Corresponding Author

Key Words: apoptosis, cell death, ischemia, necrosis, troponin
Release of the cardiac-specific isoform of troponin I (cTnI) into the systemic circulation is frequently taken as a biomarker of cardiomyocyte necrosis and used in the diagnosis of myocardial infarction. But, transient increases in blood cTnI concentrations are observed in seemingly healthy individuals following strenuous exercise, asymptomatic patients, and disease states other than acute coronary syndromes (1). The latter include short bouts of ischemia insufficient to cause infarction, heart failure, myocarditis, pulmonary embolism, arrhythmias, sepsis, and trauma. Although cardiomyocyte death is a component of some of these conditions, its involvement in others may be less prominent or questionable. Blood cTnI concentrations remain an important diagnostic test in the clinical context of acute coronary syndromes, but these observations highlight the need for caveats in their interpretation. Moreover, they raise questions regarding the cellular mechanisms that mediate cTnI release and, accordingly, the cellular processes for which it serves as a biomarker. These questions are important because increases in blood cTnI concentrations are associated with adverse cardiovascular events and mortality.
In this issue of JACC: Basic to Translational Science, Weil et al. (2) report that a mere 10 min of myocardial ischemia followed by 24 h of reperfusion is sufficient to cause release of cTnI into the blood, and then proceed to investigate mechanisms. Ischemia was induced in closed-chested pigs using a balloon catheter in the left anterior descending artery, a highly controlled and clinically relevant model. Hemodynamic and echocardiographic monitoring confirmed the expected transient changes in left ventricular end-diastolic pressure and cardiac function. Serum cTnI concentrations, assessed with a porcine-specific kit, increased from 13 ng/l at baseline to 1,021 ng/l at 24 h following reperfusion—with 38 ng/l being the 99th percentile in normal pigs.
So, why did brief ischemia followed by reperfusion precipitate cTnI release? Several hypotheses were considered, starting with the possibility that this occurred independently of cardiomyocyte death. Of relevance, a small pool of cTnI is unincorporated into sarcomeres—possibly in the cytosol—and, therefore, might be available to escape the cell if the plasma membrane were perturbed (3). Two mechanisms for the latter have been entertained: reversible membrane permeabilization (possibly through stretch-responsive integrins) and shedding of membrane “blebs.” In addition, proteolysis of troponin, induced by ischemia and increased pre-load, has been hypothesized to facilitate the exit of this protein from cardiomyocytes. Although these individual mechanisms were not investigated, the authors surmised from their data that the gradual early rise in blood cTnI concentrations and the absence of a significant gradient between coronary venous and systemic venous circulations argue against early release of a free cTnI pool.
They next turned their attention to mechanisms related to cardiomyocyte death. The classic study of Jennings et al. (4) in canine models showed that ischemia exceeding 20 min is required to induce necrosis in cardiomyocytes. Thus, it is not surprising that hearts subjected to 10 min of ischemia followed by either 1 or 24 h of reperfusion in this study failed to show evidence of necrosis by hematoxylin and eosin staining (no myocyte nuclear loss, inflammatory cell infiltration, or contraction bands). In addition, contiguous regions exhibiting loss of viability (i.e., an infarct) were not observed by staining with 2,3,5-triphenyltetrazolium chloride, a colorimetric substrate that reflects mitochondrial function. In contrast, markers of apoptosis were present in cardiomyocytes at 1 h—but not 24 h—following reperfusion. These included a 6-fold increase in terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), reflective of DNA degradation; and immunostaining for active caspase-3, a protease in the final common pathway of apoptosis. The authors concluded that cardiomyocyte apoptosis was responsible for the release of cTnI.
Is this a reasonable conclusion? One question is whether the magnitude of cardiomyocyte apoptosis can account for the amount of cTnI release? Approximately 18 cardiomyocytes/cm2 (1 in 6,000 cardiomyocytes) (J. Canty, March, 2017) were TUNEL positive in the ischemic zone at 1 h of reperfusion, while the maximum serum cTnI concentration was ∼1,000 ng/l at 24 h of reperfusion. Although this cTnI concentration is ∼50-fold higher than the 99th percentile in normal pigs, it is quite modest compared to the serum cTnI concentrations observed with reperfused porcine myocardial infarction following an hour of ischemia, which are frequently in the 30,000 to 100,000 ng/l range. Moreover, the duration that cardiomyocyte TUNEL positivity persists in this setting is not known making it impossible to extrapolate from this marker to the cumulative magnitude of cardiomyocyte death it represents. Therefore, while this issue cannot be addressed definitively, we believe that it is possible that the frequency of TUNEL-positive cardiomyocytes could account for the serum cTnI concentrations.
