Abstract
Objectives
This study compared the clinical performance of the Canadian CT Head Rule (CCHR) and the New Orleans Criteria (NOC) for detecting any traumatic intracranial lesion on computed tomography (CT) in patients with a Glasgow Coma Scale (GCS) score of 15. Also assessed were ability to detect patients with “clinically important” brain injury and patients requiring neurosurgical intervention. Additionally, the performance of the CCHR was assessed in a larger cohort of those presenting with GCS of 13 to 15.
Methods
This prospective cohort study was conducted in a U.S. Level I trauma center and enrolled a consecutive sample of mildly head-injured adults who presented to the emergency department (ED) with witnessed loss of consciousness, disorientation or amnesia, and GCS 13 to 15. The rules were compared in the group of patients with GCS 15. The primary outcome was prediction of “any traumatic intracranial injury” on CT. Secondary outcomes included “clinically important brain injury” on CT and need for neurosurgical intervention.
Results
Among the 431 enrolled patients, 314 patients (73%) had a GCS of 15, and 22 of the 314 (7%) had evidence of a traumatic intracranial lesion on CT. There were 11 of 314 (3.5%) who had “clinically important” brain injury, and 3 of 314 (1.0%) required neurosurgical intervention. The NOC and CCHR both had 100% sensitivity (95% confidence interval [CI] = 82% to 100%), but the CCHR was more specific for detecting any traumatic intracranial lesion on CT, with a specificity of 36.3% (95% CI = 31% to 42%) versus 10.2% (95% CI = 7% to 14%) for NOC. For “clinically important” brain lesions, the CCHR and the NOC had similar sensitivity (both 100%; 95% CI = 68% to 100%), but the specificity was 35% (95% CI = 30% to 41%) for CCHR and 9.9% (95% CI = 7% to 14%) for NOC. When the rules were compared for predicting need for neurosurgical intervention, the sensitivity was equivalent at 100% (95% CI = 31% to 100%) but the CCHR had a higher specificity at 80.7% (95% CI = 76% to 85%) versus 9.6% (95% CI = 7% to 14%) for NOC. Among all 431 patients with a GCS score 13 to 15, the CCHR had sensitivities of 100% (95% CI = 84% to 100%) for 27 patients with clinically important brain injury and 100% (95% CI = 46% to 100%) for five patients requiring neurosurgical intervention.
Conclusions
In a U.S. sample of mildly head-injured patients, the CCHR and the NOC had equivalently high sensitivities for detecting any traumatic intracranial lesion on CT, clinically important brain injury, and neurosurgical intervention, but the CCHR was more specific. A larger cohort will be needed to validate these findings.
Over 1.7 million traumatic brain injuries (TBIs) occur annually in the United States. Of these cases, approximately 52,000 result in death, 275,000 are hospitalized, and 1.4 million are treated and released from emergency departments (EDs) across the country.1 Over 75% of treated TBI cases are classified as mild based on their Glasgow Coma Scale (GCS) score.2 While most mild TBI patients do not require treatment, an estimated 6% to 9% have intracranial injuries and 0.4% to 1% require critical neurosurgical intervention.3,4 In these patients, computed tomography (CT) imaging has proven essential in early diagnosis and intervention.5 Although severe complications requiring neurosurgical intervention are rare in mild TBI patients, fear of the dire consequences of delayed treatment has led many to advocate for the liberal use of CT scanning in patients with mild TBI.6,7 This follows the trend of increasing CT usage in diagnosis; according to recent estimates, 62 million CT scans are performed annually in the United States.8
While increasing CT use has considerably improved diagnostic capabilities and reduced hospital admissions,9 it has also raised concern over unnecessary exposure to ionizing radiation.10–14 Although the calculation of projected cancer risk is still controversial, some studies suggest that CT scans of the head may be among the largest contributors to radiation exposure due to the frequency with which they are performed.15 There is significant consensus that efforts should be made to standardize CT usage to prevent unnecessary radiation exposure while maintaining quality of care.10,11,16,17 In an effort to increase the efficiency of CT usage in TBI cases in the ED, a number of independent clinical decision rules, such as the Canadian CT Head Rule (CCHR), the New Orleans Criteria (NOC), and the National Emergency X-Radiography Utilization Study II (NEXUS II) criteria, have been developed.18–24 The earliest and most frequently studied of these are the CCHR and NOC. To date, at least four separate studies have compared the performance of the CCHR and the NOC in identifying patients at high risk for intracranial injury and in eliminating unnecessary CT use.20–23 While both were found to be highly sensitive in detecting intracranial injuries, their specificities varied.
