Abstract
Study Objective:
Various randomized controlled trials and a meta-analysis have compared i-gel™ and laryngeal mask airway Supreme™ (LMA-S™) in adult patients and found that both the devices provided equivalent oropharyngeal leak pressure (OLP). However, no randomized controlled trial has compared air-Q™ with i-gel™ and LMA-S™ in adult patient. Hence, we designed this study to compare air-Q™ with LMA-S™ and i-gel™ in adult patients.
Materials and Methods:
A total of 75 adult patients of the American Society of Anesthesiologists physical status I/II of both sexes, between 18 and 60 years, were included in this prospective randomized controlled trial conducted in a tertiary care center. Randomization of patients was done in three equal groups according to the insertion of supraglottic airway device by a computer-generated random number sequence: group air-Q™ (n = 25), group i-gel™ (n = 25), and group LMA-S™ (n = 25). Primary outcome of this study was OLP. We also recorded time for successful placement of device, ease of device insertion, number of attempts to insert device, and ease of gastric tube insertion along with postoperative complications.
Results:
The mean ± standard deviation OLP of air-Q™, i-gel™, and LMA-S™ was 26.13 ± 4.957 cm, 23.75 ± 5.439 cm, and 24.80 ± 4.78 cm H2O (P = 0.279). The first insertion success rate for air-Q™, i-gel™, and LMA-S™ was 80%, 76%, and 92%, respectively (P = 0.353). The insertion time of air-Q™, i-gel™, and LMA-S™ was 20.6 ± 4.4, 14.8 ± 5.4, and 15.2 ± 4.7 s, respectively (P = 0.000). Time taken for air-Q™ insertion was significantly higher than time taken for i-gel™ (mean difference 5.8 s, P < 0.0001) and LMA-S™ (mean difference 5.4 s, P = 0.0001) insertion. Postoperative complications were similar with all three devices.
Conclusions:
We concluded that air-Q™, i-gel™, and LMA-S™ were equally efficacious in terms of routine airway management in adult patients with normal airway anatomy.
Keywords: Air-Q™, i-gel, laryngeal mask airway Supreme™, oropharyngeal leak pressure, randomized controlled trial
Introduction
Incidence of airway-related complications represented the most common mechanism leading to anesthesia malpractice claims, accounting for a large proportion of claims for death and brain damage across the world. Review of airway management data in the Fourth National Audit Project of the Royal College of Anesthetists and the Difficult Airway Society indicates that there is scope of improvement in the management.[1] Difficult Airway Society guideline recommends the use of laryngeal mask airway (LMA) or an intubating LMA in cases of failed intubation by direct laryngoscopy.[2] Second-generation supraglottic airway devices provide an added safety margin from aspiration by incorporation of a gastric access port. Air-Q™ (Cookgas LLC, St. Louis, USA) is a supraglottic airway device with anterior curve and mask ridges with inflatable cuff which perfectly fits to perilaryngeal structure that provides adequate oropharyngeal seal pressure, and it can also be used as a conduit for endotracheal intubation.[3] i-gel™ (Intersurgical, Wokingham, Berkshire, UK) is also a second-generation supraglottic airway devices made up of thermoelastomer gel (styrene ethylene butadiene styrene) with inbuilt gastric drain tube.[4] It consists of an anatomical firm tube section and noninflatable cuff, which seals the laryngeal inlet and prevents neurovascular compression at the larynx. It also contains epiglottic rest to prevent downfolding. LMA Supreme™ (LMA-S™, Laryngeal Mask Co. Ltd, Le Rocher, Victoria, Mahe Seychelles) is a polyvinyl chloride made, anatomically shaped, single-use supraglottic device that incorporates a gastric insufflation port.[5] Although a number of randomized controlled trials and a meta-analysis have compared i-gel™ and LMA-S™ in adult patients and found that both the device provided equivalent oropharyngeal leak pressure (OLP).[6,7,8,9] After reviewing the literature, we did not found any study till now that has compared air-Q™ with either of these two devices in adult patients. Jagannathan et al. compared air-Q™ with i-gel™ for fiberoptic-guided endotracheal intubation in children.[10] However, findings from study done in children cannot be extrapolated to the adults and vice versa.[11] Therefore, this study was designed to compare air-Q™ with LMA-S™ and i-gel™ in adult patients.
