the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the paraventricular nucleus of the hypothalamus. These hormones, in turn, trigger the secretion of adrenocorticotropin (ACTH) from the anterior pituitary, leading to the downstream production and release of glucocorticoids (particularly cortisol in humans and corticosterone in rodents) from the adrenal cortex. Under normal circumstances, these glucocorticoids down-regulate CRH, AVP, and ACTH activity in a negative feedback loop, thereby maintaining neuroendocrinological equilibrium. This negative feedback cycle is mediated by mineralocotiocoid receptors and glucocorticoid receptors, situated particularly in the hippocampus, hypothalamus, and pituitary (Gunnar & Quevedo, 2007). Exposure to chronic or severe stressors, and attendant prolonged elevations in glucocorticoid concentrations, however, can result in dysregulation of the HPA axis (Miller, Chen, & Zhou, 2007). Additionally, and paralleling the finding that early life stressors are associated with long-term changes in microbiome composition (Bailey et al., 2011; Barouei et al., 2012; O’Mahony et al., 2009), adverse childhood experiences appear to produce lasting irregularities in HPA axis functioning, which, in turn, may confer risk for subsequent mental illness (Pariante & Lightman, 2008; Rao, Hammen, Ortiz, Chen, & Poland, 2008).
Indeed, anomalous HPA axis activity has been observed for certain psychiatric conditions, particularly depression. Hypersecretion of CRH has been found for this disorder (Raison et al., 2006). Furthermore, in a meta-analytic review, depression was associated with higher basal cortisol concentrations, and a heightened response to psychosocial stressors (Lopez-Duran, Kovacs, & George, 2009). This hyperactivity of the HPA axis observed in depression is believed to be a consequence of impaired negative feedback regulation of CRH by glucocorticoids, which itself may be a product of altered glucocorticoid receptor functioning (Raison et al., 2006). Furthermore, the dexamethasone suppression test (DST) is an experimental paradigm often used to assess HPA axis functioning, based on the observation that glucocorticoid receptors have an affinity for this steroid (Pariante & Lightman, 2008). With a normally functioning HPA system, DST administration leads to a suppression of CRH and ACTH production, and a resulting decrease in circulating cortisol. In contrast, and indicative of dysfunction in the HPA axis negative feedback system, depression is associated with non-suppression of cortisol in response to the DST (Lopez-Duran et al., 2009). Consistent with this finding, experimental induction of glucocorticoid receptor resistance has been noted to produce depression-like behavior in rodents (Pariante & Lightman, 2008). Treatment with antidepressants appears to lead to resolution of this abnormal glucocorticoid receptor activity on the HPA axis, increasing glucocorticoid receptor expression and functioning (Foster & McVey Neufeld, 2013; Heuser et al., 1996; Pariante & Lightman, 2008).
Potentially mediating the relationship between psychosocial stressors and HPA dysregulation, and thereby depression, are pro-inflammatory cytokines (Maes, 1995; Turnbull & Rivier, 1999). Immunoregulatory inflammation and HPA axis dysregulation have been hypothesized to be part of the same pathophysiology underlying depression (Pariante & Lightman, 2008). Accumulating evidence lends support to the view that pro-inflammatory cytokines have a role in hyperactivation of the HPA axis (Raison et al., 2006). Specifically, immunoregulatory inflammation may be involved in HPA axis activation through the direct effect of pro-inflammatory cytokines on the brain, and indirectly through the development of glucocorticoid receptor resistance (Raison et al., 2006). Support for the first pathway comes from the finding that pro-inflammatory cytokines stimulate the release of CRH, leading to elevated concentrations of glucocorticoids (Dantzer et al., 2008; Maes, 1995). Consistent with the second possibility, IL-1α has been found to impair glucocorticoid receptor functioning through the effect of NF-κB and p38 MAPK (Raison et al., 2006; Wang, Wu, & Miller, 2004). Cytokine-induced glucocorticoid receptor resistance, in turn, may reduce the ability of glucocorticoids to regulate CRH and pro-inflammatory cytokine activity in normative negative feedback loops. Instead, the resulting increase in pro-inflammatory cytokine concentrations leads to further glucocorticoid receptor resistance and attendant CRH dysregulation to form a positive feedback cycle (Dantzer et al., 2008).