The CCHR and the NOC have been compared in Canadian, Dutch, Italian, and Australian patients, but there is a lack of published research comparing their effectiveness in U.S. EDs. In particular, the CCHR has not been prospectively studied in a U.S. cohort, and therefore there may be some apprehension among emergency physicians (EPs) in the United States in applying it to their practice. Moreover, the CCHR has used “clinically significant” intracranial injuries as their endpoint to reflect practice of Canadian neurosurgeons,25 which may not necessarily reflect the clinical practice in the United States. With a lack of conclusive research on the CCHR in the United States, the use of CCHR by U.S. EPs in mild TBI cases is left largely to individual practice.26
The purpose of this study was to compare the performance of the CCHR and the NOC in detecting all intracranial injuries following minor blunt head injury with a GCS 15 in a U.S. patient population. Additionally, the CCHR was assessed for clinically important brain injury and need for neurosurgical intervention in those presenting with GCS of 13 to 15. The purpose of this additional analysis is to test the performance of the CCHR in the population in which it had originally been derived, including those with GCS 13 and 14. Physicians were given the opportunity to rate their comfort level with each rule.
METHODS
Study Design
This was a prospective observational cohort study. The institutional review board of the study hospital approved the protocol with waiver of written informed consent.
Study Setting and Population
The study was conducted at a single tertiary care Level I trauma center in the United States with an annual ED census of 45,000 visits. The study enrolled a consecutive sample of adult patients 24 hours/day, 7 days/week presenting to the ED following a blunt minor head injury (suspected mild TBI) within 24 hours of injury.
Eligibility for suspected mild TBI was determined by the treating physician based on a definition of blunt trauma to the head resulting in either witnessed loss of consciousness, definite amnesia, or witnessed disorientation with an initial ED GCS score of 13 to 15. Ordering of the CT was based solely on physician judgment and was not required for inclusion in the study. Exclusion criteria included: 1) patients less than 18 years old; 2) minimal head injury without loss of consciousness, amnesia, or disorientation; 3) no clear history of trauma as the primary event (e.g., primary seizure or syncope); 4) an obvious penetrating skull injury or obvious depressed fracture; 5) an acute focal neurologic deficit; 6) unstable vital signs associated with major trauma; 7) a seizure before assessment in the ED; 8) a bleeding disorder or use of oral anticoagulants (e.g., warfarin); 9) returned for reassessment of the same head injury; and 10) pregnant.
Study Protocol
All patient assessments were made by board-certified EPs or by supervised emergency medicine residents. The physician assessors were trained by a 1-hour session to evaluate patients for the standardized clinical findings from the history, general examination, and neurologic status for each of the two rules. For patients transferred from another primary care facility or hospital, study assessments were undertaken after the patients had arrived at the study site. In the transferred cases every attempt was made to keep the assessment blinded. After the clinical examination and prior to the CT, physicians completed a standard data form that listed the criteria for each of the two rules. Physicians were also asked if the rule was positive or negative for NOC or low, medium, or high risk for CCHR. Patients underwent standard CT of the head according to the judgment of the treating physician. The study protocol did not alter physician practice. CT scans were interpreted by board-certified neuroradiologists who were blinded to the contents of the data collection sheet, but were aware of the patients’ clinical histories. Additionally, daily ED records were screened to identify any missed eligible patients for whom data collection forms had not been completed. For these patients, information on age, sex, and CT was recorded.
Treating physician acceptability of the decision rules was assessed as part of the standard data form for each patient upon enrolment. After completing the clinical examination and assessing the clinical variables for each rule prior to the CT scan, physicians completed a five-point Likert scale (very comfortable, comfortable, neutral, uncomfortable, and very uncomfortable) indicating their comfort level with applying the rules clinically. Specifically, physicians were asked prior to the CT being performed, “How comfortable would you be in following the Canadian CT Head Rule for this patient?” and “How comfortable would you be in following the New Orleans Criteria for this patient?”
Outcome Measures
The primary outcome for patients with a GCS 15 was “any brain injury” (any traumatic intracranial lesion) on CT, and the secondary outcomes were “clinically important brain injury” and “need for neurosurgical intervention.” The primary outcomes for patients with a GCS 13 to 15 were “clinically important brain injury” and “need for neurosurgical intervention.” Need for neurosurgical intervention was defined as either death within 7 days secondary to head injury or the need for any of the following procedures within 7 days: craniotomy, elevation of skull fracture, intracranial pressure monitoring, or intubation for head injury (shown on CT). “Clinically important brain injury” was defined as any acute traumatic lesion found on CT that would normally require admission to hospital and neurologic follow-up. This definition has been previously described in other related studies.20,25,27 All brain injuries are judged clinically important unless the patient is neurologically intact and has one of these lesions on CT: solitary contusion less than 5 mm in diameter, localized subarachnoid blood less than 1 mm thick, smear subdural hematoma less than 4 mm thick, isolated pneumocephaly, or closed depressed skull fracture not through the inner table.18
The NOC were developed to guide CT use in patients with GCS 15 and not those with GCS 13 or 14. CCHR was originally developed to guide CT use in patients with GCS 13 to 15. We specifically examined the performance of the CCHR and NOC in patients with GCS 15 to make the two rules comparable.