Materials and Methods
This randomized controlled trial was registered with the Clinical Trial Registry of India (CTRI) (CTRI/2015/03/005623) after obtaining the Institute Ethics Committee approval (NK/1738/MD/11729-30 dated September 18, 2014) and was performed at Postgraduate Institute of Medical Education and Research, Chandigarh, India, between March and August 2015. After obtained informed consent from the participants, 75 adult patients between the age of 18–60 years of either sex and the American Society of Anesthesiologists (ASA) physical status I or II who were scheduled to undergo elective surgery under general anesthesia with laryngeal mask as a primary airway device were included in the study. Patients having gastroesophageal reflux disease, prior esophageal surgery, hiatus hernia known oropharyngeal morbidities, prior history of postoperative nausea and vomiting, full stomach with risk of aspiration, body mass index >35 kg/m2, any condition which requires endotracheal intubation, and difficult airway were excluded from this study.
Randomization of patients in this study was done in three equal groups according to a computer-generated random number sequence: group air-Q™ (n = 25), group i-gel™ (n = 25), and group LMA-S™ (n = 25). Randomization sequences were kept in opaque sealed envelope, and they were only opened at the time of induction of general anesthesia by a person not involved in the study and handed over to the anesthesia team.
It was impossible to blind the device operator due to obvious technical reasons, but investigators observing the patient in the postoperative period and those analyzing the data and participants were blinded to the group allocation.
Standard ASA fasting guidelines were followed in all patients. In the operation theater, pulse oximetry finger probe, noninvasive arterial blood pressure cuff, and electrocardiograph electrodes were attached. Induction of anesthesia was done with fentanyl 2 mcg/kg and propofol 2–3 mg/kg. Once the adequate depth of anesthesia was confirmed by jaw thrust, supraglottic airway device was placed as per manufacturer recommendations. For choosing the size of the device, we used manufacturers' recommendations. After the insertion, supraglottic device cuff was inflated using aneroid manometer and was connected to the circle system of an anesthesia workstation (Datex-ohmeda S/5 Avance), and device was checked for effective ventilation with the appearance of square wave capnograph trace and bilateral chest movements on gentle manual ventilation. In case of a significant air leak or a partial or complete airway obstruction, the device was removed and reinserted. Subsequently, orogastric tube (OGT) was inserted through the drain tube. The device was considered to be failure if more than three attempts required for successful placement.
OLP of the device was determined by closing the expiratory valve of the circle system with a fresh gas inflow of 3 L/min. The aneroid manometer dial was observed as the pressure increased, and when the dial reached stability, the airway pressure was recorded. This pressure was noted as OLP of the device. Expiratory valve was released completely once the pressure exceeded 40 cm H2O. We also recorded time for successful placement[s] of device (picking the device to appearance of EtCO2 waveform), ease of device insertion (easy/moderate/difficult/impossible), number of attempts to insert device (first, second, or third), and ease of gastric tube insertion (easy/moderate/difficult/impossible). Spontaneous ventilation under general anesthesia was maintained with 50% nitrous oxide + oxygen and 1% isoflurane targeting an end-tidal minimum alveolar concentration of inhalation anesthetic agent around 1.0–1.3 and EtCO2 of 35–45 mmHg. After the surgery, the device was removed under a deep plane of anesthesia. Intra- and post-operative complications were noted.
