Abstract
Management of pregnant women with heart disease remains challenging due to the advancement of innovations in cardiac surgery and correction of complex cardiac anomalies, and more recently, with the successful performance of heart transplants, cardiac diseases are not only likely to coexist with pregnancy, but will also increase in frequency over the years to come. In developing countries with a higher prevalence of rheumatic fever, cardiac disease may complicate as many as 5.9% of pregnancies with a high incidence of maternal death. Since many of these deaths occur during or immediately following parturition, heart disease is of special importance to the anesthesiologist. This importance arises from the fact that drugs used for preventing or relieving pain during labor and delivery exert a major influence – for better or for worse – on the prognosis of the mother and newborn. Properly administered anesthesia and analgesia can contribute to the reduction of maternal and neonatal mortality and morbidity.
Keywords: Anesthesia, complex cardiac, pregnancy, rheumatic
Introduction
Management of pregnant women with heart disease remains challenging due to the advancement of innovations in cardiac surgery and correction of complex cardiac anomalies, and more recently, with the successful performance of heart transplants, cardiac diseases are not only likely to coexist with pregnancy, but will also increase in frequency over the years to come.[1,2,3,4] In developing countries with a higher prevalence of rheumatic fever, cardiac disease may complicate as many as 5.9% of pregnancies[5] with a high incidence of maternal death. Since many of these deaths occur during or immediately following parturition, heart disease is of special importance to the anesthesiologist. This importance arises from the fact that drugs used for preventing or relieving pain during labor and delivery exert a major influence – for better or for worse – on the prognosis of the mother and newborn. Properly administered anesthesia and analgesia can contribute to the reduction of maternal and neonatal mortality and morbidity.
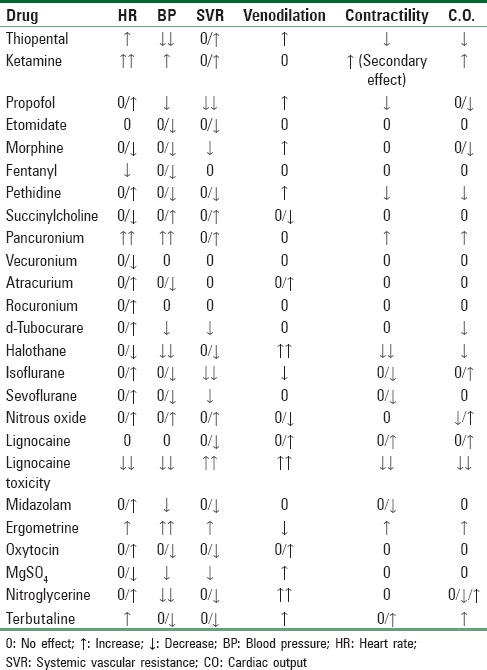
Cardiac pathology represents a wide spectrum of conditions, including congenital or acquired, functional or structural, cyanotic or noncyanotic, and endocardial, myocardial, or pericardial defects. These cardiac lesions may be associated with few symptoms in the nonpregnant state, but they become apparent for the first time in mid-to-late pregnancy as a result of the physiologic hemodynamic stresses that develop. Various structural cardiac lesions may be uncorrected, fully corrected, or partially corrected (palliated) when the pregnant patient is encountered by the anesthetist. In many cases, there is no consensus as to the optimal anesthetic technique and therefore there is no single recipe approach to their management. Although general and regional anesthesia both affect the hemodynamic changes occurring during labor and delivery, the choice of technique is often immaterial if appropriate hemodynamic goals are considered and invasive monitoring is used to attain them. The most likely cardiovascular effects of commonly utilized anesthetic and obstetric drugs are shown in Table 1.[6]
Table 1.
Cardiovascular effects of commonly used anesthetic and obstetric drugs
Overall maternal and fetal morbidity and mortality from cardiac disease are directly related to the severity of cardiac disease. Maternal mortality ranges from 0.4% in the New York Heart Association (NYHA) class I–II disease to 6.8% in class III–IV disease [Table 2].[7] The risk to the mother with congenital heart disease depends on the type of malformation and the functional impairment it produces [Table 3].[8] Major concern for a pregnant woman with a cardiac disease is cardiac decompensation because of the inability to meet the additional demands imposed by the physiologic changes of pregnancy and parturition. If present, infection, hemorrhage, and thromboembolism compound the risk. It is essential to understand the impact of the physiologic changes of pregnancy upon the specific heart lesion to properly counsel and manage these patients. Pregnant women with heart disease should be managed by a team of representatives from obstetrics and perinatology, anesthesiology, neonatology, cardiology, cardio-thoracic surgery, intensive care, nursing, and social work.[9]
Table 2.
The New York Heart Association functional capacity and objective assessment
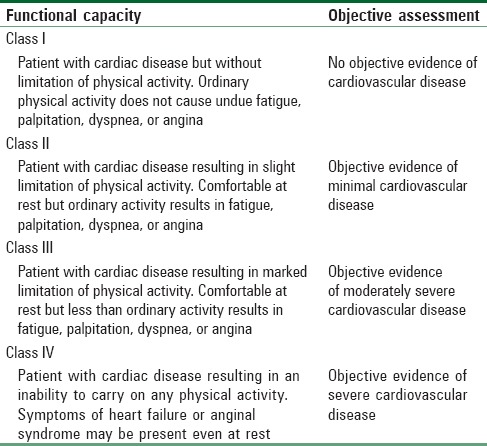
Table 3.
Maternal mortality associated with heart disease in pregnancy (Clark's classification)

Physiological Changes during Pregnancy
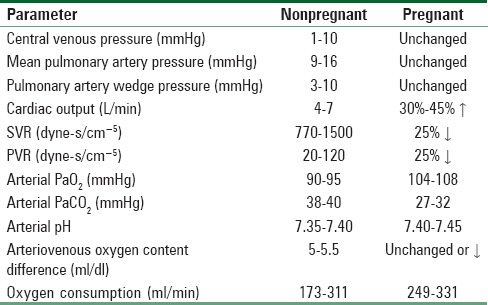
Cardio-respiratory changes, which occur throughout the pregnancy and peripartum period, may affect the well-being of women with heart disease. A comparison of normal cardio-respiratory parameters between the pregnant and nonpregnant states is shown in Table 4.[10]
Table 4.
Normal hemodynamic and ventilatory parameters in the nonpregnant and pregnant patients
Respiratory system
Minute ventilation and oxygen consumption increase during pregnancy, with a state of mild hyperventilation caused by the central action of progesterone. Increased minute ventilation results in a mild respiratory alkalosis.[11]
Blood volume
By term, plasma volume is 40%–50% higher than prepregnant levels, with increases in plasma volume exceeding those of red blood cell mass (20%–30%).[12] This disproportion produces a dilutional or physiologic anemia, which is treatable with supplements of oral iron.
Cardiac output
Cardiac output (CO) starts to increase by the 10th week of gestation and continues to rise to a peak of 30%–50% above baseline at 32 weeks' gestation. Studies suggesting significant decreases in CO during the later half of pregnancy were performed with patients at supine – a position associated with aortocaval compression.[13] There is a less significant fall in CO at this time if measurements are made in the left lateral decubitus position, because venous return is maintained.[14] The increase in CO is due to greater stroke volume in the first half of pregnancy, in contrast to the later half when CO is maintained by an increase in heart rate superimposed on an increase in stroke volume.[14,15] There is a rise in endogenous circulating catecholamines during labor, which produces a positive inotropic and chronotropic myocardial response. Left ventricular end-diastolic volume rises as a result of an expanded plasma volume, and this leads to an increase in myocardial contractility and stroke volume. CO and changes in cardiac function can be readily assessed by echocardiography in women with heart disease. Echocardiography is often very helpful in early pregnancy because it serves to provide baseline hemodynamic values with which changes in late pregnancy can be compared and therapy can be guided.
Blood pressure
The placental bed acts as a low-resistance arteriovenous shunt, and in addition, there is physiologic vasodilatation in the arterial vascular bed due to the hormonal influence of endothelial prostacyclin and circulating progesterone.[16] As a result, blood pressure (BP) usually falls during pregnancy, with an increase in pulse pressure due to a greater decrease in diastolic pressure.
Labor and delivery
Increased heart rate due to labor pains and enhanced stroke volume by improved venous return from the contracting uterus raise CO by 20%–50%. During labor, each uterine contraction is associated with a 5%–20% increase in BP.[17] The mode of anesthesia influences the hemodynamic changes during labor and delivery in a significant manner.