Data Analysis
Descriptive statistics with means and proportions were used to describe the data. Classification performance was assessed by sensitivity and specificity, with 95% confidence intervals (CIs; for differences in binomial proportions). Performance of the two decision rules was compared for 1) any intracranial lesion on CT, 2) clinically important brain injury, and 3) need for neurosurgical intervention in the cohort of patients with GCS of 15. Additionally, the CCHR was assessed for clinically important brain injury and need for neurosurgical intervention in those presenting with GCS of 13 to 15. Physician comfort level with each of the rules was recorded for each enrolled subject and is presented descriptively using proportions. Additionally, we calculated the potential CT ordering rate based on the percentage of patients who would require a head CT according to the two rules. Significance was set at 0.05. All analyses were conducted using SAS, version 8.2 (SAS Institute Inc., Cary, NC). Using the formula in Fleiss28 for inferences about a single proportion, we estimated that a sample size of 420 patients with suspected mild TBI would be required to provide 50 cases of CTs demonstrating traumatic intracranial lesions to allow a 95% CI of 91% to 100% around a sensitivity of 99%.
RESULTS
There were 656 patients from June 2002 to August 2005 with suspected TBI seen in the ED who met eligibility criteria. Of these, 431 had data forms completed by the physicians (all patients with GCS 13 to 15) and 314 (73%) had a GCS of 15. A CT head scan was performed in all but three enrolled cases (99.3% ordering rate) in those with GCS 13 to 15 and in 100% of those with GCS 15. In the larger cohort of 431 patients, 42 (9.7%) were found to have some acute traumatic intracranial abnormality on head CT, 27 (6.3%) were classified as having a clinically important brain injury, and 5 (1.2%) required neurosurgical intervention. In the cohort with GCS score 15, 22 (7%) were found to have some acute traumatic intracranial abnormality on head CT, 11 (3.5%) were classified as having a clinically important brain injury, and 3 (1%) required neurosurgical intervention. The flow chart describes the proportion of patients in each category for GCS 13–15 and for GCS 15 (Figure 1).
Figure 1.
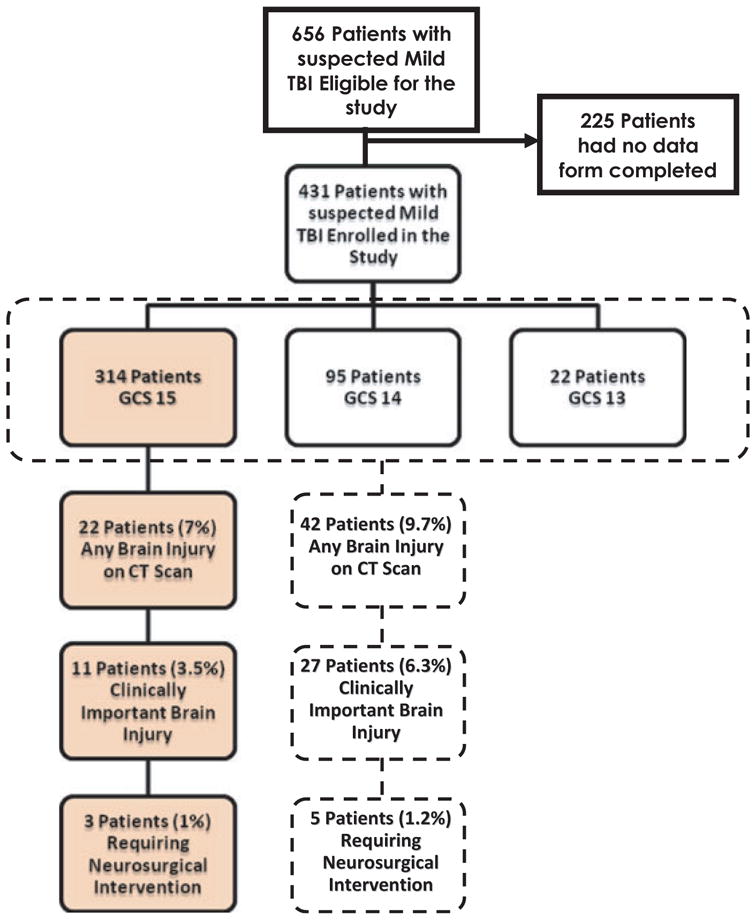
Enrollment flow chart. GCS = Glasgow Coma Scale; TBI = traumatic brain injury.