Statistical analysis
Previously, no study compared all three devices in one setting. Hence, we calculated the sample size based on mean difference of airway leak pressure as 4.9 cm H2O between i-gel™ and LMA-S™ by Chew et al.[12] The sample size came out to be 21 patients per group at a power of 80% and confidence interval of 95%. With a possible dropout of 10%, we included 25 patients per group (total of 75 patients for three groups). The statistical analysis was done using Statistical Package for Social Sciences (SPSS Inc., version 22.0 for Windows, Chicago, IL, USA). For normally distributed data, means of three groups were compared using one-way ANOVA. Proportions were compared using Chi-square tests. For comparison of time-related variables, repeated measure ANOVA followed by one-way ANOVA was applied. If P value is significant, then multiple comparisons Bonferroni's tests for three groups were applied. A two-tailed P < 0.05 was considered significant.
Results
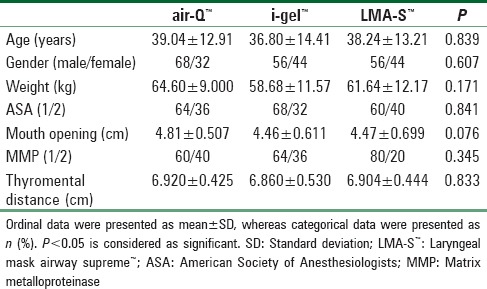
Eighty-five patients were assessed for eligibility, out of which 75 patients met inclusion criteria of this randomized trial and recruited in this trial [Figure 1]. There were two device failures in air-Q™ group and one device failure in i-gel™ group, and they were excluded from final data analysis. Demographic data including age, gender, weight, ASA, and airway parameters were comparable between all three groups [Table 1].
Figure 1.

Consort diagram
Table 1.
Comparison of baseline demographic parameters
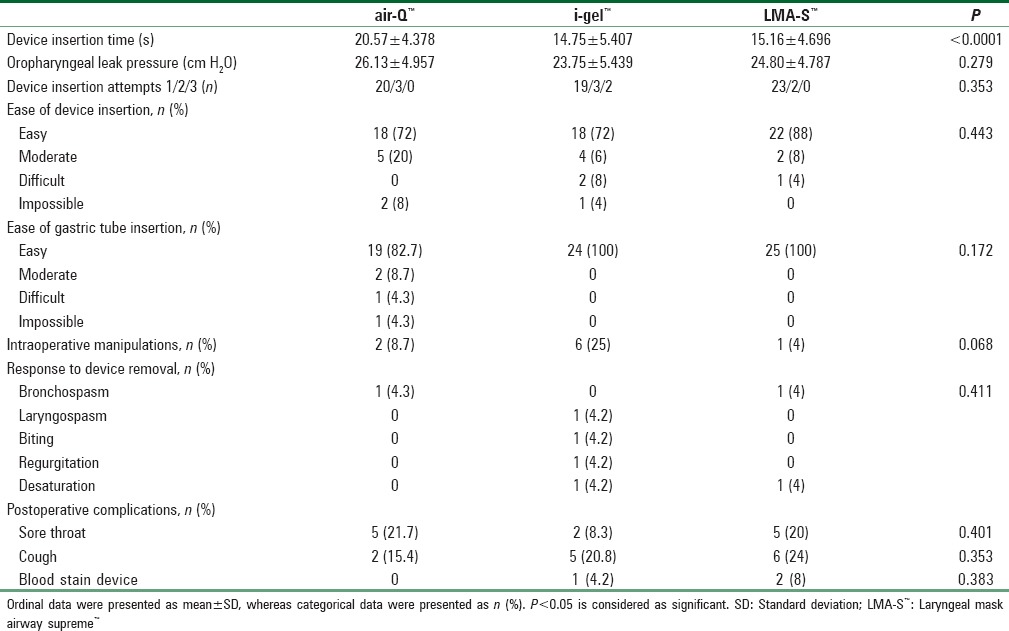
OLPs between all the three groups were comparable (26.1 ± 4.9 cm H2O in air-Q™, 23.8 ± 5.4 cm H2O in i-gel™, and 24.8 ± 4.8 cm H2O in LMA-S™, P = 0.28, one-way ANOVA). The mean ± standard deviation device insertion time taken for air-Q™, i-gel™, and LMA-S™ was 20.6 ± 4.4 s, 14.8 ± 5.4 s, and 15.2 ± 4.7 s, respectively (P < 0.0001, one-way ANOVA). Multiple comparisons Bonferroni's tests show that time taken for air-Q™ insertion was significantly higher than time taken for i-gel™ (mean difference 5.8 s, P < 0.0001) and LMA-S™ (mean difference 5.4s, P = 0.0001) insertion. The first attempt insertion success rate for air-Q™, i-gel™, and LMA-S™ was 80%, 76%, and 92%, respectively (P = 0.30, Chi-square test). Ease of insertion of device, ease of gastric tube placement, and response to the removal of device were comparable for all three devices [Table 2]. With regard to postoperative complications, postoperative sore throat was associated with air-Q™, i-gel™, and LMA-S™ were 21.7%, 8.3%, and 20%, respectively. Blood-stained device was found in two (8%) cases in LMA-S™ group, one (4.2%) in i-gel™ group, and none in air-Q™ group.
