Abstract
Background:
Local anesthetic infiltration for medical thoracoscopy has an analgesic properties for short duration. Single injection thoracic paravertebral block (PVB) provides limited analgesia.
Purpose:
Comparison between thoracic PVB performed at two or three levels with local infiltration for anesthetic adequacy in adult medical thoracoscopy as a primary outcome and postthoracoscopic analgesia and pulmonary function as secondary outcomes for adult medical thoracoscopy.
Patients and Methods:
Prospective randomized control study included 63 adult patients with exudative pleural effusion randomly divided into three groups of 21 patients: 3-level PVB, 2-level PVB group, and local infiltration group. Patients with contraindications to regional anesthesia or uncontrolled comorbidities were excluded from the study. Pain visual analog scale and spirometry were used for comparison as anesthetic adequacy in adult medical thoracoscopy as a primary outcome besides prolonged analgesia and improved pulmonary function as secondary outcomes.
Results:
The anesthetic adequacy was 95.3% in 3-level PVB group, 81% in 2-level PVB group, and 71.5% in local infiltration group. The mean sensory level was 1 ± 0.8 and 1 ± 0.6 segment above and 0.8 ± 0.6 and 0.7 ± 0.7 segment below the injected level in 3-level PVB group and 2-level PVB, respectively. VAS was statistically significant higher in local infiltration compared to the other two groups immediately postthoracoscopic and 1 h after. Two-hour postthoracoscopy, significant increase in forced vital capacity values in the three groups compared to their basal values whereas forced expiratory volume at 1 s (FEV1) only in both PVB groups.
Conclusion:
Unilateral 3-level TPVB was superior to 2-level TPVB and LA infiltration for anesthetic adequacy for patients undergoing medical thoracoscopy. Moreover, US-guided TPVB was followed by higher FEV1 values and lower pain scores during the next 12 h postthoracoscopy in comparison to local infiltration, so 3-level TPVB is an effective and relatively safe anesthetic technique for adult patients undergoing medical thoracoscopy which may replace local anesthesia.
Keywords: Local anesthesia, medical thoracoscopy, thoracic paravertebral block
Introduction
Medical thoracoscopy describes the evaluation of the pleural space in nonintubated patients, and it is the “gold standard diagnosis” of pleural effusion, especially the exudative one. Medical thoracoscopy is a minimally invasive, simple, and safe method to facilitate performing multiple thoracic interventions.[1,2]
Local anesthetic (LA) infiltration has been used popularly in medical thoracoscopy. It has benefits of making the procedure less complex with low cost. It has been used alone or as needed an intravenous (IV) sedatives and analgesics were given to decrease pain and anxiety during the procedure.[3,4] Although its popularity as an alternative method to general anesthesia, their short duration of action results in the consequent need for repeated analgesic administration.[5]
Paravertebral block (PVB) can offer a long-lasting analgesia. Thoracic paravertebral block (TPVB) has been used for postoperative analgesia as well as a sole anesthetic for unilateral surgeries such as mastectomy[6,7] and open cholecystectomy.[8] Unpredicted dermatomal block could result After a single injection PVB due to unanticipated vertical spread of LA in the paravertebral space and the more the number of PVB injection levels, the more extended and adequate effect.[6]
Hence, this study was held to detect the appropriate injection numbers of unilateral TPVB at either 2 or 3 levels that will be able to provide adequate anesthesia compared to LA infiltration applied for adult medical thoracoscopy as a primary outcome. The postthoracoscopic analgesia in the first 12 h and the variation in pulmonary function were the secondary outcomes. We hypothesized that both PVB groups will provide more adequate anesthesia for thoracoscopy than LA infiltration group, with superiority of 3-level PVB over 2-level PVB.