The mean age of enrolled patients was 38 years (range = 18 to 103 years) and the proportion of males to females was 64% to 36%. The three most common injury mechanisms were motor vehicle crashes (MVC; 52%), falls (26%), and motorcycle crashes (18%). Characteristics of the patients with GCS 13 to 15 and the subgroup with GCS 15 are presented in Table 1. Among the eligible patients not enrolled, demographic and clinical characteristics were similar to those of enrolled patients, with the proportion of males to females at 69 and 31% (p = 0.13); the most common mechanisms of injury were MVC, falls, and motorcycle crashes (p = 0.73); 76% arrived by ambulance (p = 0.23); CT was ordered in 98% (p = 0.32); and 6.2% had intracranial lesions on CT (p = 0.11).
Table 1.
Characteristics of the 431 Suspected Mild TBI Study Patients With GCS 13–15 and the Subgroup With GCS 15
| Characteristics | All Patients With GCS 13–15 (n = 431) | Patients With GCS 15 (n = 314) |
|---|---|---|
| Age (yr), mean ± SD (range) | 38.4 ± 18 (18–103) | 38.3 ± 18 (18–99) |
| Sex | ||
| Female | 155 (36) | 113 (36) |
| Male | 276 (64) | 201 (64) |
| Mechanism of injury | ||
| MVC | 223 (51.7) | 160 (51.0) |
| Motorcycle collision | 18 (4.2) | 14 (4.5) |
| Other motorized vehicles | 13 (3.0) | 8 (2.6) |
| Pedestrian struck and thrown | 5 (1.2) | 4 (1.3) |
| Pedestrian struck | 8 (1.9) | 3 (1.0) |
| Bicycle struck | 9 (2.1) | 7 (2.2) |
| Bicycle collision | 5 (1.2) | 2 (0.7) |
| Other bicycle | 5 (1.2) | 4 (1.3) |
| Fall from elevation > 10 feet/15 stairs | 13 (3.0) | 12 (3.8) |
| Fall from elevation 3–10 feet/5–15 stairs | 30 (7.0) | 22 (7.0) |
| Fall from elevation < 3 feet/5 stairs | 27 (6.3) | 21 (6.7) |
| Assault blunt object | 19 (4.4) | 15 (4.8) |
| Assault fist or feet | 30 (7.0) | 23 (7.3) |
| Diving | 1 (0.2) | 1 (0.3) |
| Fall onto head (axial load) | 2 (0.5) | 1 (0.3) |
| Contact sports (axial load) | 3 (0.7) | 3 (1.0) |
| Heavy object onto head (axial load) | 5 (1.2) | 4 (1.3) |
| Other sports | 3 (0.7) | 2 (0.6) |
| Head struck by other object | 9 (2.1) | 6 (1.9) |
| Hit head on an object | 2 (0.5) | 2 (0.6) |
| Other | 1 (0.2) | 0 (0.0) |
| MVC characteristics* | ||
| Simple rear-end MVC | 11 (2.6) | 9 (2.9) |
| Ejection from vehicle | 41 (9.5) | 21 (6.7) |
| Rollover | 51 (11.8) | 30 (9.6) |
| Death in same MVC | 6 (1.4) | 4 (1.3) |
| “Head-on” collision | 22 (5.1) | 13 (4.1) |
| Time from injury to assessment | ||
| <30 minutes | 52 (12.1) | 40 (12.7) |
| 1 hour | 139 (32.3) | 93 (29.6) |
| 2 to 3 hours | 61 (14.2) | 44 (14.01) |
| 3 to 24 hours | 62 (14.4) | 47 (15.0) |
| Arrived by ambulance | 346 (80.3) | 240 (76.4) |
| Transfer from another institution | 18 (4.2) | 15 (4.8) |
| Witnessed loss of consciousness | 147 (34.1) | 107 (34.1) |
| Amnesia | 139 (32.3) | 83 (26.4) |
| Initial GCS score | ||
| 15 | 314 (72.9) | 314 (100) |
| 14 | 94 (21.8) | – |
| 13 | 23 (5.3) | – |
| Admitted | 259 (60.1) | 169 (53.8) |
| CT of head performed | 428 (99.3) | 314 (100) |
| Skull fracture | 8 (1.9) | 5 (1.6) |
| Basal | 4 (0.9) | 1 (0.3) |
| Linear | 4 (0.9) | 4 (1.3) |
| Depressed | 0 (0.0) | 0 (0.0) |
| Any acute brain injury | 42 (9.7) | 22 (7.0) |
| Cerebral contusion | 18 (4.2) | 8 (2.6) |
| Subarachnoid hemorrhage | 21 (4.9) | 10 (3.2) |
| Subdural hematoma | 8 (1.9) | 4 (1.3) |
| Epidural hematoma | 4 (0.9) | 1 (0.3) |
| Pneumocephalus | 3 (0.7) | 2 (0.6) |
| Intracerebral hematoma | 0 (0.0) | 0 (0.0) |
| Intraventricular hemorrhage | 1 (0.2) | 1 (0.3) |
| Diffuse cerebral edema | 0 (0.0) | 0 (0.0) |
| Clinically important brain injury* | 27 (6.3) | 11 (3.5) |
| Clinically unimportant brain injury* | 15 (3.5) | 11 (3.5) |
| Neurosurgical intervention* | 5 (1.2) | 3 (1.0) |
| Craniotomy | 2 (0.5) | 0 (0.0) |
| Elevation of skull fracture | 0 (0.0) | 0 (0.0) |
| Intubation for head injury | 3 (0.7) | 3 (1.0) |
| Death secondary to head injury | 0 (0.0) | 0 (0.0) |
| Intracranial pressure monitoring | 0 (0.0) | 0 (0.0) |
Values are reported as n (%) unless otherwise noted.