Cardiopulmonary Signs and Symptoms of Normal Pregnancy
Cardiopulmonary signs and symptoms of normal pregnancy may simulate heart disease. These include easy fatigability and dyspnea. However, dyspnea is almost always limited to the awareness of breathing, rather than the uncomfortable awareness of the necessity for breathing. Hyperventilation is caused by the stimulating effects of progesterone.[18] Hyperventilation, together with an increased work of ventilation, makes many women aware of their breathing. It is also partially responsible for compensated respiratory alkalosis. Tiring easily may be due to the soporific effects of progesterone and its effects on smooth muscle. Orthopnea is more common in obese women and may be due to limitation of diaphragmatic motion.[19] Chest pain during pregnancy is most commonly due to hiatus hernia, esophageal reflux, or ribcage distension. Tachycardia is normal in pregnancy, as are premature depolarizations of atrial or ventricular origin and nonspecific ST-, T-, and Q-wave changes.[20] Syncope in later pregnancy, when the patient is supine, is usually caused by inferior vena caval (IVC) compression. Syncope sometimes occurs with the sudden assumption of the upright position, based on orthostasis.
Some cardiovascular findings on physical examination may be confusing. Peripheral edema occurs in 60%–80% of pregnant women, and is attributed in part to hemodilution, causing a fall in protein oncotic pressure and an elevation of capillary pressure as a consequence of elevated venous pressure in the legs. This peripheral edema is not associated with hepatomegaly. Prominent neck veins are related to a hyperactive cardiac state. However, mean right atrial pressure is neither elevated nor is the hepatojugular reflex positive. Pseudo-cardiomegaly is related to displacement of the apex. Heart sounds normally become increased. There is often a third heart sound due to volume loading. In addition, right ventricular outflow tract murmurs are common.[21]
Symptoms and Signs of Heart Disease in Pregnancy
Severe or progressive dyspnea, progressive orthopnea, paroxysmal nocturnal dyspnea, hemoptysis, exertional syncope, chest pain related to effort or emotion, and progressive or generalized edema indicate the presence of heart disease. Physical findings strongly suggestive of heart disease include cyanosis, clubbing, persistent neck vein distension, positive hepatojugular reflux, palpable thrill, diastolic murmurs, paradoxical splitting of cardiac sounds, true cardiomegaly, documented sustained dysrhythmias, and pulmonary hypertension
General Principles of Anesthetic Management of the Parturient with Heart Disease
Hemodynamic changes that occur in pregnancy represent a significant stress test. Hence, most women with cardiac disease who remain asymptomatic throughout pregnancy tolerate labor and delivery well. Conversely, women who are breathless with less than ordinary activity or at rest [i.e., NYHA class III and IV, as well as Groups 2 and 3 of Clark's classification listed in Table 3] usually tolerate pregnancy, labor, and delivery poorly. In these cases, when general anesthesia (GA) has to be given for operative procedures such as lower segment cesarean section (LSCS), immediate reversal from anesthesia may not be possible or desirable after surgery. Hence, postoperative ventilatory support with hemodynamic monitoring or planning of corrective procedure for the underlying cardiac lesion such as severe natural or bio-prosthetic valvular dysfunction, aortic or pulmonary artery (PA) aneurysm, and Takayasu arteritis should be considered before reversal and extubation of the parturient.[23] Factors that have an adverse effect on outcome in these cases, regardless of functional status, are listed in Table 5.
Table 5.
Factors poorly tolerated by parturients with congenital heart disease regardless of functional status

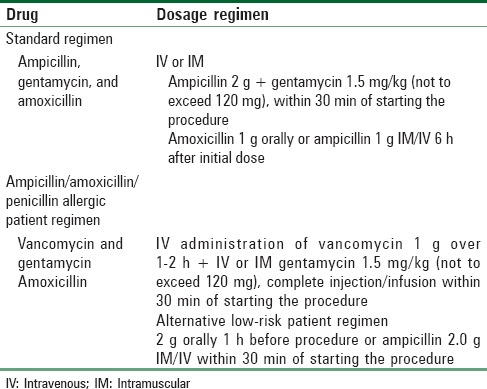
Patients in functional NYHA class III–IV categories with surgically correctable lesions should undergo surgery before pregnancy or during the second trimester of pregnancy before labor and delivery. If the woman is pregnant when first seen, conservative therapy includes bed rest in the left lateral position, prudent use of diuretics or volume loading, and infective endocarditis antibiotic prophylaxis [Table 6]. Doses of gentamycin or vancomycin should be modified, or second doses should be omitted, in women with renal dysfunction.[24] Digoxin treatment is only given where there is indication (e.g., atrial fibrillation) or heart failure with documented ventricular dysfunction. No evidence exists that digoxin helps this class of patients if they are in sinus rhythm or have normal systolic ventricular function.
Table 6.
Antibiotic prophylaxis for patients undergoing genitourinary/gastrointestinal surgery with cardiac lesions
The goals of anesthetic management during labor and delivery in pregnant women with heart disease include (1) analgesia, (2) hemodynamic monitoring, (3) optimizing cardiovascular and respiratory functions by manipulating various hemodynamic factors and tailoring anesthetic technique for maternal and fetal well-being, and (4) resuscitation including airway and ventilatory management if need arises. The concept of producing a “cardiac grid” for each patient with cardiac lesions has been introduced by Moore in 1981.[25] According to this concept, five hemodynamic factors that include (1) preload, (2) pulmonary vascular resistance (PVR), (3) systemic vascular resistance (SVR), (4) heart rate, and (5) myocardial contractility are related to patient's cardiac disease to provide optimal hemodynamic stability and rational planning of anesthetic management. These five factors are under the anesthetist's control and can be increased or decreased by manipulating the anesthetic technique or by the use of specific drugs [Table 7]. Any other significant hemodynamic factor related to the operative procedure or peripartum period, such as blood or fluid loss, may also be considered. The cardiac grid will determine the desirable hemodynamic requirements for a particular lesion. For this, the evaluation of the patient should be complete, and diagnosis of the type of cardiac lesion should not only be based on clinical findings but also should be confirmed by diagnostic aids such as echocardiography. The desired hemodynamic profile of some common lesions is shown in Table 8.[25]
Table 7.
Hemodynamic parameters under the anesthetist's control
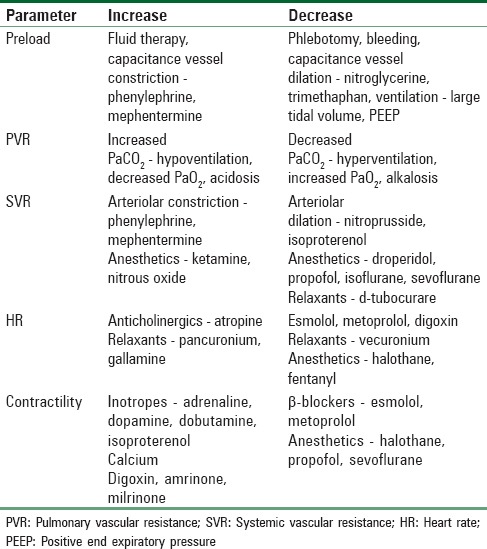
Table 8.
Cardiac grid (desired hemodynamic changes) for common cardiac lesions
The immediate postpartum period is critical, especially if pulmonary hypertension is present. Most fatalities occur in the 1st week after delivery, but others occur as late as 3–4 weeks after delivery. For this reason, invasive monitoring should not be discontinued immediately after delivery, and full therapeutic and monitoring support in a critical care area should be provided. The anesthesiologist has a role in assisting with postoperative pain management. In particular, epidural analgesia may be helpful in reducing the stress response as well as inducing favorable rheologic changes. Sympathectomy generally improves microvascular flow, and regional anesthesia decreases the risk of perioperative deep vein thrombosis in surgical patients.[26]
Peripartum Monitoring
Noninvasive BP (NIBP), electrocardiogram (ECG) for arrhythmias and heart rate, pulse oximetry, respiratory rate, signs of persistent respiratory distress, 4 hourly temperature, and input–output fluid balance are minimum mandatory monitoring required in all cardiac patients undergoing labor and delivery.[23] In case of parturients in functional class III and IV or Clark's risk Group 2 and 3, additional invasive monitoring such as intra-arterial pressure, PA pressure, PA wedge pressure, and arterial blood gas analysis may be required during trial of labor and vaginal delivery or LSCS. However, invasive monitoring techniques are no substitute for clinical evaluation of mental status, urine output, skin temperature, capillary filling, and pulse quality is vitally an important monitoring of tissue perfusion. Monitoring should be instituted in labor suit itself and should be continued to operative and postoperative room if need for LSCS arises. Contraindications to using a PA catheter include previous Mustard or Senning operation and parturients with Tetralogy of Fallot, and pulmonary vascular disease, particularly with intracardiac shunts.[27] Perioperative transesophageal echocardiography (TEE) may be required if parturient condition deteriorates and simultaneous cardiac surgery is planned.[28] Air bubble precautions should be universally taken in any cardiac patient, especially with those having shunts to prevent paradoxical embolism.