Patients and Methods
This prospective, randomized study included 63 adult patients, American Society of Anesthesiologists physical status I or II admitted to Chest Department, Mansoura University Hospital, from March 2015. Those patients were scheduled for elective unilateral rigid medical thoracoscopy for diagnosis and management of an exudative pleural effusion. Ethical approval had been obtained from Mansoura Faculty of Medicine Institutional Review Board (MFM-IRB number R/110) and Pan African Clinical Trial Registry (number PACTR201512001341395). Patients signed their written consents after detailed explanation of the study protocol. Exclusion criteria were any contraindications to regional anesthesia (coagulopathy, infection at the site of needle insertion, or anesthetic drug allergy), patients on current opioid prescription or with significant concurrent medical disease. Patients underwent previous thoracotomy or with chest deformity, sensory deficit, or had empyema were also excluded from the study.
After medical history revision, clinical examination, laboratory and chest radiological evaluation, pleural fluid aspiration for biochemical examination, acid-fast bacilli stain, total cell count, pleural fluid culture and sensitivity, cytological examination, closed pleural biopsy by Abrams needle, spirometry using smart pulmonary function test (PFT) with stress on forced vital capacity (FVC) and forced expiratory volume at 1 s (FEV1%) of the predicted was done just before thoracoscopy. The patients were instructed to use a 10 mm visual analog scale (VAS) for pain (where 0, no pain; 10, worst pain imagined). All patients were fasting 6 h for solid food.
Anesthetic procedure
Patients were monitored for heart rate (HR), mean blood pressure (MBP), and peripheral oxygen saturation (SpO2). 2–3 L/min O2 was applied through nasal cannula and IV 0.025 mg/kg midazolam was given. Under a complete aseptic technique and with accessibility of the necessary medications and resuscitation equipment, patients were anesthetized through an anesthetist not involved in monitoring and data collection. The thoracoscopic trocar point of entry was selected by ultrasound (US) evaluation to identify the point where the effusion collection is largest and the elevated position of the diaphragm which was at the 6th intercostal space.
Through sealed envelope technique, patients were randomly divided into three groups of 21 patients each according to the anesthetic technique used. Group 1 was managed by unilateral 3-level PVB (3-level PVB group), Group 2 was managed by unilateral 2-level PVB (2-level PVB group), and Group 3 was managed by local infiltration (LI group) along the site of thoracoscopy entrance.
Patients in the local infiltration group received 9 ml subcutaneous infiltration which consists of 4.5 ml of bupivacaine 0.5% plus 2 ml mepivacaine 2% diluted with normal saline at the thoracoscopy entrance site.
For both PVB groups, the same anesthetic solution was used, containing 9 ml bupivacaine 0.5% plus 4 ml mepivacaine 2% diluted with normal saline in a total volume of 18 ml. Marking of the PVB injection sites at the thoracoscopic side was between the transverse processes 2.5 cm from midline performed at T4 and T5 in the 2-level PVB group or at T3, T4, and T5 in the 3-level PVB group, where patients were sitting. Then, the subcutaneous tissue at each injection site was infiltrated with lidocaine 2%.
A 13-MHz high-frequency linear US transducer (Sonoscape, China) was placed in a vertical orientation 2–3 cm lateral to the midline to visualize the vertebral transverse processes as an interrupted thick hyperechoic lines and the parietal pleura as a hyperechoic shadow sliding with inspiration. Deep to it, the superior costotransverse ligament was detected as a multiple straight homogeneous echogenic and hypoechogenic bands. Under US visualization, an 18-gauge Tuohy needle was introduced midway between the two transverse processes in a plane approach, lateral to medial direction until the needle tip is seen close to the costotransverse ligament, as the needle was seen approaching the costotransverse ligament, loss of resistance to air was implemented to avoid pleural puncture. After negative aspiration, 6 ml of the anesthetic solution was injected slowly in each space in the 3-level PVB group, whereas 9 ml was injected at each level in the 2-level PVB group with the observation of the pleural displacement.
Sensory block was assessed using cold perception bilaterally till 30 min after injection, and in both PVB groups, the dermatomal level of sensory blockade was recorded. If adequate sensory blockade of the entrance site of thoracoscopy did not occur, patient was excluded from the study. A clinically adequate anesthesia state was identified by no need for any analgesic or sedative during thoracoscopy. If anesthesia was inadequate, identified by patients' complaint of intolerable pain during thoracoscopy, 1.5–2.5 mg/kg of propofol initial loading dose, followed by 2–10 mg/kg/h continuous IV infusion (IVI) and 1 g paracetamol were given, and the patient was excluded from the study.