If we assume that release of cTnI during cell death entails loss of plasma membrane integrity, an important fundamental issue is why this would be happening in apoptotic cardiomyocytes. In principle, cells undergoing apoptosis fragment into apoptotic bodies that maintain membrane integrity until undergoing phagocytosis by macrophages or neighboring cells. This prevents the release of intracellular molecules—including the ones that incite the intense inflammatory response typical of necrotic cell death. Thus, if these cardiomyocytes are really undergoing apoptosis, why are their plasma membranes becoming leaky? One possibility is that phagocytic cleanup is somehow defective in this context. As this parameter was not assessed in this study, we cannot exclude this explanation. However, if inadequate cleanup were the mechanism for plasma membrane breakdown, we would have expected the progressive accumulation of cellular debris and inflammation, and this was not observed at either the 1-h or 24-h time points of reperfusion.
Another possibility to explain a breakdown in plasma membrane integrity is that what began as cardiomyocyte apoptosis transitioned to secondary necrosis. To understand how this may occur, we will review apoptosis and necrosis signaling and then how they may converge (5). Both forms of cell death are mediated by 2 interconnected pathways, 1 involving cell surface death receptors and the other involving the mitochondria. Regardless of pathway, the defining molecular event in apoptosis is activation of caspases, cysteine proteases that cut certain cellular proteins to bring about cell death. In the death receptor apoptosis pathway, caspases are activated by recruitment into complexes containing death receptors and associated proteins. In the mitochondrial apoptosis pathway, caspase activation is triggered by BAX/BAK-dependent permeabilization of the outer mitochondrial membrane (OMM) allowing the release of cytochrome c into the cytosol where it stimulates events leading to caspase activation. In contrast, the defining molecular events in necrosis differ depending on the pathway. In the death receptor necrosis pathway (referred to as necroptosis), this involves activation of RIP1/3 kinases. In the mitochondrial necrosis pathway, on the other hand, the triggering event is Ca2+-induced opening of the permeability transition pore (PTP) in the inner mitochondrial membrane (IMM). This results in immediate dissipation of the proton gradient that drives adenosine triphosphate synthesis—producing energetic failure and necrosis. All 4 of these pathways—death receptor apoptosis, death receptor necrosis, mitochondrial apoptosis, and mitochondrial necrosis—play significant roles in cardiomyocyte death during heart disease. But, the relative contributions of each differ depending on the clinical context. For example, the acute injury of myocardial infarction with reperfusion (initiated by prolonged ischemia—not the 10 min employed in this study) involves mostly necrotic cell death that is mediated by both death receptor and mitochondrial pathways. In contrast, cardiomyocyte death during remodeling and heart failure involves mainly apoptosis.
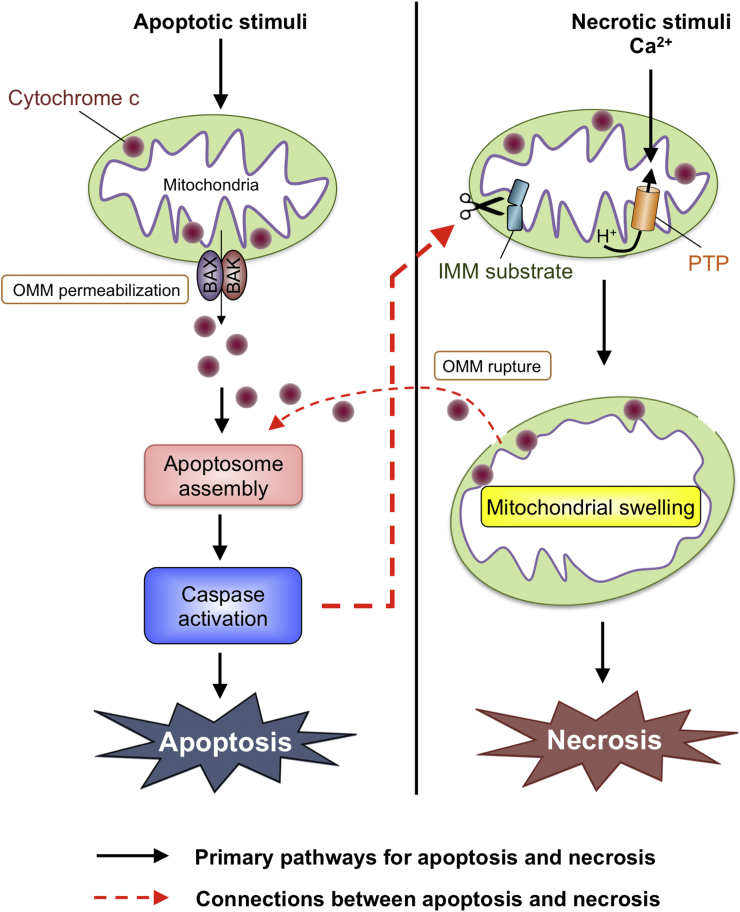