Anticoagulation in Pregnancy
Anticoagulant therapy is provided in pregnancy as long-term thrombolysis in patients with a history of thromboembolism or as prophylaxis in patients with valvular heart disease or prosthetic heart valves. Oral anticoagulant therapy during pregnancy is contraindicated. Warfarin therapy in the first trimester is associated with an increased incidence of fetal death and birth defects (warfarin embryopathy), and there are reports of neonatal central nervous system abnormalities from warfarin later in pregnancy.[29] In addition, prematurity and low birth weights are more frequent in pregnancies when the mother has received oral anticoagulants during the gestational period.[30] Many have abandoned the concept of re-instituting oral anticoagulation for the mid-trimester.[31] The exception is patients with mechanical valves in whom some recommend warfarin after 13 weeks of gestation, in combination with low-dose aspirin (60–80 mg/day), because of concerns about the efficacy of heparin in preventing systemic embolism.[32] Warfarin is given in the postpartum period and appears safe in women who breastfeed. No active drug has been found in breast milk or the blood stream of breastfed infants.[31,33]
Heparin is the drug of choice during pregnancy because it is a large molecule that does not cross the placenta. Numerous regimens for heparin administration have been devised. The activated partial thromboplastin time (APTT) is monitored closely because heparin requirements increase as pregnancy progresses.[32] Most physicians now recommend subcutaneous heparin q12h throughout the entire pregnancy, maintaining APTT at 1.5–2 times of control levels. Adverse pregnancy outcomes are less with heparin compared with warfarin. Complications such as heparin-induced osteoporosis or thrombocytopenia are rare.
There is interest in using low molecular weight heparin (LMWH) during pregnancy because it has a long half-life, allowing once-a-day dosing, and it is more bioavailable after subcutaneous injection. With a molecular weight of 4000-5000 daltons, it does not enter the fetal circulation.[32] LMWH appears to be a safe alternative to unfractionated heparin as an anticoagulant during pregnancy.[33]
When valvular surgery is required, tissue valves (bioprostheses) should be considered in women of childbearing age. The valves are associated with improved pregnancy outcome and they help to avoid the problem of anticoagulation during pregnancy.[34,35] However, thromboembolic phenomena can still occur, and the long-term durability of bioprostheses is poor, with an increased need for subsequent valve replacement (35% patients in one series).[36] This finding helps explain why some physicians believe that a mechanical valve (especially the newer, less thrombogenic valves), with aggressive heparinization throughout pregnancy, is appropriate.[37]
Other treatments such as streptokinase and urokinase are relatively contraindicated in pregnancy because of reports of placental abruption and postpartum hemorrhage.[37] Streptokinase has been given with success to treat prosthetic mitral valve thrombosis during pregnancy.[38] The thrombosis was confirmed by echocardiography and cinefluoroscopy at 28 weeks' gestation in a woman with a history of progressive exertional dyspnea. Streptokinase, 250,000 IU, was administered as an intravenous (IV) infusion over 30 min followed by 100,000 IU/h for 24 h. Valve function returned to normal within 18 h of commencing treatment.
Pregnancy and successful delivery have been described in a woman with triple heart valve prostheses (aortic, mitral, and tricuspid).[39] The patient was on oral warfarin therapy throughout her pregnancy until 32 weeks, when it was discontinued and IV heparin was commenced. At 34 weeks, a cesarean section was performed using GA. The only adverse sequelae were frequent maternal cardiac dysrhythmias that were treated with verapamil and mexiletine. Follow-up revealed no neonatal complications.[39]
Classification of Cardiac Diseases Seen in Pregnancy
-
Valvular lesions due to rheumatic heart disease
- Mitral stenosis
- Mitral insufficiency
- Aortic insufficiency
- Aortic stenosis (AS)
- Mixed valvular lesions
-
Congenital heart disease
-
Congenital heart disease without shunt
-
General:
- Dextrocardia
- Cardiomyopathy
- Heart block
-
Left sided:
- Mitral valve prolapse
- AS
- Coarctation of aorta
- Asymmetric septal hypertrophy
-
Right sided:
- Pulmonary stenosis
- Ebstein's complex
- Idiopathic dilatation of PA
-
-
Congenital heart disease with shunt
-
Acyanotic (left → right) with increased pulmonary blood flow:
- Atrial septal defect (ASD)
- Ventricular septal defect (VSD)
- Patent ductus arteriosus (PDA)
- Anomalous pulmonary venous drainage.
-
Cyanotic (right → left)
-
Decreased pulmonary blood flow:
-
PA pressure normal or decreased
-
Enlarged right ventricle (RV) – Tetralogy of Fallot
- Pulmonary stenosis with reversed interatrial shunt
- Enlarged left ventricle (LV) – Tricuspid atresia.
-
-
PA pressure elevated
- Eisenmenger's syndrome of PDA, ASD, VSD
-
-
Increased pulmonary blood flow:
- Transposition of great vessels
- Persistent truncus arteriosus.
-
-
-
-
Other heart diseases:
- Coronary artery disease
- Primary pulmonary hypertension
- Pericarditis
- Pregnancy after cardiac surgery (valvular/corrective/palliative)
- Cardiomyopathy
- Pregnancy postcardiac transplant.
Cardiac dysrhythmias and conduction defects during pregnancy.
Valvular Lesions during Pregnancy
Chronic mitral or aortic regurgitations (volume overload lesions) are usually well tolerated during pregnancy if patients remain in NYHA class I or II. Parturients with severe valvular regurgitation or stenosis do not tolerate hemodynamic changes associated with pregnancy. Any woman with symptomatic stenotic lesions warrants very close attention and possible surgical correction during pregnancy.
Mitral stenosis
Mitral stenosis accounts for 90% of rheumatic heart disease in pregnancy, with 25% of the patients developing symptoms for the first time during late pregnancy. Relative obstruction across the valve increases as pregnancy advances because of the greater blood volume, heart rate, and CO. Increased obstruction leads to pulmonary venous congestion, it may produce pulmonary edema. Pure or dominant mitral stenosis is the most common pathology associated with acute pulmonary edema in pregnancy, followed by aortic valve disease and/or primary myocardial disease.[20] Thrombi within the enlarged left atrium represent a major therapeutic challenge because of the risks and side effects of anticoagulation. Affected women require bed rest, careful diuresis, and immediate correction of dysrhythmias. Heparin administration throughout pregnancy should be considered in those with chronic or paroxysmal atrial fibrillation, left atrial (LA) thrombus, or prior embolic history. Intractable heart failure or hemoptysis is an indicator for urgent surgical intervention, which includes balloon valvuloplasty. During delivery and the immediate postpartum period, the pulmonary capillary wedge pressure (PCWP) may increase by as much as 10 mmHg and thus is a critical time for such patients. A postpartum increase in PCWP is most likely in the presence of severe multiple sclerosis (MS) (i.e., functional class III and 1V).[40] The principal anesthetic considerations for mitral stenosis[41] are as follows:
Prevent rapid ventricular rates
Maintain sinus rhythm
Avoid large, rapid falls in SVR
Prevent increases in central blood volume, however, optimal LA preload to be maintained
Avoid hypoxemia and/or hypoventilation, both of which may increase PA pressures and cause RV failure.