Research relating the enteric microbiome to HPA axis functioning was stimulated by the finding that germ-free mice (i.e., those without enteric microbes) exhibited exaggerated ACTH and corticosterone responses to stress (Sudo et al., 2004). This exaggerated stress response can in part be reversed with the administration of Bifidobacterium infantis, and importantly, enteric colonization must occur during a sensitive early developmental window for normative HPA axis functioning to emerge (Sudo et al., 2004). Germ-free mice appear to exhibit reduced anxiety, however, a finding that runs counter to what would be expected with a hyperactive HPA system (Cryan & Dinan, 2012). Perhaps more clearly suggestive of the potential influence of the enteric microbiota on HPA axis functioning, a recent study found LPS to stimulate the production of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, and consequently, glucocorticoids (Glennon, Kaunzner, Gagnidze, McEwen, & Bulloch, 2015). Furthermore, administration of probiotics consisting of Lactobacillus spp. during early stress exposure in rats has been found to normalize basal corticosterone concentrations (Foster & McVey Neufeld, 2013). Congruent with this finding, prophylactic administration of rats with the probiotic Lactobacillus farciminis results in decreased intestinal permeability and prevents HPA dysregulation in response to stress (Ait-Belgnaoui et al., 2012). Additionally, Lactobacillus spp. leads to reductions in corticosterone concentrations in rodents subjected to early maternal separation (Dinan et al., 2015). Although Bifidobacterium infantis was found to reduce stress response with maternal separation paradigm, corticosterone levels remained unchanged (Dinan et al., 2015), perhaps suggestive of a degree of specificity in the bacterial species involved in modulating the HPA axis. In a rare human study, albeit with healthy volunteers, a combination of Lactobacillus helveticus and Bifidobacterium longum resulted in lowered urinary free cortisol (Messaoudi, Violle, et al., 2011). Also supportive of potential microbial influences on the HPA axis, ingestion of a prebiotic compound led to a reduction in waking cortisol in healthy humans (Schmidt et al., 2015). Administration of Lactobacillus spp. and Bifidobacterium spp., however, yielded no effect on HPA axis activity in a recent randomized control trial with humans (Mohammadi et al., in press), suggestive of the need for more research to clarify the specific microbes that may potentially modulate HPA axis activity.
Vagus nerve
Finally, a vagal-mediated pathway may also be involved in the link between stress-induced dysbiosis, cytokine activity, and psychiatric illness. Cardiac vagal control (CVC; i.e., respiratory-associated changes in heart rate) is often assessed in the psychological literature as a physiological index of emotion regulation, especially in response to stress. CVC has been studied particularly with depression, with lower heart rate variability being found to be associated with this disorder (Kemp et al., 2010; Raison et al., 2006). Moreover, vagus nerve stimulation is FDA-approved as an adjunctive treatment for refractory depression (US Food and Drug Administration, 2005).
Recent evidence has also emerged implicating the vagus nerve as a neural pathway in the relationship between the enteric microbiota and mental health. Specifically, the anti-depressant and anxiolytic properties and associated neurochemical effects of Lactobacillus rhamnosus are absent in vagotomized rodents (Bravo et al., 2011). Vagotomy in rodents also appears to prevent colitis-induced anxiety-like behavior (Bercik, Park, et al., 2011). Furthermore, administration of the probiotic Bifidobacterium longum requires an intact vagus nerve to exert an anxiolytic effect. Contrastingly, antibiotic-induced alterations in the enteric microbiota appear to produce behavioral and neurochemical changes through pathways independent of vagus nerve function, suggesting pathways other than the vagus nerve may exist between the enteric microbiota and the brain (e.g., HPA axis hyperactivation; Bercik, Denou, et al., 2011; Dinan et al., 2015). Although it is not yet entirely clear how the enteric microbiota activates the vagus nerve, pro-inflammatory cytokines may be one potential mechanism. Indeed, stimulation of the vagus nerve has been observed to occur in response to pro-inflammatory cytokines (e.g., IL-1β and TNF-α), as well as endotoxins (e.g., LPS) that produce a pro-inflammatory immunological response (Collins & Bercik, 2009).
