Abstract
Introduction:
Although the Nuss procedure provides excellent cosmetic results for the correction of pectus excavatum, the provision of analgesia following such procedures can be challenging.
Methods:
The current study retrospectively reviews our experience over a 2.5 year period with thoracic epidural analgesia (TE), paravertebral blockade (PVB), and intravenous opioids delivered via patient-controlled analgesia (PCA) to provide postoperative analgesia.
Results:
The study cohort included 30 patients (mean age = 15.6 ± 1.5 years), 15 of whom were treated with PCA, 8 with TE, and 7 with PVB. There were no significant differences in pain scores between the 3 groups at any time point during the first 3 postoperative days. Compared to PCA, the PVB group had lower opioid consumption over the first 24 hours of hospitalization by 1.7 mg/kg morphine equivalents (95% CI of difference: 0.1, 3.3; p=0.035); but had higher opioid consumption by 2.0 mg/kg morphine equivalents than the TE group (95% CI of difference: 0.3, 3.7; p=0.024). There were no differences in opioid consumption between PVB and PCA or between PVB and TE at 48 or 72 hours. The number of intraoperative hypotension episodes was significantly lower in the PCA group when compared to the PVB group (p=0.001), with no difference between the PVB and TE groups.
Conclusions:
The use of regional anesthesia should be considered a viable option for the relief of postoperative pain in pediatric patients following the Nuss procedure albeit with a higher incidence of intraoperative hemodynamic effects. A randomized, prospective, study powered to compare all 3 techniques against one another would be necessary to confirm the significance of these findings.
Keywords: Nuss procedure, paravertebral blockade, pectus excavatum, thoracic epidural analgesia
Introduction
Repair of pectus excavatum using the Nuss procedure, or thoracoscopic approach, is a common pediatric surgical procedure associated with severe postoperative pain.[1] The issues of postoperative pain management in patients who have undergone this surgical repair have been previously demonstrated.[2,3,4] These studies confirm the difficulties of ensuring adequate postoperative pain control. Two common modalities for postoperative pain management include patient-controlled analgesia (PCA) and continuous thoracic epidural infusions (TE), but these pain management modalities have yielded mixed results.[5,6,7,8] A large institutional report described 21 years of experience with TE as the mainstay for postoperative pain management following the Nuss procedure in more than 1000 patients.[9] However, the practice had changed at the time of publication, following two cases of lower extremity paralysis associated with TE use. Although subsequent detailed investigation determined that TE was not at fault, the practice for the provision of postoperative analgesia remained the exclusive use of PCA.
An alternative to TE for providing analgesia following thoracic surgery is continuous paravertebral blockade (PVB), which involves peripheral, spinal nerve block as the nerves exit the spinal canal. PVB is considered less invasive than TE because the central neural axis (spinal cord) is not accessed, thereby limiting the potential for spinal cord damage and paralysis. When compared to TE, PVB may be also associated with fewer adverse effects, such as nausea, vomiting, urinary retention, and systemic hypotension.[10,11] Pain control with PVB has been shown to be at least equivalent to TE following various surgical procedures involving the thoracic dermatomes.[12,13,14] However, a few studies have assessed the efficacy of PVB in providing analgesia following the Nuss procedure and comparing it to other modalities.[15] This study tests the hypothesis that continuous PVB provides comparable postoperative analgesia to that achieved with TE and PCA, with a lower adverse effect profile.
Methods
This study was approved by the Institutional Review Board (IRB 13-00702) at Nationwide Children's Hospital (Columbus, Ohio) and was registered at clinicaltrials.gov (NCT02009267). Requirements for informed consent were waived due to the retrospective observational study design. The comprehensive pain service database at our hospital was reviewed for all patients undergoing the Nuss procedure over a 2.5 year period, including the year 2011 through 2013. Patients were excluded if pectus excavatum repair was accomplished using a surgical approach other than the Nuss procedure, such as the Ravitch procedure. Variables analyzed over the initial 72-h postoperative period included opioid consumption, pain scores, incidence of nausea and vomiting, and frequency of anti-emetic use. Numeric pain scores using a scale of 0–10 were obtained on arrival to postanesthesia care unit (PACU); on discharge to the ward; and throughout the subsequent hospital stay. Measurements were averaged during the first three consecutive 24-h postoperative periods. The time of discharge from the PACU to first oral intake was noted. Intraoperative adverse effects, including the occurrence of intraoperative hypotension (≥30% decrease of systolic blood pressure from baseline) and the need for catecholamine administration, were determined.