Carefully titrated epidural analgesia for labor and delivery is the principal anesthetic issue. The concomitant use of invasive hemodynamic monitors is to be recommended in symptomatic parturients or in those with critical stenosis (mitral valve area <0.6 cm2).[42] Hemmings et al. found that epidural analgesia with low concentration of local anesthetic and opioid mixture during the first stage of labor caused a beneficial reduction of PVR and SVR, lowered PA pressures, and returned CO to baseline levels.[42] Ngan Kee et al. found that combined spinal epidural analgesia using intrathecal fentanyl followed by dilute bupivacaine-fentanyl epidural infusion is a useful technique for providing analgesia and maintaining hemodynamic stability.[43] Both epidural and GA have been described for cesarean delivery. However, in one case report, alfentanil provided hemodynamic stability and allowed for immediate postoperative extubation but caused neonatal respiratory depression.[41] Epidural anesthesia with 0.5% bupivacaine has been given successfully in women undergoing urgent cesarean delivery for hemodynamic deterioration from severe MS.[44] Each patient was placed in 15° head down position to maintain the PCWP at 25 mmHg.
Aortic stenosis
AS is found rarely in women of childbearing age, and when present in a moderately severe form, has been associated with high fetal and maternal mortality.[45] However, a reappraisal of congenital AS in pregnancy demonstrated satisfactory outcomes in 25 pregnancies with no mortality in any of the 13 women concerned.[46] Almost 25% of the women were classified as having severe stenosis (<0.7 cm2), and deterioration in cardiac status occurred in 27% of the group studied. All patients were offered epidural anesthesia for pain relief or operative delivery. The cesarean section rate was 30%. Pulse oximetry, peripheral arterial and venous lines, and antibiotic prophylaxis were utilized in all patients.
In pregnant patients with AS, transvalvular gradients increase progressively throughout pregnancy, as a consequence of more blood volume and less SVR. Such increases can result in syncope, angina, and reduced perfusion to the placenta and fetus. Bed rest in the left lateral decubitus position and avoidance of negative inotropes are recommended.
Augmented preload with IV fluids may be of benefit in maintaining a fixed stroke volume. However, fluid loads in the presence of hypervolemia of pregnancy may exacerbate pulmonary congestion consequent to LV failure. Other anesthetic considerations in the parturient with AS include maintaining sinus rhythm and avoiding bradycardia and sudden changes to the SVR.
In some cases, percutaneous balloon aortic valvuloplasty is performed during pregnancy and is reported to be associated with good maternal and fetal outcomes.[47] Valvuloplasty is usually reserved for cases in which the aortic valve area is 0.3 cm2 or less; valvuloplasty can usually increase the area to about 1.0 cm2.
Case reports have advocated carefully titrated epidural anesthesia or combined spinal epidural anesthesia for labor and delivery in parturient women with severe AS.[48,49,50] These reports argue against GA for women with severe AS because of concern that induction of GA and tracheal intubation may result in hypertension and tachycardia, which decrease CO and coronary blood flow. In some cases, the CO is low because of a high SVR associated with pain and anxiety. A gradual reduction in SVR associated with induction of epidural anesthesia serves to improve CO in the presence of a fixed stroke volume, assuming that the filling pressures are adequate. Some rationalize not using epinephrine with the epidural local anesthetic solution,[44,48] whereas others have used it in the test dose in parturients with cardiac disease.[49,51]
Care should be exercised when administering oxytocin to these patients because a large bolus can cause hypotension and tachycardia and increases pulmonary arterial pressure.[49] Slow infusion of a dilute oxytocin solution is usually well tolerated. As a general rule, care should be exercised in parturients with cardiac disease when administering other uterotonic agents such as ergometrine, which can induce systemic hypertension, and prostaglandin F2α, which has the potential to cause severe pulmonary hypertension if injected directly into the circulation in a large dose.[52]
If a general anesthetic technique is employed, an opioid-based anesthetic is useful when LV function is compromised.[53] However, there is a concern that the slow induction period associated with this technique increases the risk of pulmonary aspiration. Any well-managed anesthetic technique is suitable if the risks and benefits are acknowledged, drugs are titrated carefully, and invasive monitoring is employed as a guide to appropriate therapy in the event of adverse hemodynamic changes.
Mixed valvular lesions
Mixed valvular lesions present the dilemma of which lesion to treat. As a rule, therapy should be directed to the management of the dominant lesion (i.e., that with the most significant hemodynamic consequences). For example, if a woman presents with moderate-to-severe mitral regurgitation with mild mitral stenosis, management should be designed to treat the regurgitant lesion even if this conflicts with the usual management of the mitral stenosis.
In a case report, a woman with moderate-to-severe mitral regurgitation and mild mitral stenosis was successfully managed with epidural analgesia for induced labor and ventouse-assisted vaginal delivery.[54] Invasive monitoring of radial artery pressure and PCWP allowed precise, continuous measurement of hemodynamic variables and appropriate fluid and drug therapy. Similar reports have described the successful use of epidural analgesia for labor and delivery in women with combined mitral and aortic regurgitation[55] and combined mitral and AS.[56]
Congenital Heart Deseases
Intracardiac shunts
Congenital heart disease with intracardiac shunts falls into two main categories, cyanotic and acyanotic lesions. (see classification of heart disease in pregnancy). It is the impact of the shunts on cardiac chambers that is often evaluated to determine clinical significance and prognosis. For example, does the pregnant women with an ASD defect have right ventricular hypertrophy or not? The optimal cardiovascular parameters required in parturients with intracardiac shunts during induction and maintenance of anesthesia are listed in Table 9.[57]
Table 9.
Optimal cardiovascular parameters for parturients with an intracardiac shunt
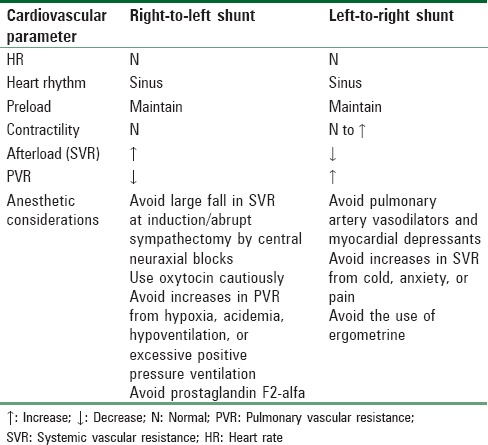
Acyanotic heart lesions
Atrial septal defect is the most common acyanotic heart lesion and the one most likely to be missed on screening. Women with left-to-right shunts, without pulmonary hypertension, can tolerate pregnancy well because of the associated physiologic decrease in SVR. In one series, 19% of pregnancies in women with left-to-right shunts were complicated, by congestive heart failure (CHF). This rate of heart failure (usually right ventricular failure) was reduced to 1% in similar patients after surgical correction of the lesion.[58] However, the success in reducing CHF depends on the size of the shunt both before and after surgical correction.
Cyanotic heart lesions
A congenital heart lesion characterized by right-to-left shunt is associated with recirculation of desaturated blood. Peripheral cyanosis occurs when more than 5 g/dL of unsaturated hemoglobin is present. Cyanosis varies directly with hematocrit level. An anemic parturient woman with poor oxygen saturation may not manifest cyanosis, whereas a woman with polycythemia may appear even cyanotic at higher oxygen saturations.[59]
Cyanotic lesions are more likely to be associated with the following:
CHF
Worsening of functional status
Preterm labor
Fewer live births
Small-for-dates neonates.
Overt maternal cyanosis is associated with fetal wastage of about 50%. In one series, a deterioration of fetal, but not maternal, condition was the factor that limited continuation of the pregnancy.[60] In Tetralogy of Fallot, and similar lesions with obstructed pulmonary outflow tracts, a reduction in SVR and BP is best treated with a pure α-agonist such as phenylephrine. If there is obstruction of the RV outflow tract, catecholamines release from inadequate pain relief, light GA, and/or exogenous catecholamines release associated with stress or anxiety should be avoided. Infundibular obstruction can be relieved by inhalation anesthetics such as halothane and sevoflurane and short-acting β-blockers (e.g., esmolol). Hypovolemia, hypercarbia, acidosis, rise in PVR (large tidal volume and rapid rate-controlled ventilation increase PVR), and fall in SVR are not tolerated.[57]
Eisenmenger syndrome
This is any condition in which there is a large communication between the systemic and pulmonary circulations with pulmonary hypertension, bi-directional shunt, and cyanosis. Over time, those lesions with large left-to-right shunts, high pulmonary blood flow, and normal PVR may develop right-to-left or bi-directional flow (shunt reversal). Currently, there is no way of predicting who will develop this Eisenmenger reaction. Such lesions include large ASDs or VSDs, large PDAs, and excessively large surgical systemic pulmonary anastomoses, either from palliation or definitive repair. When shunt reversal occurs, there is an increase in PVR resulting from structural changes in the pulmonary vasculature. These changes lead to an increase in PVR until PA pressures finally exceed systemic pressures.