Thoracoscopy
Medical thoracoscopy was performed while patients were in the lateral decubitus with the affected side up. An 11 mm rigid rod lens telescope (STORZ) was introduced in the pleural cavity; suction of all fluids was done; examination of the pleural space, cutting and removal of adhesions, and thickened pleura, and biopsy was done using forceps through forceps channel; and intercostal tube (30 F) was inserted in the pleural cavity. The aspirated pleural fluid was examined biochemically, acid-fast bacilli stain, total cell count, culture and sensitivity, and cytological examination with recording of the final pathologic diagnosis. The thoracoscopic procedure duration (from skin incision till putting the adhesive tape) was recorded.
After thoracoscopy, the patients were monitored for HR, MBP, and SpO2. Any observed complication was managed and recorded.
Two hours postthoracoscopy, PFT was repeated. VAS was measured; immediately postthoracoscopy, 1, 6, and 12 h later. Pain requiring analgesia when VAS >3 was treated with IVI of 1 g paracetamol given up to four times a day.
Sample size
Based on pilot study data from patients receiving local infiltration to detect the success rate of anesthetic adequacy (our primary outcome variable), it was 70%. We assumed a 25% increase in anesthetic adequacy in the 2-level PVB group would be clinically significant. According to these data, a sample size of 17 patients per group assuming an α error of 0.05, β error of 0.2, and a power of 80%. Allowing 10% dropout, so 19 patients were needed in each group.
Data collection
Data entry and analyses were performed using SPSS statistical package version 21 (SPSS, Inc., Chicago, IL, USA). The data were examined for normal distribution using Shapiro–Wilks test. Paired t-test was conducted to evaluate the impact of time on the mean of continuous variable in each group. Parametric data were reported as mean ± standard deviation (SD), whereas nonparametric categorical data were reported as frequency and percentage or median (interquartile range) then Kruskal–Wallis test was used for comparisons. For normally distributed continuous parametric variables among the groups, one-way analysis of variance was used to compare means ± SD with Post Hoc analysis for internal comparisons between groups where P < 0.0167 was considered statistical significance level as α level set at 0.0167 otherwise P < 0.05 is considered statistically significant level.
Results
One case (4.7%) in the 3-level PVB group, four cases (19%) in the 2-level PVB group, and six cases (28.5%) in the local infiltration group required IV anesthetic due to pain detected after starting thoracoscopy and they were excluded from the study. Hence the anesthetic adequacy was 95.3% in 3-level PVB group, 81% in 2-level PVB group, and 71.5% in local infiltration group. Furthermore, one patient in 2-level PVB complained of mild pain once during the use of diathermy, but the thoracoscopic procedure was completed with no further pain or anesthetic requirement. Figure 1 shows the consort flowchart of the studied patients with total number of 52 patients followed up and analyzed.
Figure 1.