GCS = Glasgow Coma Scale; MVC = motor vehicle collision; TBI = traumatic brain injury.
Some patients have more than one characteristic.
The proportions of variables from each clinical decision rule that were present in enrolled subjects are presented in Table 2. In the subset of patients with GCS 15, the most frequently cited variable from the CCHR was “dangerous mechanism” (51.6% of patients), and from the NOC, was “trauma above the clavicles” (60.8% of patients).
Table 2.
Proportion of Variables in Each Clinical Decision Rule in Enrolled Subjects
| Variables | GCS 13–15 (n = 431) | GCS 15 (n = 314) |
|---|---|---|
| CCHR (%) | ||
| High risk (Total) | 145 (33.6) | 61 (19.4) |
| GCS score < 15 at 2 hours after injury | 72 (16.7) | 6 (1.9) |
| Suspected open or depressed skull fracture | 31 (7.2) | 18 (5.7) |
| Any sign of basal skull fracture | 14 (3.3) | 5 (1.6) |
| Vomiting ≥ 2 episodes | 15 (3.5) | 11 (3.5) |
| Age ≥ 65 years | 37 (8.6) | 27 (8.6) |
| Medium risk (total) | 172 (39.9) | 147 (46.8) |
| Amnesia before impact ≥ 30 minutes | 141 (32.7) | 83 (26.4) |
| Dangerous mechanism | 245 (56.9) | 162 (51.6) |
| Low risk (total) | 114 (26.5) | 106 (33.8) |
| NOC (%) | ||
| GCS < 15 | 117 (27.1) | 0 (0.0) |
| Drug or alcohol intoxication | 108 (25.1) | 68 (21.7) |
| Headache | 158 (36.7) | 119 (37.9) |
| Age > 60 years | 37 (8.6) | 27 (8.6) |
| Vomiting | 20 (4.7) | 13 (4.14) |
| Persistent anterograde amnesia | 120 (27.8) | 76 (24.2) |
| Seizure | 3 (0.7) | 2 (0.6) |
| Trauma above the clavicle | 265 (61.5) | 191 (60.8) |
CCHR = Canadian CT Head Rule; GCS = Glasgow Coma Scale; NOC = New Orleans Criteria.
In patients with a GCS of 15, Tables 3A, 3B, and 3C compare the sensitivities, specificities, and CT scanning rates of the two rules for: 1) any traumatic intracranial lesion on CT, 2) clinically important brain injury, and 3) need for neurosurgical intervention. Sensitivities were all 100%; specificities were higher for CCHR than for NOC. Among all 431 patients with a GCS score 13 to 15, the CCHR had sensitivities of 100% (95% CI = 84% to 100%) for 27 patients with clinically important brain injury and 100% (95% CI = 46% to 100%) for five patients requiring neurosurgical intervention (Table 4).
Table 3.