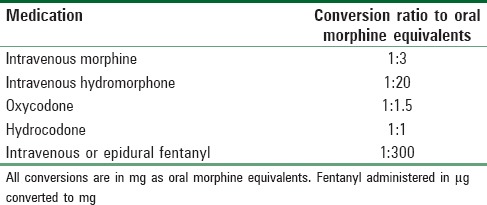
The primary objective of this study was to compare postoperative opioid consumption after the Nuss procedure between patients receiving PVB and TE and between patients receiving PVB and PCA. Cumulative postoperative opioid consumption was reported in oral morphine equivalents over 24-h periods for the first 72 h. Fentanyl delivered through the neuraxial route was considered equivalent to intravenous fentanyl and converted to oral morphine equivalents using defined scales [Table 1].[16,17] Secondary objectives were to determine whether the PVB group differed from the TE or PCA groups in pain scores, the incidence of nausea and vomiting, the frequency of antiemetic administration, time to first oral intake after PACU discharge, intraoperative hypotension, and intraoperative administration of catecholamine.
Table 1.
Opioid conversion ratios used to calculate total daily utilization
For patients receiving regional anesthesia, PVBs were placed bilaterally using sterile ultrasound technique at the level of T4–T5 or T5–T6. PVBs were initially dosed intraoperatively using ropivacaine 0.5% at 1.5 mg/kg per catheter not to exceed 3 mg/kg total dose. Ropivacaine 0.1% or 0.2% was infused postoperatively at 0.25 mg/kg/h per side, not to exceed 0.5 mg/kg/h for the total dose. PVBs were supplemented with PCA after PACU recovery in all cases. TEs were placed using midline loss of resistance technique at T5, T6, or T7 level. TEs were dosed with either ropivacaine 0.1% or bupivacaine 0.125% with fentanyl 2 μg/ml not to exceed a local anesthetic total dose of 0.5 mg/kg/h. Patients receiving TE therapy did not receive PCA in the initial postoperative period. If analgesia was deemed inadequate, a PCA was initiated. At that time, fentanyl was removed from the TE catheter infusion.
Data were summarized as means and standard deviations for normally-distributed continuous variables, as medians with interquartile ranges for nonnormally-distributed continuous variables, and as counts with percentages for categorical variables. Independent t-tests were used to compare opioid consumption and pain scores at each time point between PVB and TE and between PVB and PCA. Wilcoxon rank-sum tests (PVB vs. TE and PVB vs. PCA) were used to compare time to first oral intake and intraoperative episodes of hypotension. Fisher's exact tests were used to compare the intraoperative use of catecholamine, incidence of nausea or vomiting, and the use of antiemetic agents between PVB and TE and between PVB and PCA. P < 0.05 was considered statistically significant.
Results
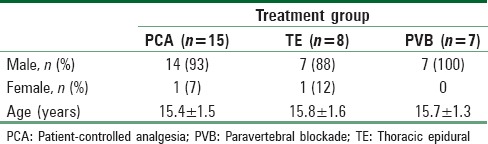
The study cohort included 31 patients undergoing thoracoscopic repair of pectus excavatum via the Nuss procedure and receiving postoperative analgesia through TE, PVB, or PCA. One patient who had received analgesia via PVB was excluded as one of the infusing catheters was accidentally dislodged in the PACU. The patient was subsequently changed to PCA therapy for postoperative analgesia. Of the remaining thirty patients, 15 were treated with PCA, eight with TE, and seven with PVB. The majority of patients were male, and the mean age of patients in the study cohort was 15.6 ± 1.5 years [Table 2].
Table 2.
Study cohort characteristics
Clinical characteristics of the 3 groups are summarized in Table 2. There were no significant differences in pain scores between the three groups at any time point during the study [Table 3]. Compared to PCA, the PVB group had lower opioid consumption over the first 24 h of hospitalization by 1.7 mg/kg morphine equivalents (95% confidence interval [CI] of difference: 0.1, 3.3; P = 0.035); but had higher opioid consumption by 2.0 mg/kg morphine equivalents than the TE group [Table 3; 95% CI of difference: 0.3, 3.7; P = 0.024]. There were no differences in opioid consumption between PVB and PCA or between PVB and TE at 48 or 72 h [Table 3 and Figure 1]. There were no differences in the occurrence of nausea or vomiting at any time point when comparing the three groups. There was no difference in the use of antiemetic agents and the time from PACU discharge until first oral intake among the 3 groups. The number of intraoperative hypotensive episodes was significantly lower in the PCA group when compared to the PVB group (P = 0.001), with no difference between the PVB and TE groups. All patients in the PVB group received at least one dose of a catecholamine to treat hypotension, whereas only one patient in each of the PCA and TE groups received a dose of catecholamine intraoperatively.