An integrative model
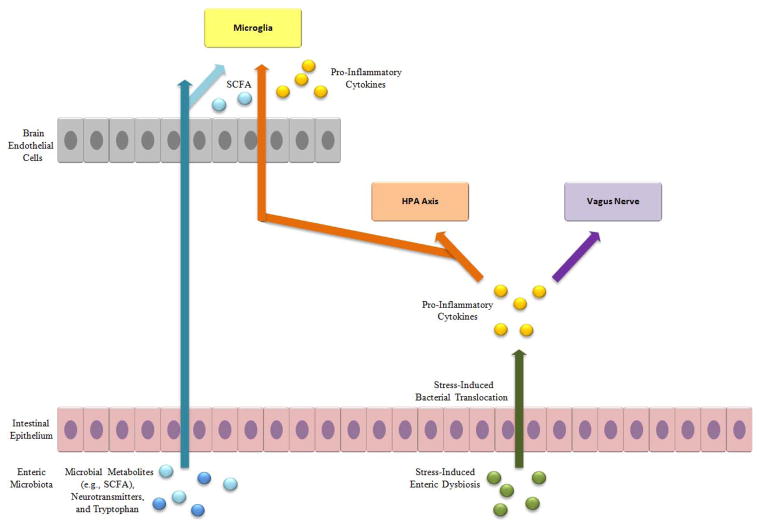
Drawing on these different lines of research, an integrative conceptual model of the relation between the enteric microbiome and mental illness is proposed with the view of guiding future work in this area (see Figure 1). According to this model, in the case of a healthy enteric microbiota, microbial by-products (e.g., SCFAs, such as butyrate, as well as neurotransmitters and the serotonin precursor tryptophan) are directly involved in maintaining health brain functioning, being able to cross the blood-brain barrier. The microbial by-product tryptophan is synthesized into serotonin after crossing this barrier. SCFAs, in particular, are involved in tightening the junctions between cells in the blood-brain barrier, and in proper microglia development and functioning.
Figure 1.
Schematic diagram of an integrative conceptual model of potential immunoregulatory, endocrinal, and neural pathways underlying the relationship between psychosocial stressors, the enteric microbiota, and stress-related psychiatric illness.
The enteric microbiota is disturbed by psychosocial stressors (i.e., enteric dysbiosis), particularly in terms of a reduction in bifidobacteria and lactobacilli, and lead to increased permeability of the intestinal epithelium, which, in turn, permits bacterial translocation across the intestinal mucosa into the MLNs and bloodstream. The stress-induced enteric dysregulation confers risk for psychiatric illness through immunoregulatory, endocrinal, and neural pathways. Specifically, enteric dysfunction, especially as manifested by the presence of LPS in translocated bacteria, stimulates the production of circulating pro-inflammatory cytokines (e.g., IL-1α, IL-1β, IL-6, and TNF-α). These pro-inflammatory cytokines cause dysregulation of the HPA system by stimulating overproduction of CRH and glucocorticoid resistance. This immune activation also mediates the effect of stress-induced enteric dysfunction through a vagal afferent pathway. The dysregulation of the HPA system and vagus nerve activation, in turn, lead to elevated risk for stress-related psychiatric disorders. Finally, proinflammatory cytokines are also able to migrate across the blood-brain barrier, stimulating a neuroinflammatory reaction from microglia, which draw monocytes from the peripheral immune system. It should be noted that this proposed model is by no means exhaustive in delineating the potential mediating pathways linking enteric dysregulation and mental illness. Rather, the emphasis in the current model is on potential mechanisms that have been linked to stress and implicated in stress-related psychiatric conditions, particularly depression.