For patients with congenital heart disease, Eisenmenger syndrome represents an end stage for which the only treatment is heart–lung transplantation. Avoiding further increases in PA pressures in such patients is therefore of critical importance. If BP and oxyhemoglobin saturation (SaO2) fall despite volume replacement, titration of drugs with predominantly chronotropic and inotropic effects is preferable to drugs with peripheral vascular effects (e.g., dopamine rather than phenylephrine), because the latter may increase PVR. In an emergency, phenylephrine can be given to increase pulmonary blood flow because the elevation in PVR is usually small and the direction of the shunt is dependent on the ratio of PVR to SVR.
Women with primary pulmonary hypertension should not become pregnant because the mortality rate is extremely high. This idiopathic condition affects mostly young females, and pregnancy causes a marked increase in already elevated PA pressures, which worsens as pregnancy advances. The RV pressure increases and volume overload occur with RV dilatation and tricuspid regurgitation. Consequent to these changes, the interventricular septum may shift to the left, resulting in a reduction in LV function and CO. Epidural analgesia has been successfully administered in the intrapartum management of such patients.[61,62,63,64,65] The reduction of pulmonary artery pressure by the use of nitric oxide, nebulized iloprost, and prostaglandin infusion is not found to be associated with improved survival.[66]
Hematological changes
A physiologic response to hypoxemia in a woman with a cyanotic lesion is polycythemia, which is a useful compensation up to an hematocrit level of 60%. An increase in blood viscosity beyond this level negates any advantages that an increase in hematocrit brings in terms of oxygen delivery. Symptoms of hyperviscosity include headache, sluggish mentation, disorientation, double vision, fatigue, muscle weakness, myalgia, and paresthesias.[67] Polycythemia contributes to tissue ischemia in low-flow states because of the greater blood viscosity, and this can lead to thrombosis in situ. A maternal hematocrit level >60%, SaO2 < 80%, RV hypertension, and syncopal episodes are all poor prognostic signs in pregnant women with cyanotic congenital heart disease.[68] Hematocrit should be maintained between 50% and 60% by phlebotomy, if signs and symptoms of hyperviscosity syndrome appear.
Patient monitoring
Patients with congenital heart disease present with a range of clinical symptoms, and the natural history of each lesion varies. Severity is not based solely on the NYHA classification because exercise intolerance may neither be related to heart failure nor can it predict the ability to tolerate pregnancy. An understanding of the pathophysiology of a particular lesion is required because significant variations in morphology among patients may exist. Echocardiography is helpful in assessing the functional characteristics of the anomaly at the time of admission. It also provides an accurate assessment of ventricular function and a comparison of the relative systemic and pulmonary blood flows.
All catheter and infusion systems should be air free to prevent paradoxical embolism. This is especially critical in cyanotic patients, in whom entrainment of even small amounts of air can be disastrous. For labor and delivery, a right radial artery catheter, double-lumen central venous pressure catheter, ECG, and pulse oximeter are mandatory for symptomatic parturient women and those with known borderline myocardial or valvular function on echocardiography. Pulse oximetry can be inaccurate as an absolute value in cyanotic lesions. However, changes in SaO2 over time (trends) are accurate and useful.[23]
The diagnosis of a right-sided lesion does not always preclude the use of a PA catheter. Indeed, the measurement of filling pressures in the systemic ventricle can provide good information throughout labor and delivery. The soluble guanylate cyclase can be utilized in women with Ebstein's anomaly or pulmonary stenosis versions of transposition. However, its value is questionable, when the right heart is atretic or in Eisenmenger's syndrome because of difficulty associated with insertion and removal.[69] End-tidal carbon dioxide (EtCO2) level recording is a rough but continuous monitoring of ventilation-perfusion (V/Q) matching. TEE is a helpful monitor when available.[28]
Anesthetic options
General and regional anesthetic techniques have been employed successfully in parturients with congenital heart disease.[61,62,63,64,65,70,71] However, the number of published cases is small, and one must consider the possibility that cases with adverse outcomes are rarely reported.
GA, with endotracheal intubation, provides airway protection. Mechanical ventilation eliminates the work of breathing and may reduce oxygen consumption, thereby improving arterial oxygen content. The complications of controlled mechanical ventilation include decreased venous return as well as ventricular dysfunction, compression of pulmonary vessels, hypoxemia, hypo- or hyper-carbia, and acidemia. Critical issues, such as mode and frequency of ventilation, peak inspiratory pressure, fraction of inspired oxygen, ETCO2, and blood gas analysis, are seldom reported in published cases describing the anesthetic management of parturient women with congenital heart disease. The choice of anesthetic drugs may not be of primary importance. If the hemodynamic effect of the lesion is worsened by tachycardia, anesthetic drugs that are known to cause an increase in heart rate through vagolysis (e.g., pancuronium) should not be administered [Table 1]. IV drugs administered to patients with right-to-left shunt have a more immediate and pronounced effect because they reach the circulation sooner. However, inhaled anesthetics have a delayed onset in patients with right-to-left shunt.
Regional anesthesia allows spontaneous respiration with little disruption of V/Q relationships, which may be critical in parturient women with complex heart lesions. Epidural catheter techniques offer continuous, titrated anesthesia or analgesia, and double catheters (one directed cephaloid, the other caudal) offer versatility at the time of delivery. Saline should be used to determine the loss of resistance when locating the epidural space and to avoid accidental IV injection of air and paradoxical systemic air embolism. Moreover, this also decreases the incidence of patchy or partial block. Some investigators use epinephrine in the test dose,[49,51] whereas others avoid it.[44,48] Patients with congenital heart lesions may have an increased sensitivity to the neurotoxic effects of local anesthetics. There has been some argument in the past about the suitability of subarachnoid (spinal) anesthesia in patients with severe cardiac disease.[72,73] Most consider that it should rarely, if ever, be given to parturients with intracardiac shunts because a sudden decrease in SVR worsens cyanosis by increasing right-to-left shunt. The decrease in venous return further reduces pulmonary blood flow and promotes preferential flow to the systemic circulation, which also worsens cyanosis. Mean BP is decreased, and myocardial oxygen supply is reduced. The tendency is to add oxygen at this time, whereas an increase in SVR is much more appropriate. Administration of oxygen to patients with fixed pulmonary hypertension and more than 30% of right-to-left shunt fraction is irrelevant, in that it does not improve systemic oxygen saturation.
In women with valvular stenotic lesions and fixed stroke volumes, a sudden drop in SVR may be harmful as a result of increasing the gradient and impairing the myocardial blood flow. However, subarachnoid opioids have been given successfully in women with complex heart lesions during labor, because they produce effective analgesia with minimal sympathetic block.[74,75,76] This may not be the case with intrathecal meperidine because it can exhibit a local anesthetic effect in higher doses. Sufentanil should be administered with some caution because a number of parturients exhibit a fall in BP after a 10 μg intrathecal dose, although the fall is not usually profound. Intrathecal fentanyl combined with pudendal/saddle block has been given successfully for vaginal delivery in a parturient with Eisenmenger syndrome.[77] In her case, peripheral SaO2 was improved by the analgesic technique.
Maternal bearing down, compression and decompression of the IVC, delivery, placenta removal, hemorrhage, and autotransfusion all have unpredictable effects on intravascular volume and overall hemodynamic status, independent of the anesthetic technique in patients with complex congenital cardiac lesions. Hence, parturients with complex lesions should be monitored continuously and managed accordingly.