Table 3.
Clinical outcome characteristics of the study groups
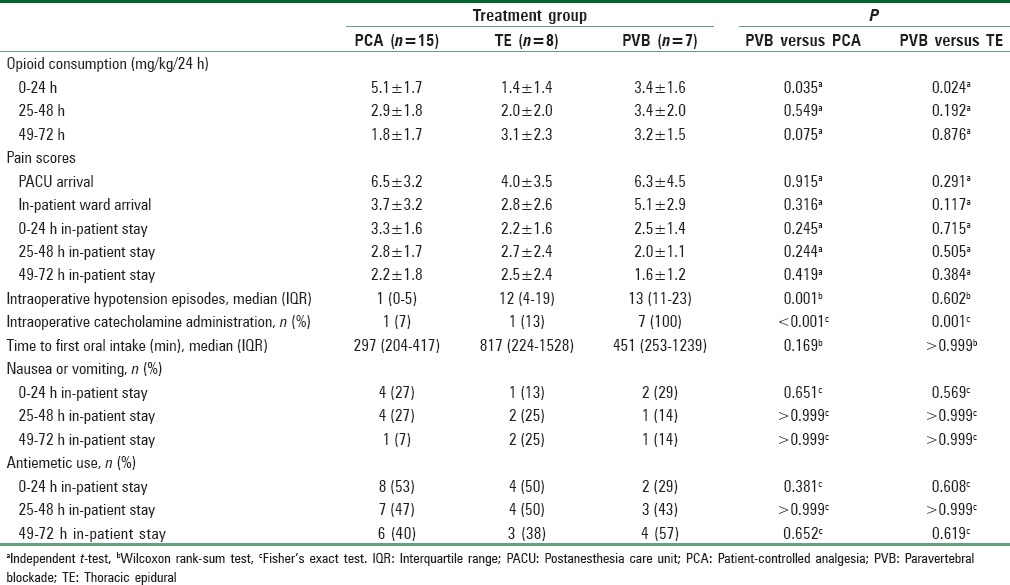
Figure 1.
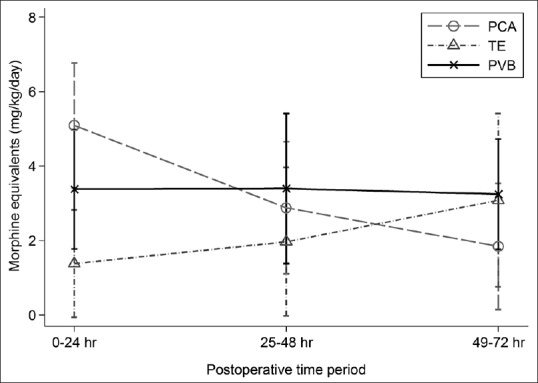
Opioid requirements expressed as morphine equivalents in mg/kg/day. The data are presented as the mean ± standard deviation at 0–24 h, P < 0.05 when comparing thoracic epidural analgesia or paravertebral blockade to patient-controlled analgesia
Discussion
The adequate treatment of postoperative pain after the Nuss procedure remains challenging. Three potential strategies used for pain management following this procedure include PVB, TE, and PCA. Several studies have found equivocal results when comparing PCA and TE following the Nuss procedure in children, whereas there are limited data on the efficacy and safety of PVB in this setting. Our retrospective analysis found a limited benefit of PVB compared to PCA or TE, which was manifested only as a reduction in opioid consumption during the first 24 h of postoperative care. However, no difference was noted in pain scores at any time during the postoperative period; and beyond the initial 24-h period among the three techniques.