Table 2.
Performance of the airway devices in the present study
Discussion
Principal finding of this study is that air-Q™, i-gel™, and LMA-S™ provide similar OLP in adults under general anesthesia. However, insertion of air-Q™ took significantly longer time than other two devices. Safety of any supraglottic airway device is determined mainly by its OLP and also indicates the feasibility of positive-pressure ventilation.[13,14] A number of randomized clinical trials and a meta-analysis also reported that OLP between i-gel™ and LMA-S™ is similar.[6,7,8,9,15] On the contrary to our findings, Chew et al. and Ragazzi et al. reported that OLP in cases of LMA-S™ is higher than i-gel™.[12,16] On the other hand, reported mean OLP in adult patients in case of air-Q™ ranges between 19 cm and 30 cm H2O.[17,18,19] However, it should be remembered that reported OLP for a supraglottic airway device depends on several factors such as use of muscle relaxant, methods of measurement, and type of ventilation (controlled vs. spontaneous respiration). We have found that time taken for air-Q™ insertion was significantly more than i-gel™ as well as LMA-S™ and insertion time is similar between i-gel™ and LMA-S™. A number of RCTs also reported similar insertion time between LMA-S™ and i-gel™.[8,12,20] Theiler et al. reported that LMA-S™ insertion is significantly shorter in patients with cervical collar.[21] Air-Q™ insertion time reported in this study is similar to the previous reports by Karim and Swanson and Galgon et al.[11,19] However, as the mean difference in insertion time is around 5 s between air-Q™ and i-gel™ or LMA-S™, actual clinical significance of this small difference is questionable. We have found a similar first insertion success rate between all three devices, and Chen et al. also found similar first insertion success rate between i-gel™ and LMA-S™.[9] In contrast to our results, Ragazzi et al. showed that first attempt success rate of LMA-S™ was significantly higher than i-gel™ (77% vs. 54%).[16] The reason for this difference could be due to device insertion by inexperienced operators as they mentioned. We have found that subjective ease of OGT insertion was comparable between all three devices, and overall success rates of OGT insertion were similar. One incidence of failure to insert OGT happened in air-Q™ group. We encountered slight difficulty in passing OGT through air-Q™ as it passes behind the cuff. The advantage of this technique was useful in suctioning supracuff area. Although many studies done on air-Q™, most of these studies did not mention about gastric tube insertion success rate. Recently published study done by Youssef et al. alone mentioned that OGT insertion success rate in air-Q™ was 93%, which matched, with our study.[22] However, they did not mention about the method of OGT insertion. In consistency with our result, Ragazzi et al. and Theiler et al.[21] mentioned that subjective ease of insertion of OGT was similar between i-gel™ and LMA-S™, whereas Teoh et al. reported that the OGT insertion was subjectively easier for LMA-S™ than i-gel™ which was in contrast to our results.[16,21,20] Although statistically insignificant, i-gel™ group had less postoperative sore throat than air-Q™ and LMA-S™. This could be due to soft consistency of i-gel cuff material. As sore throat is uncommon, a larger sample size would be required to found a statistical difference.