Cardiopulmonary bypass during pregnancy
Leyse et al. were the first to employ extracorporeal circulation during open-heart surgery in a woman at 18 weeks' gestation with congenital AS.[78] The woman did well, but she delivered an abnormal fetus with multiple congenital anomalies near term. Currently, cardiac operations with cardiopulmonary bypass (CPB) during pregnancy can be performed with reasonable safety for both mother and fetus,[79,80] a fact that is supported in a survey by the Society of Thoracic Surgeons. They reported only one maternal death from 68 procedures requiring CPB and a fetal survival rate higher than 80%.[81] The well-being of the mother and fetus must be considered, although the best interests of the two may not always coincide. Optimal therapy for one may be inappropriate for the other during CPB. As a general principle, surgery requiring CPB should be delayed until the second trimester or later.[82,83] Continuous intraoperative fetal monitoring is required to detect fetal bradycardia on bypass. One review describes a case in which successful treatment of fetal bradycardia on bypass was obtained by increasing the perfusion rate (pump flows).[84] High normal range perfusion pressures during CPB should be kept at 60 mmHg or more to compensate for the lack of autoregulation in the uteroplacental unit.[83] Hypothermia to 32°C on bypass is usually kept and not lowered below because lower temperatures have the potential to cause fetal dysrhythmias and fetal cardiac arrest.[83]
Another problem associated with CPB during pregnancy is severe postpartum hemorrhage. This was described in a report of an emergency mitral valve replacement immediately after cesarean section.[85] Lamarra et al. described the use of aprotinin, a protease inhibitor, in a similar situation. They claimed that the surgical procedure was greatly simplified, and aprotinin was an important factor in an uncomplicated outcome.[86]
Parturients with Surgically Corrected Congenital Heart Disease
Successful surgical repair of a heart lesion before pregnancy results in a significant improvement in maternal and neonatal outcomes. Palliated lesions, however, are still associated with an increased risk to mother and fetus during pregnancy.[87] In one series of women with unrelieved cyanosis, only 42% of pregnancies resulted in a live-born child. This compares with 72% live births in women whose cyanosis was relieved by palliative surgery.[88] However, in all cyanotic women, the children were usually small for gestational age and immature. In mothers made acyanotic by surgery, the rate of successful pregnancies is comparable to that of all groups of mothers with congenital heart disease and children of normal size.[88] A significant difference in pregnancy outcome is seen in women with poor cardiac status, regardless of whether palliation has occurred. Asymptomatic women have more successful outcomes with fewer cardiac or obstetric complications.
Surgical shunts can overcome severe obstruction to pulmonary blood flow. The goal is to maintain adequate arterial oxygen content when oxygenated pulmonary venous blood returns to the heart and mixes with systemic venous blood. The problem is in creating a shunt that does not produce excessive pulmonary blood flow. A list of common surgical procedures for congenital heart disease is shown in Table 10.
Table 10.
Common surgical procedures for congenital heart disease
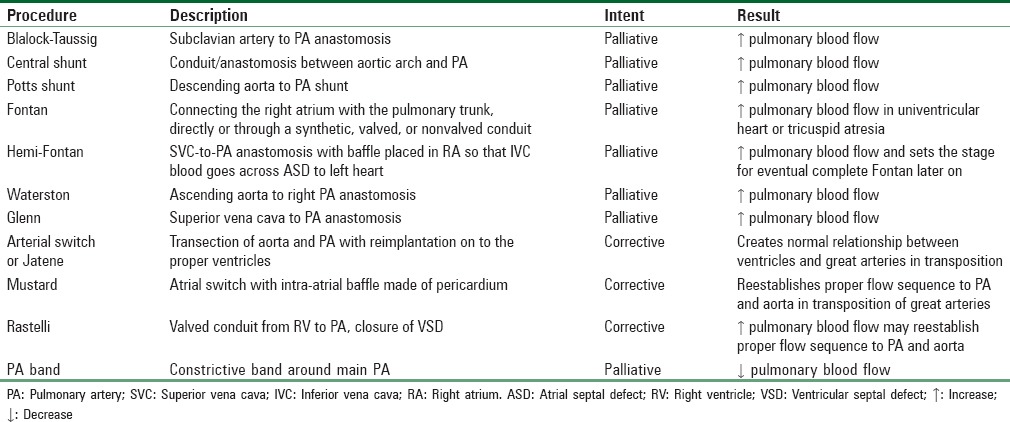
GA was recommended for cesarean birth in one case report of a woman with a single ventricle and pulmonary atresia who had undergone multiple palliative surgical repairs.[89] At 7 months of age, she received a Blalock–Taussig shunt for an apparent Tetralogy of Fallot. This shunt was preferentially performed on the side opposite to the aortic arch to prevent kinking, but it can be created on either side. The shunt overcomes severe obstruction to the pulmonary circulation. At 11 years of age, she developed worsening cyanosis and fatigue. Cardiac catheterization at that time showed a closed shunt, pulmonary atresia, ductal stenosis, reduced pulmonary blood flow, double-outlet RV, and severe hypoplastic left heart. A successful Pott's anastomosis was performed at this stage. She presented 12 years later, at 28 weeks' gestation, with severe CHF. She improved with conservative therapy (bed rest, oxygen, digoxin, and diuretics), but her condition worsened at 32 weeks' gestation. An opioid-based GA was given for cesarean delivery, and both mother and baby did well. Since pulmonary circulation was supplied almost entirely through a shunt of fixed diameter, it was thought that avoiding a fall in SVR and maintaining an adequate preload was paramount. Increases in PVR from acidosis, pain, anxiety, high inspiratory pressure, and positive end-expiratory pressure are best avoided in such cases.
Fontan procedure has been employed in patients with congenital tricuspid atresia and a wide range of congenital lesions that are associated with a single functional ventricle. In this procedure, right side of the heart can be bypassed utilizing cavopulmonary connections. Fontan procedure is often preceded by some form of palliative surgery. The main pathophysiological consideration in these patients is that systemic venous return reaches the pulmonary circulation without ventricular augmentation (i.e., Fontan's circulation represents a passive conduit). Hence, adequate central blood volume and pressure are necessary to perfuse the pulmonary circulation. PVR and SVR are other critical determinants of patients' well-being. It can be argued that general anesthetic techniques should be avoided for such cases, because they use potential myocardial depressants that may adversely affect PVR. Moreover, positive pressure ventilation impedes venous return, thereby impairing pulmonary blood flow. A further consideration is that atrial fibrillation can be present and may be difficult to treat. Instrumentation of the right heart may trigger atrial fibrillation, which is a true emergency in these patients. Therefore, cardioversion equipment and antiarrhythmic drugs should be immediately available.
Three publications report the anesthetic management of 17 pregnant women who had previously undergone Fontan repairs.[90,91,92] These reports describe epidural anesthesia and continuous intrathecal anesthesia employing a microcatheter, for cesarean birth, and successful low-dose combined spinal epidural or epidural analgesia for labor and vaginal delivery. Regional techniques avoid deleterious increases in PVR that can be associated with intubation of the trachea, intermittent positive-pressure ventilation, and GA. Ensuring adequate preload, uterine displacement, and slow titration of local anesthetics compensates for the potentially deleterious effects of venodilatation from regional anesthesia. The rate of lowering of SVR is important. Gradual induction of epidural anesthesia is associated with slower rates of decline in SVR. Cautious volume replacement can compensate for the vasodilatation associated with a reduction in afterload. Conversely, a sudden profound sympathectomy from single-dose intrathecal anesthesia is contraindicated in this situation.
Other forms of complex malformations that are palliated with Fontan circulation include left atrio-ventricular valve atresia, double-inlet left and RVs, pulmonary atresia, intact ventricular septum and hypoplastic RV, and hypoplastic right or LV in biventricular hearts with VSD (with or without straddling atrio-ventricular valve).[93] It is likely that more parturients with Fontan repairs will be seen in the future.
Cardiomyopathies
Cardiomyopathy can be classified as hypertrophic, restrictive, or dilated.
Hypertrophic cardiomyopathies with or without obstruction
Idiopathic hypertrophic subvalvular stenosis (IHSS) requires maintenance of SVR to prevent cavity collapse and outflow tract obstruction. Obstruction to LV outflow is caused by a hypertrophic muscle mass at the base of the interventricular septum and a systolic anterior motion of the anterior leaflet of the mitral valve. This condition is intriguing in that there may not be a resting obstruction but only a potential obstruction, given the right conditions. In addition, there is an apical obliterative variety that does not have a subaortic pressure gradient. If, as usually occurs, a subaortic pressure gradient is present, the ventricle is less compliant and passive ventricular filling during diastole is reduced. It follows that atrial contraction becomes an important factor in increasing ventricular end-diastolic volume. Factors that affect the degree of obstruction include systolic volume of the ventricle (preload), force of the ventricular contraction, and transmural pressure distending the outflow tract. The obstruction is decreased by large systolic volumes, which distend the outflow tract, reduced ventricular contractility, and high aortic pressure. Conversely, the obstruction is increased by small systolic volumes, which are associated with reduced preload, high ventricular contractility, and low aortic pressure associated with a decrease in afterload.