Sensitivity and Specificity of the Rules Among 314 Patients With GCS 15 Relative to the Three Outcome Measures
| CCHR | NOC | |||
|---|---|---|---|---|
|
|
|
|||
| Result of Assessment | Injury | No Injury | Injury | No Injury |
| A. Any TBI Evident on CT | ||||
| Positive (per rule) | 22 | 186 | 22 | 262 |
| Negative (per rule) | 0 | 106 | 0 | 30 |
| Sensitivity, % (95% CI) | 100 (82–100) | 100 (82–100) | ||
| Specificity, % (95% CI) | 36.3 (31–42) | 10.2 (7–14) | ||
| Potential CT rate | 66.2% | 90.4% | ||
| B. Clinically Important Brain Injury | ||||
| Positive (per rule) | 11 | 197 | 11 | 273 |
| Negative (per rule) | 0 | 106 | 0 | 30 |
| Sensitivity, % (95% CI) | 100 (68–100) | 100 (68–100) | ||
| Specificity, % (95% CI) | 35.0 (30–41) | 9.9 (7–14) | ||
| Potential CT rate | 66.2% | 90.4% | ||
| C. Neurosurgical Intervention | ||||
| Positive (per rule) | 3 | 60 | 3 | 291 |
| Negative (per rule) | 0 | 251 | 0 | 30 |
| Sensitivity, % (95% CI) | 100 (31–100) | 100 (31–100) | ||
| Specificity, % (95% CI) | 80.7 (76–85) | 9.6 (7–14) | ||
| Potential CT rate | 20.1% | 90.4% | ||
CCHR = Canadian CT Head Rule; NOC = New Orleans Criteria; TBI = traumatic brain injury.
Table 4.
Sensitivity and Specificity of the CCHR Among 431 Patients With GCS 13–15 Relative to Two Outcome Measures
| Canadian CT Head Rule for “Clinically Important” Brain Injury | Canadian CT Head Rule for “Neurosurgical Intervention” | |||
|---|---|---|---|---|
|
|
|
|||
| Result of Assessment | Injury | No Injury | Injury | No Injury |
| Positive (per CCHR), n | 27 | 290 | 5 | 142 |
| Negative (per CCHR), n | 0 | 114 | 0 | 284 |
| Sensitivity, % (95% CI) | 100 (84–100) | 100 (46–100) | ||
| Specificity, % (95% CI) | 28.2 (24–33) | 66.7 (62–71) | ||
CCHR = Canadian CT Head Rule.
Twenty attending EPs performed 81% of all evaluations, and house officers completed 19%. A comparison of how comfortable the EPs were in applying each of the two decision rules clinically is shown in Table 5. The majority of responses were in the “very comfortable” category for both rules, although in the cohort with GCS 15, physicians rated being either “very comfortable” or “comfortable” with the NOC more frequently than the CCHR (81.5% vs. 70.4%, respectively; p = 0.001). Additionally, physicians felt either “very uncomfortable” or “uncomfortable” with the NOC less frequently than the CCHR (5.1% vs. 11.5%, respectively; p = 0.009). For each case, the physicians rated their comfort level twice, once for the CCHR and once for NOC, and the level of intraphysician agreement (a single physician assessing each rule) was 59% (95% CI = 54% to 64%) with a kappa statistic κ of 0.41 (95% CI = 0.33 to 0.48). In other words, physicians gave each rule the same comfort rating in 60% of cases.
Table 5.
Level of EP Comfort With Each Decision Rule
| GCS 13–15 (n = 431) | GCS 15 (n = 314) | |||
|---|---|---|---|---|
|
|
|
|||
| How comfortable are you using this rule on this patient? | CCHR | NOC | CCHR | NOC |
| Very comfortable | 172 (39.9) | 215 (49.9) | 115 (36.6) | 142 (45.2) |
| Comfortable | 149 (34.6) | 149 (34.6) | 106 (33.8) | 114 (36.3) |
| Neutral/unsure | 69 (16.0) | 51 (11.8) | 57 (18.2) | 42 (13.4) |
| Uncomfortable | 33 (7.7) | 13 (3.0) | 30 (9.6) | 13 (4.1) |
| Very uncomfortable | 8 (1.9) | 3 (0.7) | 6 (1.9) | 3 (1.0) |
Values are reported as n (%)
GCS = Glasgow Coma Scale; CCHR = Canadian Computed Tomography Head Rule; NOC = New Orleans Criteria.
DISCUSSION
In this prospective study conducted at a Level I trauma center in the United States, a comparison of the clinical decision rules for use of CT in patients with minor head injury showed that both the CCHR and the NOC were highly sensitive for three important outcome measures: 1) any traumatic intracranial lesion on CT, 2) “clinically important” brain lesions, and 3) requiring neurosurgical intervention for patients with a GCS score of 15. We found sensitivities of both rules to reach 100% in all categories, consistent with the high sensitivities described in prior studies.20–23 The specificity of the CCHR was, however, higher than that of the NOC and could potentially lead to lower use of CT imaging.