This study had several limitations. First, our study findings might not applicable to patients with difficult airway as this study was done in patients with normal airway. Second, it was impossible to blind the device operator, which could lead to bias. Third, our study was conducted in nonparalyzed patients, so our results might not applicable to patients with paralyzed patients. Finally, we measured OLP immediately after insertion of device in neutral position, but we did not measure OLP in different positions and in different times as cuff seal may change overtime.
Conclusions
We have found that performance of air-Q™, i-gel™, and LMA-S™ is comparable in adult anesthetized patients with normal airway; however, air-Q™ insertion took longer time than rest of the two devices.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cook TM, Woodall N, Frerk C Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br J Anaesth. 2011;106:617–31. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 2.Henderson JJ, Popat MT, Latto IP, Pearce AC Difficult Airway Society. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675–94. doi: 10.1111/j.1365-2044.2004.03831.x. [DOI] [PubMed] [Google Scholar]
- 3.Kleine-Brueggeney M, Nicolet A, Nabecker S, Seiler S, Stucki F, Greif R, et al. Blind intubation of anaesthetised children with supraglottic airway devices AmbuAura-i and Air-Q cannot be recommended: A randomised controlled trial. Eur J Anaesthesiol. 2015;32:631–9. doi: 10.1097/EJA.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 4.I-gel User-guide. [Last accessed on 2011 Nov 29]. Available from: http/www.i-gel.com/lib/docs/userguides/i-gel_User_Guide_English.pdf .
- 5.Cook TM, Gatward JJ, Handel J, Hardy R, Thompson C, Srivastava R, et al. Evaluation of the LMA Supreme™ in 100 non-paralysed patients. Anaesthesia. 2009;64:555–62. doi: 10.1111/j.1365-2044.2008.05824.x. [DOI] [PubMed] [Google Scholar]
- 6.Benger J, Coates D, Davies S, Greenwood R, Nolan J, Rhys M, et al. Randomised comparison of the effectiveness of the laryngeal mask airway Supreme, i-gel and current practice in the initial airway management of out of hospital cardiac arrest: A feasibility study. Br J Anaesth. 2016;116:262–8. doi: 10.1093/bja/aev477. [DOI] [PubMed] [Google Scholar]
- 7.Eschertzhuber S, Brimacombe J, Kaufmann M, Keller C, Tiefenthaler W. Directly measured mucosal pressures produced by the i-gel™ and laryngeal mask airway Supreme™ in paralysed anaesthetised patients. Anaesthesia. 2012;67:407–10. doi: 10.1111/j.1365-2044.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Rim JC, Kim H, Lee JH, Chung CJ. Comparison of i-gel® and LMA Supreme® during laparoscopic cholecystectomy. Korean J Anesthesiol. 2015;68:455–61. doi: 10.4097/kjae.2015.68.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Jiao J, Cong X, Liu L, Wu X. A comparison of the performance of the I-gel™ vs.the LMA-S™ during anesthesia: A meta-analysis of randomized controlled trials. PLoS One. 2013;8:e71910. doi: 10.1371/journal.pone.0071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagannathan N, Sohn L, Ramsey M, Huang A, Sawardekar A, Sequera-Ramos L, et al. A randomized comparison between the i-gel™ and the air-Q™ supraglottic airways when used by anesthesiology trainees as conduits for tracheal intubation in children. Can J Anaesth. 2015;62:587–94. doi: 10.1007/s12630-014-0304-9. [DOI] [PubMed] [Google Scholar]