When the LV outflow tract is narrowed, CO falls and mitral regurgitation may occur because the mitral valve becomes the point of relief for the buildup of ventricular pressure. Mild-to-moderate IHSS is usually well tolerated because the increase in LV end-diastolic volumes associated with pregnancy reduces outflow tract obstruction. Increases in heart rate and decreases in SVR counteract this effect. Outflow obstruction is exacerbated during the third trimester in the supine position and during labor, delivery, and early puerperium. Adverse effects during labor and delivery include increases in heart rate and contractility from endogenous catecholamines (pain and anxiety). Tachycardia limits diastolic filling, which decreases LV end-diastolic volumes. Increased contractility raises LV outflow tract obstruction. A decrease in venous return from the Valsalva maneuver can occur with pushing. The benefits of an epidural infusion of dilute local anesthetic with opioid for labor are obvious in this situation.
β-blockade is given to treat LV outflow obstruction by reducing cardiac contractility and heart rate. The anesthetic management of labor and delivery can be complex because β-blocker therapy may have been discontinued during pregnancy owing to concern that it may cause fetal bradycardia and intrauterine growth retardation. A case report described the use of esmolol in a parturient with IHSS at term gestation.[94] Esmolol is a short-acting agent that can have an adverse impact on the fetus. In this case, it caused persistent neonatal hypotonia, hypotension, hypoglycemia, and bradycardia. Another report described a case of fetal bradycardia following maternal administration of esmolol that required an emergency cesarean section.[95] Labetalol in 0.25 mg/kg increments, up to a total dose of 1 mg/kg, may be preferable.
Spinal and epidural anesthesia cause a reduction in SVR, which has the potential to increase the outflow obstruction; therefore, they are relatively contraindicated. One report describes the adverse impact on the left ventriculoaortic gradient, and hence coronary perfusion, in a woman with IHSS who received a subarachnoid anesthetic for an orthopedic procedure.[96] The successful administration of GA for cesarean section has been described in women with IHSS.[96] Although volatile anesthetic agents are beneficial in that they produce a reduction in myocardial contractility, they should be administered cautiously to avoid a marked fall in SVR (halothane is superior to isoflurane, because it has less impact on SVR) and concerns of postpartum hemorrhage. GA, for cesarean section, and lumbar epidural analgesia, for vaginal delivery, have both been associated with postpartum pulmonary edema in parturients with IHSS who did not receive invasive hemodynamic monitoring.[97] These patients should be closely monitored with a PA catheter for worsening outflow obstruction resulting from the diuresis that occurs in the first 48 h postpartum.
The critical determinants of a good outcome are careful titration of anesthetic agents, adequate volume loading, and prompt replacement of blood loss guided by invasive hemodynamic monitors. Ephedrine is contraindicated in the treatment of hypotension associated with IHSS due to the risk of tachycardia and increased inotropy. Phenylephrine in 50 μg increments is the treatment of choice for hypotension following sympathetic blockade. Large doses of phenylephrine should be avoided to prevent reduction in placental perfusion. Although some believe that oxytocin is relatively contraindicated in parturients with IHSS, a dilute oxytocin infusion will likely be well tolerated.[98]
Restrictive cardiomyopathies
Restrictive cardiomyopathy is a rare entity representing the end stage of myocarditis or an infiltrative process of the myocardium, such as amyloidosis or hemochromatosis. Restrictive cardiomyopathy mimics constrictive pericarditis and is characterized by impaired ventricular filling and poor contractility. CO is maintained initially by an increase in heart rate and filling pressure, but not by an increase in myocardial contractility. The anesthetic management depends on whether the dominant feature of the disease is restrictive ventricular filling or impaired ventricular function. Monitoring includes ECG for heart rate and ischemic changes and PA catheter to record filling pressures and CO.
TEE should be employed when available. Therapy is directed toward providing adequate ventricular filling, heart rate, and myocardial contractility. β-agonists such as isoproterenol or dobutamine are the inotropic agents of choice, because in addition to increasing the ejection fraction, they raise heart rate and usually decrease SVR. Epidural anesthesia is preferred over GA because myocardial depressants can be avoided and positive-pressure ventilation can decrease venous return under GA.
Dilated cardiomyopathies
Idiopathic dilated cardiomyopathy may be present in pregnant patients with a reported incidence of 5–8/100,000 live births per year.[99] This has poorer outcome as compared to peripartum cardiomyopathy, also known as “cardiomyopathy of pregnancy.”[99,100] Diagnostic criteria for the later condition are the development of heart failure within the last month of pregnancy or 5 months' postpartum in the absence of prior heart disease with no determinable cause (such as alcoholism, protein deficiency, or acute viremia) and there must be echocardiographical indication of left ventricular failure such as ejection fraction <45% or fractional shortening <30% and end-diastolic dimension >2.7 cm/m2 body surface area.[101] The incidence for this is estimated to be between 1 in 3000 and 1 in 4000 pregnancies. Unexplained dilated cardiomyopathy should be treated as heart failure, with or without congestion.[99,100,102] Symptoms may occur suddenly, but often cardiac failure develops insidiously. Symptoms may resolve spontaneously in 20%–40% of cases with little or no treatment. Peripartum cardiomyopathy can be associated with pulmonary hypertension, however, may result in multiorgan failure.[101,103] Women present with fatigue, dyspnea, edema, and occasionally chest pain with hemoptysis. They often have a raised jugular venous pressure, cardiomegaly, and gallop rhythm. Echocardiography reveals hypokinetic dilated ventricles with normal valves. Serial echocardiography to monitor LV function throughout the pregnancy and postpartum period is recommended.[104] Postmortem studies show enlarged hearts that are soft and flabby with a dilated myocardium and variable endocardial thickening or areas of myocardial necrosis.
Treatment is empirical with diuretics, inotropes, afterload reducers, and anticoagulants. In one series, 78% of women with peripartum cardiomyopathy had evidence of myocarditis.[105] Resolution of the myocarditis was associated with a significant improvement in LV function. Resolution can occur spontaneously without loss of cardiac function. Immunosuppressive therapy with oral prednisone and azathioprine for 6–8 weeks has improved LV function and prognosis in women with myocarditis and LV dysfunction.[105]
Continuous venovenous hemofiltration has been successful in treating severe cardiomyopathy after failure of conventional therapy.[106] It is achieved by circulating blood over a highly permeable hemodialysis membrane but without exposure to an osmotic dialysis gradient. Serum is filtered across the membrane by hydrostatic pressure. The membrane allows passage of fluids and solutes with a molecular weight of <20,000 and so produces a filtrate with a composition-approximating serum. Heart transplantation is reserved for severe cases unresponsive to all medical therapy.
Mortality can range from 30% to 60%, but the prognosis is more grave if the cardiac size has not returned to normal within 6 months of delivery. Death results from failure to improve cardiac function or from cerebral or pulmonary embolism. The rate of recurrence is uncertain but is more likely if the cardiac size is slow to return to normal.
Anesthetic management depends on the clinical status of the parturient and the mode of delivery. In one report of a woman who had pulmonary edema in an earlier pregnancy, serial echocardiography revealed persistent cardiomegaly in the next confinement. Successful outcome was achieved with epidural anesthesia and noninvasive hemodynamic monitoring for cesarean delivery.[107] In another report wherein cardiomyopathy presented atypically, a parturient with no history or physical signs of heart disease suffered a cardiac arrest at induction of GA for emergency cesarean delivery.[108] After resuscitation and delivery of the child, the diagnosis was confirmed by echocardiography, but no etiology for the cardiomyopathy was determined.
Coronary artery disease
The incidence of myocardial infarction in pregnancy is rare. However, with increase in maternal age and associated risk factors for CAD such as smoking, obesity, and hyperlipidemia in pregnant women, its incidence is on a rise. It is more common during third trimester and puerperium. Early coronary angiography is indicated to detect the cause and further management. The use of aspirin, clopidogrel, and prophylactic heparin precludes the use of regional anesthesia for cesarean section while GA with the use of boluses of esmolol, lignocaine, and remifentanyl as an infusion to control the pain and cardiovascular parameters has been shown to cause minimal neonatal depression.
Cardiac dysrhythmia
Cardiac dysrhythmia found during pregnancy is mostly benign in nature. Abnormalities in cardiac conducting tissue and rheumatic heart disease are the main causes of serious dysrhythmias. A recent onset of atrial fibrillation in pregnancy is associated with a high incidence of CHF, embolization, and mortality. To control the ventricular rate, verapamil, digoxin, quinidine, and beta adrenergic blocking agents are frequently used. IV adenosine (3–12 mg) is the treatment of choice for termination of supraventricular tachycardia, but direct current cardioversion can be used effectively without any harm to fetus.