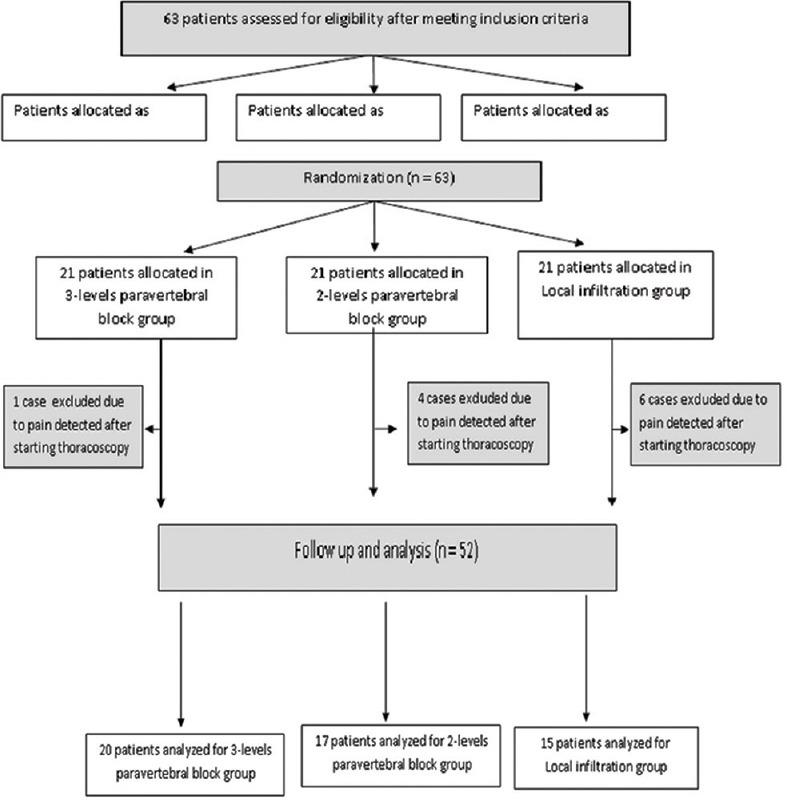
Consort flowchart of the studied groups
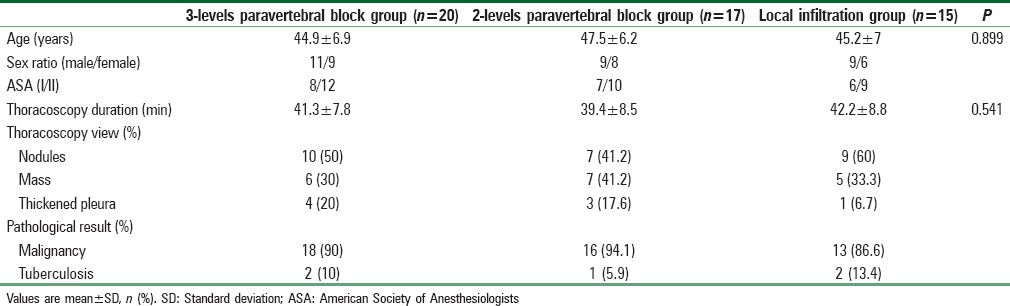
Demographic data, thoracoscopic duration, view, and pathological diagnosis did not differ among the three groups [Table 1].
Table 1.
Demographic and thoracoscopic data of the studied groups
All cases in both PVB groups had a unilateral spread of the block. The mean sensory level was 1 ± 0.8 and 1 ± 0.6 segment above and 0.8 ± 0.6 and 0.7 ± 0.7 segment below the injected level in 3-level PVB group and 2-level PVB, respectively.
The MBP increased significantly 15 min after starting thoracoscopy from the basal values in local infiltration group [Figure 2]. A significant decrease in MBP from the baseline was recorded only in one patient in 3-level PVB group 15 minutes after the block, which necessitates the administration of IV fluids and 10 mg ephedrine, then the procedure was performed successfully in the lateral position.
Figure 2.
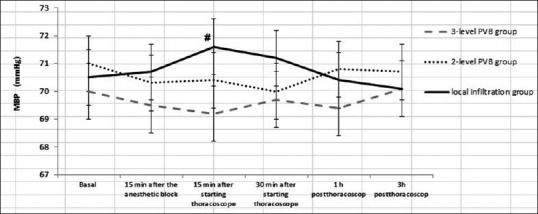
Mean arterial blood pressure of the studied groups
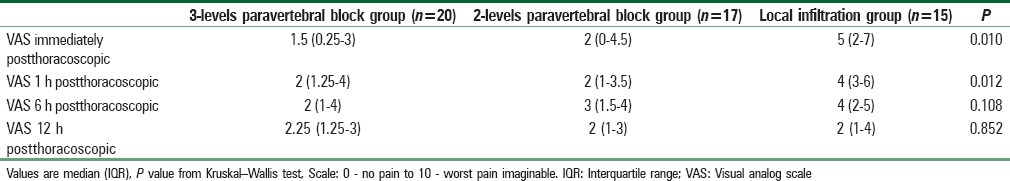
Table 2 summarized VAS immediately postthoracoscopic then after 1, 6, and 12 h. VAS was significantly higher in the local infiltration group compared to the other two PVB groups immediately postthoracoscopic (P = 0.010) and 1 h after (P = 0.010).