We also assessed the performance of the CCHR in patients with a GCS 13 to 15 for “clinically important brain injury” and for predicting the need for neurosurgical intervention. In this larger cohort, the CCHR had 100% sensitivity for identifying clinically important brain injury and patients requiring neurosurgical intervention. Although some patients were transferred from other hospitals, only a few of those patients had undergone CT imaging prior to arrival. The majority were transferred from other facilities for evaluation of other injuries such as orthopedic injuries. Of the 18 transfers only five had head CTs included with their transfer, and the treating physicians did not know the status of the patients’ brain injuries prior to their assessments.
Although in previous validation studies, Stiell et al.20 noted that the rules perform similarly in detecting “clinically significant brain injuries,” our study assessed this prospectively in a U.S. trauma center using “any traumatic intracranial lesion on CT” as our primary endpoint. Earlier studies comparing the two decision rules may have led to apprehension among U.S. EPs about using the CCHR clinically, because not all intracranial lesions were included in the outcome, just those deemed “clinically important” by an expert panel.
The specificities of the rules in our study also confirm the results of prior studies, with the CCHR having a significantly higher specificity than the NOC for all brain injuries and need for neurosurgical intervention, although the magnitude of the difference we found was lower than those previously reported20,29 and likely reflective of the smaller sample size. Our calculated specificities for “all intracranial lesions on CT” of 34% and 10.2% for the CCHR and NOC, respectively, indicate that the CCHR has more potential for reducing the CT ordering rate in U.S. EDs without compromising sensitivity. Further validation in a larger cohort is warranted to narrow the CIs.
There are several advantages of this study over some of the previous studies performed comparing these decision rules. First, almost all patients had neuroimaging performed (99%), and surrogate measures of brain injury were not used to classify patients. This was not mandated by the study, but rather is usual practice for this Level I trauma center. Second, the primary outcome measure was any traumatic intracranial injury on CT and not just “clinically important” brain injury. This endpoint affects patient management in many Level I trauma centers in the United States. Third, the study was conducted prospectively and not as a secondary or post hoc analysis. Last, Canadian EPs are familiar and comfortable with the CCHR, but the comfort level of U.S. EPs has not been assessed to this extent before.
We also assessed physician willingness to use these rules in practice by asking participating physicians about their level of comfort in applying the decision instruments. EPs were more apprehensive about applying the CCHR than they were the NOC. A larger proportion of EPs felt comfortable applying the NOC (81.5%) than the CCHR (70.4%) and fewer felt uncomfortable applying the NOC (5.1%) than the CCHR (11.5%). This may be a reflection of the lack of validation in a U.S. population or that the CCHR was developed for “clinically significant” lesions on CT and not for all intracranial injuries. The NOC was derived and validated in the United States, yet virtually every patient had a CT scan of the head performed. This could be a manifestation of the practice patterns at this Level I trauma center or perhaps of the apprehension to apply these decision rules secondary to the medicolegal climate, interpreting elements of the rules, or other barriers beyond the scope of this discussion.
LIMITATIONS
Enrollment required that the EPs voluntarily assess patients for the study and complete the data forms in the midst of their busy clinical duties, and therefore it was not feasible to enroll all eligible cases. However, a comparison of the characteristics of enrolled and non-enrolled cases revealed that the two groups were very similar and no selection bias could be detected. The CIs were wider than those reported in other studies and reflect the smaller size of our study. Further validation in a larger cohort is warranted to narrow the CIs. Despite this, no patients with intracranial lesions on CT were missed by either rule. This study was conducted at a single Level I trauma center and may not be generalizable to other centers. Nonetheless, this trauma setting is typical of many trauma centers around the country, with an array of emergency and trauma physicians trained in different parts of the country. Additionally, patients were selected according to explicit and transportable inclusion criteria, rather than on the subjective decision of physicians to order CT imaging. A large spectrum of injury severity was also enrolled to ensure a heterogenous sample.
CONCLUSIONS
In a U.S. sample of minor head injury patients with Glasgow Coma Scale scores of 15, the Canadian CT Head Rule and the New Orleans Criteria had equivalent high sensitivities for detecting any traumatic intracranial injury on CT and for determining the need for neurosurgical intervention. The Canadian CT Head Rule, however, had a higher specificity and showed greater potential to lower neuroimaging rates. A larger study in the United States will be required to validate these results.
Footnotes
Presented at the Society for Academic Emergency Medicine Annual Meeting, Chicago, IL, May 2007.