Conclusions
The research to date is consistent with the possibility that stress-induced enteric dysfunction may heighten risk for psychiatric illness through a combination of immunoregulatory, endocrinal, and neural mechanisms. There is need for future studies directly examining the interrelationship between these processes, particularly in terms of mediational relationships. Furthermore, much of the existing research has been conducted with animal models, and to a lesser degree, healthy human subjects. Although such work is important, more research is required with psychiatric populations to determine the relevance of these potential mechanisms to clinically meaningful phenomena. Elucidating the relation between the enteric microbiome and stress-related manifestations of mental illness, as well as the processes underlying this relationship, is clinically important insofar as it may be a promising avenue for the development of a novel class of treatments for these conditions. It is worth noting within this context that there is some preliminary evidence in a rodent study (Messaoudi, Violle, et al., 2011) that probiotics may be free of some of the cognitive side-effects and addictive properties of some currently available psychopharmacological medications, which is suggestive of their potential safety and tolerability as a form of treatment. With the recent stagnation in the development of psychopharmacological agents for treating mental illness (Hyman, 2012; Insel, 2015; Miller, 2010), the need for the development of new treatment options is more pressing than ever.
Acknowledgments
Preparation of this manuscript was supported in part by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH101138. The content is solely the responsibility of the author and does not necessarily represent the official views of the funding agency.
References
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Bharwani A, Lee KR, Fox M, Thomson JA. Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neuroscience and Biobehavioral Reviews. 2015;51:164–188. doi: 10.1016/j.neubiorev.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Rudensky AY. Microbial metabolites control gut inflammatory responses. Proceedings of the National Academy of Sciences. 2014;111:2058–2059. doi: 10.1073/pnas.1323183111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault-Bréard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R, Rousseau G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. British Journal Of Nutrition. 2012;107:1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Developmental Psychobiology. 1999;35:146–155. [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain, Behavior, and Immunity. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Parry NMA, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infection and Immunity. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsgaard Bendtsen KM, Krych L, Sørensen DB, Pang W, Nielsen DS, Josefsen K, Hansen LH, et al. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PloS One. 2012;7:e46231. doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouei J, Moussavi M, Hodgson DM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS ONE. 2012;7:e46051. doi: 10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Cognitive models of depression. Journal of Cognitive Psychotherapy. 1987;1:5–37. [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. European Journal Of Clinical Nutrition. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609.e1–3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology And Motility. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annual Review of Medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, Taylor CM, Welsh DA, Berthoud HR. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biological Psychiatry. 2015;77:607–615. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. PNAS. 2011;108(Suppl):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HMB, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: Implications for treatment. Annual Review of Neuroscience. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Levinson AJ, Daskalakis ZJ. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neuroscience and Biobehavioral Reviews. 2011;35:818–825. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behavior. Nature Reviews Neuroscience. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. Journal of Neuroscience. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota–gut–brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? Journal of Physiology. 2014;592:2989–2997. doi: 10.1113/jphysiol.2014.273995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bella JM, Bao Y, Gloor GB, Burton JP, Reid G. High throughput sequencing methods and analysis for microbiome research. Journal of Microbiological Methods. 2013;95:401–414. doi: 10.1016/j.mimet.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation: An experimental laboratory investigation. Psychological science. 2009;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: How gut microbes shape human behavior. Journal of Psychiatric Research. 2015;63:1–9. doi: 10.1016/j.jpsychires.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Elwood LS, Hahn KS, Olatunji BO, Williams NL. Cognitive vulnerabilities to the development of PTSD: A review of four vulnerabilities and the proposal of an integrative vulnerability model. Clinical Psychology Review. 2009;29:87–100. doi: 10.1016/j.cpr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends in Neurosciences. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Fraher MH, O’Toole PW, Quigley EMM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nature Reviews Gastroenterology & Hepatology. 2012;9:312–322. doi: 10.1038/nrgastro.2012.44. [DOI] [PubMed] [Google Scholar]