Table 2.
Visual analog scale scores for pain degree
As regards perioperative complications, one case had pneumothorax in the 2-level PVB group detected by bedside US after the block. There was postthoracoscopic nausea in two patients in 3-level PVB group and one patient in both 2-level PVB and local infiltration groups and all of those patients improved after treated by IV metoclopramide 10 mg.
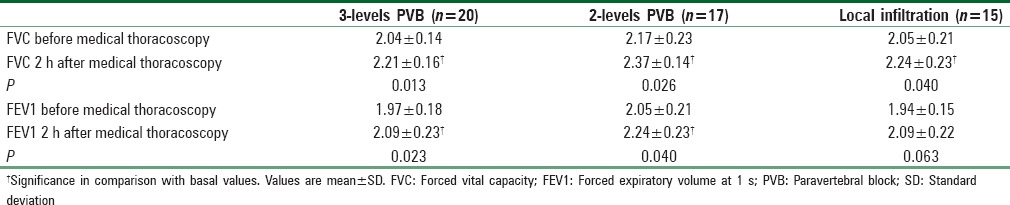
Two-hour postthoracoscopy, both FVC and FEV1 values displayed a significant increase in the three groups compared to their basal values [Table 3]. There were no statistically significant differences among the three groups as regard FVC and FEV1 either before the thoracoscopy or after it. As regard FVC, there was a statistically significant increase in all the three groups after thoracoscopy versus before it (P = 0.013, 0.026, and 0.040). As regard FEV1, there was a statistically significant increase in both PVB groups after thoracoscopy versus before it (P = 0.023 and 0.040); however, there was no statistically significant increase in Local infiltration group after thoracoscopy versus before it (P = 0.063).
Table 3.
Forced vital capacity and forced expiratory volume at 1 s of the studied groups
Discussion
In this study, the 3-level US-guided TBVB was the most appropriate regional anesthetic technique during medical thoracoscopy and was superior to both local infiltration anesthesia and 2-level TBVB. Moreover, both TBVB groups provided better postprocedure analgesia and an improvement in pulmonary function which was evidenced with better VAS, stable hemodynamics during the procedure and postoperatively.
Our study showed that TBVB was adequate anesthetic technique for medical thoracoscopy. This goes in line with the adequacy of TPVB as a sole anesthesia for breast surgery in the study of Naja et al.[9] Another study involving patients undergoing herniorrhaphy, single injection PVB was administrated at T11 level using 12 mL of 2% mepivacaine. The PVB provided adequate anesthesia in 60% of the studied patients and had covered seven dermatomes.[10] TBVB provided a good postoperative analgesia in both groups after thoracoscopy up to 12 h after the procedure with lower VAS than local infiltration. This lower VAS in both PVB groups approves the results of previous studies showing that TPVB reduced the severity of postoperative pain after breast surgeries.[9,11] This is in agreement with another study which showed that a multilevel PVB provided adequate analgesia for breast surgery.[12] Furthermore, in a meta-analysis of 15 randomized controlled trials, it was concluded that PVB provided optimal postoperative pain control with little adverse effects compared with other strategies for postoperative pain management.[13] This sufficient analgesia can be explained by the preemptive effect of the PVB with reduction of the nociceptive input to the central nervous system with attenuation of central sensitization; therefore less pain is perceived.[14]
The more injected levels in TPVB, the more the extent of anesthetic coverage and the more the analgesic duration which may extend up to 72 h post-injection but with increased invasiveness of the procedure which is accompanied by significant patient discomfort and possibly more side effects.[6] This explained the effectiveness of 3-level PVB group for anesthetic adequacy in comparison to 2-level PVB group. The mean sensory dermatomal segments blocked in the 3-level PVB group were 1.6 segments above and 1 segment below the level of injection, whereas it was 0.9 above and 0.8 below in 2-level PVB group. This contradicts the results of Cheema et al., 2003,[15] on patients with chronic pain. Higher mean sensory level of 2.2 segments above and 1.4 segments below the level of PVB injection was found in their study which could be explained by the type and volume of the LA as they used almost 10–15 ml bupivacaine 0.5%, mixed with 80 mg Depo-Medrone. In our literature, two types of LAs (bupivacaine and mepivacaine) were used to speed the onset with prolonged duration. In both PVB groups, there was no evidence of bilateral blockade. A TPVB injection may remain localized to the injected level or it may spread to the contiguous levels above and below, whereas it is uncommon to spread to the contralateral side.[16]