Previous reports by McBride presented strong evidence that TE reduced the requirements for parenteral opioids following surgical repair of pectus excavatum deformity.[4] Similar results with improved analgesia demonstrated by lower pain scores were reported in a prospective study by Weber et al.[7] However, in our study cohort, a difference in opioid consumption among the three groups was observed only in the immediate 24-h postoperative interval, but not beyond this period. The absence of differences between TE and PVB beyond the first 24 postoperative hours was consistent with findings previously reported by Hall Burton and Boretsky.[15]
In addition, no difference in the incidence of postoperative nausea and vomiting (PONV) was noted in this study. Given the retrospective nature of the current study, we evaluated the use of the antiemetic medication, ondansetron, as a surrogate marker for PONV. The literature has previously reported mixed results when evaluating the incidence of PONV of these three analgesic techniques. No differences in side effects between TE and PVB were reported by Hall Burton and Boretsky, whereas a meta-analysis by Scarci et al. demonstrated PVB's superior side effect profile, with a lower incidence of nausea compared to patients receiving TE following thoracic surgery.[15,18]
However, we found that the incidence of intraoperative hypotension was significantly greater in patients receiving PVB or TE when compared to PCA. Furthermore, it appears that the magnitude of hypotension was greater with PVB than with either TE or PCA, as all of the patients receiving PVB required treatment of hypotension with a vasoactive agent, compared to only one patient in each of the PCA and TE groups (P = 0.021 compared to TE and P < 0.001 compared to PCA). Although both TE and PVB can lead to a decrease in blood pressure due to the resultant sympathectomy caused by the local anesthetic agent, it appears that the magnitude of this effect may have been greater with PVB, given the frequent use of vasoactive agents to reverse the changes in blood pressure. However, no perioperative sequelae were noted related to the hypotension. No neurological complications associated with the placement of catheters or the administered medications were noted.
Although there was a decrease in opioid consumption with regional anesthesia techniques over PCA for the first 24 h after surgery for correction of pectus excavatum, neither regional anesthesia technique was clearly superior to PCA in terms of opioid consumption, pain control, or side-effects during the entire 72 h postoperative follow-up. While TE is generally regarded as the gold standard for providing analgesia following thoracic procedures, less invasive techniques such as PVB may be an alternative when there are specific contraindications to TE.[19] Neurological complications related to placement of TE, albeit infrequent, have been reported, and constitute the basis for a change in practice in the treatment of postoperative pain following the Nuss procedure in some pediatric institutions.[15]
Limitations of the current study include its retrospective nature and the limited patient cohort. TE and PVB infusions were not standardized regarding the specific type or total hourly dose of the local anesthetic agent and opioid. In addition, the on-demand bolus technique now available with newer catheter infusion pumps was not used at the time of this study. Therefore, patients received only a fixed continuous infusion and were not able to supplement the TE or PVB infusion. Furthermore, the use of supplemental, nonopioid adjuvants (ketorolac or acetaminophen) was not standardized. The timing of pain assessment was also not standardized and therefore, we could not determine if the scores were determined at rest or during periods of dynamic pain such as work of deep breathing, coughing; or while performing activities of daily living.
Conclusion
Regional anesthesia remains a viable option for the relief of postoperative pain in pediatric patients following the Nuss procedure. Both PVB and TE decreased opioid consumption during the immediate postoperative period, and remain an effective treatment for postoperative analgesia. PVB can be considered an alternative to TE for cases of mild anticoagulation, or when there are concerns regarding the potential for neuraxial complications, as PVB is considered a peripheral nerve block technique. PCA may be considered as stand-alone therapy for pain relief in Nuss procedure patients given its comparable efficacy to regional techniques beyond the 1st postoperative day. A randomized, prospective, study powered to compare all three techniques against one another would be necessary to confirm the significance of these findings.
Financial support and sponsorship
This research was supported by Clinical and Translational Science Award grant UL1TR001070 provided by the National Institutes of Health via The Ohio State University Center for Clinical and Translational Science.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the invaluable assistance of Ms. Julie Rice and Mr. Usama Awan for their multiple contributions, and the support given to this project by the staff of the Department of Anesthesia and Pain Medicine at Nationwide Children's Hospital. Thank you.