- Galley JD, Bailey MT. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes. 2014;5:390–396. doi: 10.4161/gmic.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Current Molecular Medicine. 2008;8:274–281. doi: 10.2174/156652408784533760. [DOI] [PubMed] [Google Scholar]
- Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon E, Kaunzner UW, Gagnidze K, McEwen BS, Bulloch K. Pituitary dendritic cells communicate immune pathogenic signals. Brain, Behavior, and Immunity. 2015;50:232–240. doi: 10.1016/j.bbi.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The Neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Bruce AE, Lumley MN. The role of childhood abuse and neglect in the sensitization to stressful life events in adolescent depression. Journal of Abnormal Psychology. 2006;115:730–741. doi: 10.1037/0021-843X.115.4.730. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Brozovich FA, Rapee RM. A cognitive behavioral model of social anxiety disorder: Update and extension. In: Hofmann SG, DiBartolo PM, editors. Social anxiety: Clinical, developmental, and social perspective. 2. New York, NY: Elsevier; 2010. pp. 395–422. [Google Scholar]
- Heuser IJ, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, Yassouridis A, et al. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. American Journal of Psychiatry. 1996;153:93–99. doi: 10.1176/ajp.153.1.93. [DOI] [PubMed] [Google Scholar]
- Hill J, Pickles A, Rollinson L, Davies R, Byatt M. Juvenile- versus adult-onset depression: Multiple differences imply different pathways. Psychological Medicine. 2004;34:1483–1493. doi: 10.1017/S0033291704002843. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Revolution stalled. Science Translational Medicine. 2012;4:155cm11, 155cm11. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- Insel TR. The NIMH experimental medicine initiative. World Psychiatry. 2015;14:151–153. doi: 10.1002/wps.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Archives of General Psychiatry. 2002;59:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Jones JC, Barlow DH. The etiology of posttraumatic stress disorder. Clinical Psychology Review. 1990;10:299–328. doi: 10.1016/0272-7358(90)90064-H. [DOI] [Google Scholar]
- Kaplan BJ, Rucklidge JJ, Romijn A, McLeod K. The emerging field of nutritional mental health: Inflammation, the microbiome, oxidative stress, and mitochondrial function. Clinical Psychological Science. 2015;3:964–980. doi: 10.1177/2167702614555413. [DOI] [Google Scholar]
- Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Martin A, King RA, Charney D. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biological Psychiatry. 2001;49:980–1001. doi: 10.1016/S0006-3223(01)01127-1. [DOI] [PubMed] [Google Scholar]
- Kemeny ME. Psychobiological responses to social threat: Evolution of a psychological model in psychoneuroimmunology. Brain, Behavior, and Immunity. 2009;23:1–9. doi: 10.1016/j.bbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kiliaan AJ, Saunders PR, Bijlsma PB, Berin MC, Taminiau JA, Groot JA, Perdue MH. Stress stimulates transepithelial macromolecular uptake in rat jejunum. American Journal of Physiology. 1998;275:G1037–1044. doi: 10.1152/ajpgi.1998.275.5.G1037. [DOI] [PubMed] [Google Scholar]
- Klauke B, Deckert J, Reif A, Pauli P, Domschke K. Life events in panic disorder—An update on “candidate stressors. Depression and Anxiety. 2010;27:716–730. doi: 10.1002/da.20667. [DOI] [PubMed] [Google Scholar]
- Knowles SR, Nelson EA, Palombo EA. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: A possible mechanism underlying susceptibility to illness. Biological Psychology. 2008;77:132–137. doi: 10.1016/j.biopsycho.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Goldman D, Buzas B, Hodgkinson C, Leibenluft E, Nelson E, Sankin L, et al. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. NeuroImage. 2010;53:952–961. doi: 10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- Liu RT, Miller I. Life events and suicidal ideation and behavior: A systematic review. Clinical Psychology Review. 2014;34:181–192. doi: 10.1016/j.cpr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiology & Behavior. 1998;65:63–68. doi: 10.1016/S0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Annals of the New York Academy of Sciences. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinology Letters. 2008;29:117–124. [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. Journal of Affective Disorders. 2012;141:55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Benninson L, Ursell L, Greenwood BN, et al. Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1β and IL-18 but not IL-6, IL-10 or MCP-1. PLoS ONE. 2012;7:e50636. doi: 10.1371/journal.pone.0050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: Paradigm shift in neuroscience. Journal of Neuroscience. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM. Life stressors as risk factors in depression. Clinical Psychology: Science and Practice. 1998;5:291–313. doi: 10.1111/j.1468-2850.1998.tb00151.x. [DOI] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication II: Associations with persistence of DSM-IV disorders. Archives of General Psychiatry. 2010;67:124–132. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Neufeld KA, Luczynski P, Dinan TG, Cryan JF. Reframing the teenage wasteland: Adolescent microbiota-gut-brain axis. Canadian Journal of Psychiatry. 2016;61:214–221. doi: 10.1177/0706743716635536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Neufeld K-A, Luczynski P, Seira Oriach C, Dinan TG, Cryan JF. What’s bugging your teen? The microbiota and adolescent mental health. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2016.06.005. (in press) [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal Of Nutrition. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- Miller G. Is pharma running out of brainy ideas? Science. 2010;329:502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosomatic Medicine. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research review: The role of cytokines in depression in adolescents: a systematic review. Journal of Child Psychology and Psychiatry. 2013;54:816–835. doi: 10.1111/jcpp.12080. [DOI] [PubMed] [Google Scholar]
- Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Adab Z, et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutritional Neuroscience. doi: 10.1179/1476830515Y.0000000023. (in press) [DOI] [PubMed] [Google Scholar]
- Murphy MLM, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clinical Psychological Science. 2013;1:30–40. doi: 10.1177/2167702612455743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterology & Motility. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Needham BL, Epel ES, Adler NE, Kiefe C. Trajectories of change in obesity and symptoms of depression: The CARDIA study. American Journal of Public Health. 2010;100:1040–1046. doi: 10.2105/AJPH.2009.172809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EMM, Cryan JF, et al. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biological Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Pariante C, Lightman S. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathogens. 2009;1:6–6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiology of Stress. doi: 10.1016/j.ynstr.2016.03.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature Reviews Gastroenterology & Hepatology. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M. Toward a true bacteriotherapy for Clostridium difficile infection. New England Journal of Medicine. 2015;372:1566–1568. doi: 10.1056/NEJMcibr1500270. [DOI] [PubMed] [Google Scholar]
- Russell WR, Hoyles L, Flint HJ, Dumas ME. Colonic bacterial metabolites and human health. Current Opinion in Microbiology. 2013;16:246–254. doi: 10.1016/j.mib.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host & Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders PR, Hanssen NP, Perdue MH. Cholinergic nerves mediate stress-induced intestinal transport abnormalities in Wistar-Kyoto rats. American Journal of Physiology. 1997;273:G486–490. doi: 10.1152/ajpgi.1997.273.2.G486. [DOI] [PubMed] [Google Scholar]
- Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. American Journal of Physiology. 1994;267:G794–799. doi: 10.1152/ajpgi.1994.267.5.G794. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterology & Motility. 2014;26:1615–1627. doi: 10.1111/nmo;12427. [DOI] [PubMed] [Google Scholar]
- Schmidt C. Mental health: Thinking from the gut. Nature. 2015;518:S12–S15. doi: 10.1038/518S13a. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PWJ. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology. 2015;232:1793–1801. doi: 10.1007/s00213-014-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Smith PA. The tantalizing links between gut microbes and the brain. Nature. 2015;526:312–314. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. American Journal of Physiology Gastrointestinal and Liver Physiology. 2001;280:G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annual Review of Clinical Psychology. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behavior, and Immunity. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes, Brain, and Behavior. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. Journal of Physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Human Microbiome Project Consortium. A framework for human microbiome research. Nature. 2012a;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012b;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. 1401.e1–4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The Human Microbiome Project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiological Reviews. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. New Device Approval: VNS Therapy System - P970003s050. 2005 Retrieved from www.fda.gov/cdrh/mda/docs/p970003s050.html.
- Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Advances in Experimental Medicine and Biology. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Molecular Psychiatry. 2004;9:65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareie M, Johnson-Henry K, Jury J, Yang P, Ngan B, McKay DM, Soderholm JD, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]