Although still incompletely defined, bidirectional connections exist between apoptosis and necrosis signaling in both death receptor and mitochondrial pathways. For example, opening of the PTP in the IMM during necrosis can lead to caspase activation (Figure 1). This is thought to be mediated by cytochrome c release resulting from rupture of the OMM during necrosis as opposed to BAX/BAK-dependent OMM permeabilization as during apoptosis. OMM rupture is likely the consequence of mitochondrial swelling induced by the massive inflow of water into the hyperosmolar mitochondrial matrix when the PTP opens. Conversely—and possibly relevant to this study—caspase activation during apoptosis may lead to necrosis through cleavage of IMM substrates that precipitate PTP opening directly or indirectly through the generation of oxidative stress (Figure 1). Although portions of these apoptosis-necrosis connections have been studied, these pathways require further definition and testing.
Figure 1.
Potential Connections Between Apoptosis and Necrosis Signaling in the Mitochondrial Pathway
Apoptosis: BAX and BAK induce permeabilization of the outer mitochondrial membrane (OMM). This allows the release of cytochrome c to the cytosol, where it facilitates assembly of a multiprotein complex called the apoptosome in which caspase activation takes place. Necrosis: Ca2+ triggers opening of the permeability transition pore (PTP) in the inner mitochondrial membrane (IMM). This precipitates loss of the IMM proton gradient resulting in cessation of adenosine triphosphate synthesis. Connections: Caspases activated during apoptosis may cleave IMM substrates facilitating PTP opening and secondary necrosis (bolded, dashed, red line). Conversely, PTP opening during primary necrosis may cause mitochondrial swelling and OMM rupture, resulting in cytochrome c release and caspase activation (non-bolded, dashed, red line).
In conclusion, this study by Weil et al. (2) provides important information. First, it establishes definitively that a short duration of ischemia typical of an angina episode is adequate to cause a cTnI leak. Second, it raises the possibility that cTnI release can result from focal cardiomyocyte apoptosis with eventual plasma membrane breakdown. Additional work in cell systems and animal models will be needed to test this mechanism.
Acknowledgments
The authors appreciate critical comments from Dr. David J. Lefer. The authors thank the Wilf family for their generous support.
Footnotes
Ms. Amgalan was supported by an American Heart Association Predoctoral Fellowship (15PRE25080032). Dr. Kitsis was supported by grants from the National Institutes of Health (R01HL128071, R01HL130861, R01CA17091), Department of Defense (DOD) (PR151134P1), American Heart Association (15CSA26240000), Fondation Leducq (RA15CVD04), Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease, and the Wilf Family Cardiovascular Research Institute. Dr. Pekson has reported that he has no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Giannitsis E., Katus H.A. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol. 2013;10:623–634. doi: 10.1038/nrcardio.2013.129. [DOI] [PubMed] [Google Scholar]
- 2.Weil B.R., Young R.F., Shen X. Brief myocardial ischemia produces cardiac troponin I release and focal myocyte apoptosis in the absence of pathological infarction in swine. J Am Coll Cardiol Basic Trans Science. 2017;2:105–114. doi: 10.1016/j.jacbts.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White H.D. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–2408. doi: 10.1016/j.jacc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Jennings R.B., Sommers H.M., Herdson P.B., Kaltenbach J.P. Ischemic injury of myocardium. Ann NY Acad Sci. 1969;156:61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinidis K., Whelan R.S., Kitsis R.N. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]