Medical thoracoscopy is used by the pulmonologist for diagnostic procedure by taking biopsies from the parietal and the visceral pleura or even performing lung biopsy. It is also used for the management of large pleural effusion other than doing adhesiolysis of adhesions in the presence of a loculated or infected pleural space or talc pleurodesis in patients with secondary pneumothorax. It is mostly performed under local anesthesia, especially for whom unsuitable for more invasive techniques such as video-assisted thoracoscopy under general anesthesia; however, it was associated with marked postthoracoscopic pain and patient discomfort.[17] Despite LA infiltration is an easy technique, it provides short pain free period limited to the early postoperative period[18] which was noted by a significant higher VAS in the local infiltration group immediately after thoracoscopy and 1 h later compared to both PVB groups which can be explained by the stronger attenuation of the stress response in the PVB groups than local anesthesia. The weak ability of local infiltration to attenuate the stress response could also explain the significant increase in MBP 15 min after starting thoracoscopy in local infiltration group in our study.
Postthoracoscopic FVC and FEV1 values increased compared to the basal values in the three groups which can be explained by the improvement in the chest conditions after thoracoscopy. The improvement in FEV1 values was significantly higher in both PVB after thoracoscopy versus before it. This is similar to the finding of Davies et al.[19] who demonstrated that TPVB provided more improvements in respiratory function in addition to good analgesia with less side effect (especially on hemodynamic) after thoracic surgeries compared to epidural analgesia.
In the past, TPVB was performed blindly based on the bony landmarks and loss of resistance detected while the needle piercing the superior costotransverse ligament almost 1–1.5 cm from the superior border of vertebral transverse process. The nerve stimulator could guide PVB injection close to the nerve in the dorsal part of the vertebral space after obtaining muscle contractions.[9] The proximity of the thoracic paravertebral space to the lung leads to a risk of pneumothorax (0%–6.7%).[6] US-guided TPVB has been used popularly to decrease the risk of pleural puncture as it allows visualization of the needle tip entry and the spread of LA in the paravertebral space.[20] This could explain that only one case had pneumothorax in the 2-level PVB group. Although pneumothorax induced after TPVB could not be considered as a problem in this literature since chest tube was inserted at the end of the thoracoscopic procedure.
The limitations of our study are US alone cannot guarantee 100% prevention of pneumothorax despite it allows accurate determination of the paravertebral space and observation of the spread of LA in real time. Furthermore, no opioids were added to the anesthetic mixture which may improve the postoperative analgesia. Future studies may implement a combination of US and nerve stimulator for more optimal access and success to the paravertebral space more close to the nerve roots. Furthermore, we may use adjuvants to the LA for more prolonged periods of analgesia with low side effect profile.
Last but not least, unilateral 3-level US-guided TPVB was more preferred to the 2-level TPVB and the LA infiltration for patients undergoing medical thoracoscopy as an anesthetic technique. It provided better anesthesia and more prolonged postoperative analgesia than the two other techniques. Moreover, both US-guided TPVB was associated with better pulmonary function than local anesthesia with improved FEV1.