References
- 1.Kelly RE, Jr, Shamberger RC, Mellins RB, Mitchell KK, Lawson ML, Oldham K, et al. Prospective multicenter study of surgical correction of pectus excavatum: Design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg. 2007;205:205–16. doi: 10.1016/j.jamcollsurg.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Densmore JC, Peterson DB, Stahovic LL, Czarnecki ML, Hainsworth KR, Davies HW, et al. Initial surgical and pain management outcomes after Nuss procedure. J Pediatr Surg. 2010;45:1767–71. doi: 10.1016/j.jpedsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Futagawa K, Suwa I, Okuda T, Kamamoto H, Sugiura J, Kajikawa R, et al. Anesthetic management for the minimally invasive Nuss procedure in 21 patients with pectus excavatum. J Anesth. 2006;20:48–50. doi: 10.1007/s00540-005-0367-4. [DOI] [PubMed] [Google Scholar]
- 4.McBride WJ, Dicker R, Abajian JC, Vane DW. Continuous thoracic epidural infusions for postoperative analgesia after pectus deformity repair. J Pediatr Surg. 1996;31:105–7. doi: 10.1016/s0022-3468(96)90329-2. [DOI] [PubMed] [Google Scholar]
- 5.Butkovic D, Kralik S, Matolic M, Kralik M, Toljan S, Radesic L. Postoperative analgesia with intravenous fentanyl PCA vs.epidural block after thoracoscopic pectus excavatum repair in children. Br J Anaesth. 2007;98:677–81. doi: 10.1093/bja/aem055. [DOI] [PubMed] [Google Scholar]
- 6.Soliman IE, Apuya JS, Fertal KM, Simpson PM, Tobias JD. Intravenous versus epidural analgesia after surgical repair of pectus excavatum. Am J Ther. 2009;16:398–403. doi: 10.1097/MJT.0b013e318187de3e. [DOI] [PubMed] [Google Scholar]
- 7.Weber T, Mätzl J, Rokitansky A, Klimscha W, Neumann K, Deusch E Medical Research Society. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg. 2007;134:865–70. doi: 10.1016/j.jtcvs.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 8.St. Peter SD, Weesner KA, Weissend EE, Sharp SW, Valusek PA, Sharp RJ, et al. Epidural vs. patient-controlled analgesia for postoperative pain after pectus excavatum repair: A prospective, randomized trial. J Pediatr Surg. 2012;47:148–53. doi: 10.1016/j.jpedsurg.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 9.Kelly RE, Goretsky MJ, Obermeyer R, Kuhn MA, Redlinger R, Haney TS, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg. 2010;252:1072–81. doi: 10.1097/SLA.0b013e3181effdce. [DOI] [PubMed] [Google Scholar]
- 10.Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, et al. Asystematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107:1026–40. doi: 10.1213/01.ane.0000333274.63501.ff. [DOI] [PubMed] [Google Scholar]
- 11.Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs. epidural blockade for thoracotomy – A systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96:418–26. doi: 10.1093/bja/ael020. [DOI] [PubMed] [Google Scholar]
- 12.Casati A, Alessandrini P, Nuzzi M, Tosi M, Iotti E, Ampollini L, et al. Aprospective, randomized, blinded comparison between continuous thoracic paravertebral and epidural infusion of 0.2% ropivacaine after lung resection surgery. Eur J Anaesthesiol. 2006;23:999–1004. doi: 10.1017/S0265021506001104. [DOI] [PubMed] [Google Scholar]
- 13.Richardson J, Sabanathan S, Jones J, Shah RD, Cheema S, Mearns AJ. A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth. 1999;83:387–92. doi: 10.1093/bja/83.3.387. [DOI] [PubMed] [Google Scholar]
- 14.Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15:288–92. doi: 10.1053/jcan.2001.23271. [DOI] [PubMed] [Google Scholar]
- 15.Hall Burton DM, Boretsky KR. A comparison of paravertebral nerve block catheters and thoracic epidural catheters for postoperative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr Anaesth. 2014;24:516–20. doi: 10.1111/pan.12369. [DOI] [PubMed] [Google Scholar]
- 16.Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19:286–97. doi: 10.1097/00002508-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Guinard JP, Carpenter RL, Chassot PG. Epidural and intravenous fentanyl produce equivalent effects during major surgery. Anesthesiology. 1995;82:377–82. doi: 10.1097/00000542-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Scarci M, Joshi A, Attia R. In patients undergoing thoracic surgery is paravertebral block as effective as epidural analgesia for pain management? Interact Cardiovasc Thorac Surg. 2010;10:92–6. doi: 10.1510/icvts.2009.221127. [DOI] [PubMed] [Google Scholar]
- 19.Visoiu M, Yang C. Ultrasound-guided bilateral paravertebral continuous nerve blocks for a mildly coagulopathic patient undergoing exploratory laparotomy for bowel resection. Paediatr Anaesth. 2011;21:459–62. doi: 10.1111/j.1460-9592.2010.03511.x. [DOI] [PubMed] [Google Scholar]