The authors have no relevant financial information or potential conflicts of interest to disclose.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention; [Accessed Oct 23, 2011]. Traumatic Brain Injury in the United States. Available at: http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2003. [Google Scholar]
- 3.af Geijerstam JL, Britton M. Mild head injury–mortality and complication rate: meta-analysis of findings in a systematic literature review. Acta Neurochir (Wien) 2003;145:843–50. doi: 10.1007/s00701-003-0115-1. [DOI] [PubMed] [Google Scholar]
- 4.Jeret JS, Mandell M, Anziska B, et al. Clinical predictors of abnormality disclosed by computed tomography after mild head trauma. Neurosurgery. 1993;32:9–15. doi: 10.1227/00006123-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Shackford SR, Wald SL, Ross SE, et al. The clinical utility of computed tomographic scanning and neurologic examination in the management of patients with minor head injuries. J Trauma. 1992;33:385–94. doi: 10.1097/00005373-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Stein SC, Ross SE. Mild head injury: a plea for routine early CT scanning. J Trauma. 1992;33:11–3. [PubMed] [Google Scholar]
- 7.Stein SC, Burnett MG, Glick HA. Indications for CT scanning in mild traumatic brain injury: a cost-effectiveness study. J Trauma. 2006;61:558–66. doi: 10.1097/01.ta.0000233766.60315.5e. [DOI] [PubMed] [Google Scholar]
- 8.Mettler FA, Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95:502–7. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Keir SL, Seymour J, et al. What is the best imaging strategy for acute stroke? Health Technol Assess. 2004;8:iii, ix–x, 1–180. doi: 10.3310/hta8010. [DOI] [PubMed] [Google Scholar]
- 10.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 11.Fayngersh V, Passero M. Estimating radiation risk from computed tomography scanning. Lung. 2009;187:143–8. doi: 10.1007/s00408-009-9143-9. [DOI] [PubMed] [Google Scholar]
- 12.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–78. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 13.Heilbrun ME, Chew FS, Tansavatdi KR, Tooze JA. The role of negative CT of the abdomen and pelvis in the decision to admit adults from the emergency department after blunt trauma. J Am Coll Radiol. 2005;2:889–95. doi: 10.1016/j.jacr.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Livingston DH, Loder PA, Koziol J, Hunt CD. The use of CT scanning to triage patients requiring admission following minimal head injury. J Trauma. 1991;31:483–7. [PubMed] [Google Scholar]
- 15.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz DT. Counter-point: are we really ordering too many CT scans? West J Emerg Med. 2008;9:120–2. [PMC free article] [PubMed] [Google Scholar]
- 17.Stiell IG, Wells GA, Vandemheen K, et al. Variation in ED use of computed tomography for patients with minor head injury. Ann Emerg Med. 1997;30:14–22. doi: 10.1016/s0196-0644(97)70104-5. [DOI] [PubMed] [Google Scholar]
- 18.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT head rule for patients with minor head injury. Lancet. 2001;357:1391–6. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
- 19.Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343:100–5. doi: 10.1056/NEJM200007133430204. [DOI] [PubMed] [Google Scholar]
- 20.Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT head rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294:1511–8. doi: 10.1001/jama.294.12.1511. [DOI] [PubMed] [Google Scholar]
- 21.Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005;294:1519–25. doi: 10.1001/jama.294.12.1519. [DOI] [PubMed] [Google Scholar]
- 22.Rosengren D, Rothwell S, Brown AF, Chu K. The application of North American CT scan criteria to an Australian population with minor head injury. Emerg Med Australas. 2004;16:195–200. doi: 10.1111/j.1742-6723.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 23.Stein SC, Fabbri A, Servadei F, Glick HA. A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann Emerg Med. 2009;53:180–8. doi: 10.1016/j.annemergmed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Mower WR, Hoffman JR, Herbert M, Wolfson AB, Pollack CV, Jr, Zucker MI. Developing a decision instrument to guide computed tomographic imaging of blunt head injury patients. J Trauma. 2005;59:954–9. doi: 10.1097/01.ta.0000187813.79047.42. [DOI] [PubMed] [Google Scholar]
- 25.Stiell IG, Lesiuk H, Vandemheen K, et al. Obtaining consensus for the definition of “clinically important” brain injury in the CCC study [abstract] Acad Emerg Med. 2000;7:572. [Google Scholar]
- 26.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, et al. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52:714–48. doi: 10.1016/j.annemergmed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Stiell IG, Lesiuk H, Wells GA, et al. The Canadian CT head rule study for patients with minor head injury: rationale, objectives, and methodology for phase I (derivation) Ann Emerg Med. 2001;38:160–9. doi: 10.1067/mem.2001.116796. [DOI] [PubMed] [Google Scholar]
- 28.Fleiss JL. Statistical Methods for Rates and Proportions. 2. New York, NY: John Wiley & Sons; 1981. [Google Scholar]
- 29.Stein SC, Ross SE. Minor head injury: a proposed strategy for emergency management. Ann Emerg Med. 1993;22:1193–6. doi: 10.1016/s0196-0644(05)80989-8. [DOI] [PubMed] [Google Scholar]