In conclusion 3-level TPVB was the recommended as an adequate regional anesthetic technique for medical thoracoscopy owing to lower postoperative pain score and better pulmonary function the an other techniques.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ernst A, Hersh CP, Herth F, Thurer R, LoCicero J, 3rd, Beamis J, et al. Anovel instrument for the evaluation of the pleural space: An experience in 34 patients. Chest. 2002;122:1530–4. doi: 10.1378/chest.122.5.1530. [DOI] [PubMed] [Google Scholar]
- 2.Maskell NA, Butland RJ Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax. 2003;58(Suppl 2):ii8–17. doi: 10.1136/thorax.58.suppl_2.ii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diacon AH, Wyser C, Bolliger CT, Tamm M, Pless M, Perruchoud AP, et al. Prospective randomized comparison of thoracoscopic talc poudrage under local anesthesia versus bleomycin instillation for pleurodesis in malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1445–9. doi: 10.1164/ajrccm.162.4.2002030. [DOI] [PubMed] [Google Scholar]
- 4.Nezu K, Kushibe K, Tojo T, Takahama M, Kitamura S. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest. 1997;111:230–5. doi: 10.1378/chest.111.1.230. [DOI] [PubMed] [Google Scholar]
- 5.Fredman B, Zohar E, Tarabykin A, Shapiro A, Mayo A, Klein E, et al. Bupivacaine wound instillation via an electronic patient-controlled analgesia device and a double-catheter system does not decrease postoperative pain or opioid requirements after major abdominal surgery. Anesth Analg. 2001;92:189–93. doi: 10.1097/00000539-200101000-00036. [DOI] [PubMed] [Google Scholar]
- 6.Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–5. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Terheggen MA, Wille F, Borel Rinkes IH, Ionescu TI, Knape JT. Paravertebral blockade for minor breast surgery. Anesth Analg. 2002;94:355–9. doi: 10.1097/00000539-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Beyaz SG, Özocak H, Ergönenç T, Erdem AF. The thoracic paravertebral block performed for open cholecystectomy operation in order to anesthesia: Two cases. Anesth Essays Res. 2014;8:239–42. doi: 10.4103/0259-1162.134521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naja MZ, Ziade MF, Lönnqvist PA. Nerve-stimulator guided paravertebral blockade vs.general anaesthesia for breast surgery: A prospective randomized trial. Eur J Anaesthesiol. 2003;20:897–903. doi: 10.1017/s0265021503001443. [DOI] [PubMed] [Google Scholar]
- 10.Saito T, Gallagher ET, Cutler S, Tanuma K, Yamada K, Saito N, et al. Extended unilateral anesthesia.new technique or paravertebral anesthesia? Reg Anesth. 1996;21:304–7. [PubMed] [Google Scholar]
- 11.Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ. Single-injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesth Analg. 2004;99:1837–43. doi: 10.1213/01.ANE.0000136775.15566.87. [DOI] [PubMed] [Google Scholar]
- 12.Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: A randomized double-blind study. Anesth Analg. 2007;105:1848–51. doi: 10.1213/01.ane.0000286135.21333.fd. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: A meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105:842–52. doi: 10.1093/bja/aeq265. [DOI] [PubMed] [Google Scholar]
- 14.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 15.Cheema S, Richardson J, McGurgan P. Factors affecting the spread of bupivacaine in the adult thoracic paravertebral space. Anaesthesia. 2003;58:684–7. doi: 10.1046/j.1365-2044.2003.03189_1.x. [DOI] [PubMed] [Google Scholar]
- 16.Agnoletti V, Piraccini E, Corso R, Avino F, Rotondo C, Maitan S, et al. Methylene blue diffusion after multilevel thoracic paravertebral blocks. J Cardiothorac Vasc Anesth. 2011;25:e5–6. doi: 10.1053/j.jvca.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Rahman NM, Ali NJ, Brown G, Chapman SJ, Davies RJ, Downer NJ, et al. Local anaesthetic thoracoscopy: British thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii54–60. doi: 10.1136/thx.2010.137018. [DOI] [PubMed] [Google Scholar]
- 18.Sidiropoulou T, Buonomo O, Fabbi E, Silvi MB, Kostopanagiotou G, Sabato AF, et al. A prospective comparison of continuous wound infiltration with ropivacaine versus single-injection paravertebral block after modified radical mastectomy. Anesth Analg. 2008;106:997–1001. doi: 10.1213/ane.0b013e31816152da. [DOI] [PubMed] [Google Scholar]
- 19.Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs. epidural blockade for thoracotomy – A systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96:418–26. doi: 10.1093/bja/ael020. [DOI] [PubMed] [Google Scholar]
- 20.Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001;56:1184–8. doi: 10.1046/j.1365-2044.2001.02084-2.x. [DOI] [PubMed] [Google Scholar]