- 11.Karim YM, Swanson DE. Comparison of blind tracheal intubation through the intubating laryngeal mask airway (LMA Fastrach™) and the air-Q™. Anaesthesia. 2011;66:185–90. doi: 10.1111/j.1365-2044.2011.06625.x. [DOI] [PubMed] [Google Scholar]
- 12.Chew EE, Hashim NH, Wang CY. Randomised comparison of the LMA Supreme with the I-Gel in spontaneously breathing anaesthetised adult patients. Anaesth Intensive Care. 2010;38:1018–22. doi: 10.1177/0310057X1003800609. [DOI] [PubMed] [Google Scholar]
- 13.Keller C, Brimacombe JR, Keller K, Morris R. Comparison of four methods for assessing airway sealing pressure with the laryngeal mask airway in adult patients. Br J Anaesth. 1999;82:286–7. doi: 10.1093/bja/82.2.286. [DOI] [PubMed] [Google Scholar]
- 14.Maitra S, Khanna P, Baidya DK. Comparison of laryngeal mask airway Supreme and laryngeal mask airway Pro-Seal for controlled ventilation during general anaesthesia in adult patients: Systematic review with meta-analysis. Eur J Anaesthesiol. 2014;31:266–73. doi: 10.1097/01.EJA.0000435015.89651.3d. [DOI] [PubMed] [Google Scholar]
- 15.Beleña JM, Núñez M, Vidal A, Gasco C, Alcojor A, Lee P, et al. Randomized comparison of the i-gel(TM) with the LMA Supreme (TM) in anesthetized adult patients. Anaesthesist. 2015;64:271–6. doi: 10.1007/s00101-015-0020-z. [DOI] [PubMed] [Google Scholar]
- 16.Ragazzi R, Finessi L, Farinelli I, Alvisi R, Volta CA. LMA Supreme™ vs.i-gel™ – A comparison of insertion success in novices. Anaesthesia. 2012;67:384–8. doi: 10.1111/j.1365-2044.2011.07002.x. [DOI] [PubMed] [Google Scholar]
- 17.Bakker EJ, Valkenburg M, Galvin EM. Pilot study of the air-Q intubating laryngeal airway in clinical use. Anaesth Intensive Care. 2010;38:346–8. doi: 10.1177/0310057X1003800217. [DOI] [PubMed] [Google Scholar]
- 18.Joffe AM, Liew EC, Galgon RE, Viernes D, Treggiari MM. The second-generation air-Q intubating laryngeal mask for airway maintenance during anaesthesia in adults: A report of the first 70 uses. Anaesth Intensive Care. 2011;39:40–5. doi: 10.1177/0310057X1103900106. [DOI] [PubMed] [Google Scholar]
- 19.Galgon RE, Schroeder KM, Han S, Andrei A, Joffe AM. The air-Q(®) intubating laryngeal airway vs.the LMA-ProSeal(TM): A prospective, randomised trial of airway seal pressure. Anaesthesia. 2011;66:1093–100. doi: 10.1111/j.1365-2044.2011.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teoh WH, Lee KM, Suhitharan T, Yahaya Z, Teo MM, Sia AT. Comparison of the LMA Supreme vs.the i-gel in paralysed patients undergoing gynaecological laparoscopic surgery with controlled ventilation. Anaesthesia. 2010;65:1173–9. doi: 10.1111/j.1365-2044.2010.06534.x. [DOI] [PubMed] [Google Scholar]
- 21.Theiler LG, Kleine-Brueggeney M, Kaiser D, Urwyler N, Luyet C, Vogt A, et al. Crossover comparison of the laryngeal mask Supreme and the i-gel in simulated difficult airway scenario in anesthetized patients. Anesthesiology. 2009;111:55–62. doi: 10.1097/ALN.0b013e3181a4c6b9. [DOI] [PubMed] [Google Scholar]
- 22.Youssef MM, Lofty M, Hammad Y, Elmenshawy E. Comparative study between LMA Proseal™ and Air-Q™ Blocker for ventilation in adult eye trauma patients. Egypt J Anaesth. 2014;30:227–33. [Google Scholar]