Pregnancy postcardiac transplant
Since cardiac transplantation has become an accepted mode of therapy for many end-stage heart diseases, a number of patients with cardiac transplant have been reported to have carried pregnancy to term and delivered vaginally or by cesarean section successfully.[3,109,110]
Patients with organ transplants must be maintained on immunosuppressive therapy to prevent rejection. This usually includes cyclosporine, steroids (prednisone), and antibiotics, and these must be continued throughout the pregnancy. Strict attention to sterile technique is especially important. Prophylactic antibiotics and “stress dose” of steroids are indicated before anesthesia and surgery. Cyclosporine concentrations may change abruptly after delivery due to acute changes in maternal blood volume and should be followed with serial blood levels.[111] Despite exposure to immunosuppressive therapy, fetal effects appear to be minimal.[112]
Periodic transvenous right atrial biopsy is performed to follow the evidence of allograft cardiac rejection. When performed during pregnancy, care should be taken to provide supplemental oxygen and avoid aorto-caval compression during the procedure. These include uterine displacement and use of lead shield and not a lead apron applied directly to mother's abdomen for fetal protection, if fluoroscopy is used.
In the absence of allograft rejection, the transplanted heart usually works well during pregnancy, labor, and delivery. However, it is completely devoid of direct autonomic innervations. The baseline heart rate is usually slightly increased in cardiac transplant patients since the normal dominant underlying vagal tone is not expressed in them. Cardiac transplant recipients can increase and regulate their CO in response to exercise (though the response is slower), probably through the Starling mechanism by regulating their preload and afterload. Hence, in these patients, maintaining preload is especially important.
Only direct-acting sympathomimetic agents affect the heart rate (atropine is ineffective and isoprenaline is effective). Unlike normal patients, sympathectomy associated with high central neuraxial blocks will not cause bradycardia. These patients may be more sensitive to beta-mimetics (adrenaline being clinically significant) due to upregulation of receptors. Camann et al.[110] reported the onset of profound tachycardia in postcardiac transplant cesarean delivery, with the use of 1:200,000 adrenaline in 2% lignocaine for epidural anesthesia. The patient in their case required IV esmolol to correct the profound tachycardia that occurred in postcardiac transplant caesarean delivery.
In the absence of allograft rejection, noninvasive monitoring including NIBP, ECG, finger pulse oximetry for parturient, and fetal heart rate monitoring is usually adequate during labor and delivery. Invasive monitoring (central venous line, PA catheter) poses risks of infection and arrhythmias that need to be weighed against the useful information to be gained.
The avoidance of endogenous catecholamines associated with pain is important. The induction of continuous epidural analgesia with low-concentration local anesthetic solution with opioid early in labor is optimal. This also allows the level to be brought up rapidly in the event of an obstetric emergency, thus avoiding the need for general or single-shot spinal anesthesia and uncontrolled hemodynamic changes associated with these techniques. Continuous epidural or continuous spinal anesthesia techniques allow careful titration and greater control of cardiovascular parameters. BP should be maintained with volume loading and ephedrine or phenylephrine or mephentermine. Central neuraxial narcotics are advantageous.
If GA is used, thiopental is likely to have exaggerated depressant effects on transplanted heart. Ketamine may cause excessive tachycardia due to change in β-receptor sensitivity. A high-dose fentanyl technique or etomidate will produce the most stable induction, though onset time will be longer with narcotics.
Conclusion
Obstetric anesthesiologists should not be dogmatic about the choice of anesthesia for parturients with heart disease. Each case should be assessed individually, with special attention paid to the functional impairment. An understanding of the hemodynamics associated with the structural lesion and the appropriate use of invasive monitors are most important in providing optimal conditions for labor and delivery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Carabello BA, Crawford FA., Jr Valvular heart disease. N Engl J Med. 1997;337:32–41. doi: 10.1056/NEJM199707033370107. [DOI] [PubMed] [Google Scholar]
- 2.Perloff JK. Pregnancy and congenital heart disease. J Am Coll Cardiol. 1991;18:340–2. doi: 10.1016/0735-1097(91)90583-u. [DOI] [PubMed] [Google Scholar]
- 3.Gei AF, Hankins GD. Cardiac disease and pregnancy. Obstet Gynecol Clin North Am. 2001;28:465–512. doi: 10.1016/s0889-8545(05)70214-x. [DOI] [PubMed] [Google Scholar]
- 4.Morini A, Spina V, Aleandri V, Cantonetti G, Lambiasi A, Papalia U. Pregnancy after heart transplant: Update and case report. Hum Reprod. 1998;13:749–57. doi: 10.1093/humrep/13.3.749. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin JF. Peripartal heart disease. Clin Obstet Gynecol. 1975;18:125–31. doi: 10.1097/00003081-197509000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Bowdle TA, Horita A, Kharasch ED, editors. The Pharmacologic Basis of Anaesthesiology: Basic Science and Practical Applications. New York: Churchill Livingstone; 1994. [Google Scholar]
- 7.AHA Medical/Scientific Statement. 1994 revisions to classification of functional capacity and objective assessment of patients with diseases of the heart. Circulation. 1994;90:644–5. [PubMed] [Google Scholar]
- 8.Clark SL. Structural cardiac disease in pregnancy. In: Clark SL, Cotton DB, Phelan JP, editors. Critical Care Obstetrics. Oradell, NJ: Medical Economics Books; 1987. p. 92. [Google Scholar]
- 9.Ramin SM, Maberry MC, Gilstrap LC., 3rd Congenital heart disease. Clin Obstet Gynecol. 1989;32:41–7. doi: 10.1097/00003081-198903000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Gonik B. Intensive care monitoring of the critically ill pregnant patients. In: Creasy RK, Resnik R, editors. Maternal-Fetal Medicine: Principles and Practice. Philadelphia: WB Saunders; 1989. p. 850. [Google Scholar]
- 11.Prowse CM, Gaensler EA. Respiratory and acid-base changes during pregnancy. Anesthesiology. 1965;26:381–92. doi: 10.1097/00000542-196507000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393–9. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lees MM, Taylor SH, Scott DB, Kerr MG. A study of cardiac output at rest throughout pregnancy. J Obstet Gynaecol Br Commonw. 1967;74:319–28. doi: 10.1111/j.1471-0528.1967.tb03956.x. [DOI] [PubMed] [Google Scholar]
- 14.Ueland K, Novy MJ, Peterson EN, Metcalfe J. Maternal cardiovascular dynamics. IV. The influence of gestational age on the maternal cardiovascular response to posture and exercise. Am J Obstet Gynecol. 1969;104:856–64. [PubMed] [Google Scholar]
- 15.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161(6 Pt 1):1439–42. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 16.Goodman RP, Killam AP, Brash AR, Branch RA. Prostacyclin production during pregnancy: Comparison of production during normal pregnancy and pregnancy complicated by hypertension. Am J Obstet Gynecol. 1982;142:817–22. doi: 10.1016/s0002-9378(16)32525-x. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks CH. The hemodynamics of a uterine contraction. Am J Obstet Gynecol. 1958;76:969–82. doi: 10.1016/0002-9378(58)90181-9. [DOI] [PubMed] [Google Scholar]
- 18.Lyons HA, Antonio R. The sensitivity of the respiratory center in pregnancy and after the administration of progesterone. Trans Assoc Am Physicians. 1959;72:173–80. [Google Scholar]
- 19.Kliegman RM, Gross T. Perinatal problems of the obese mother and her infant. Obstet Gynecol. 1985;66:299–306. [PubMed] [Google Scholar]
- 20.Lang RM, Borow KM. Heart disease. In: Barron WM, Lindheimer MD, editors. Medical Disorders during Pregnancy. St. Louis: Mosby Year Book; 1991. p. 148. [Google Scholar]
- 21.Elkayam U, Gleischer N, editors. Cardiac Problems in Pregnancy. 2nd ed. New York: Alan R. Liss; 1990. Changes in cardiac findings during normal pregnancy; p. 31. [Google Scholar]
- 22.Burrow GN, Ferris T. Medical Complications during Pregnancy. 3rd ed. Philadelphia: WB Saunders; 1988. [Google Scholar]
- 23.Johnson MD, Saltzman DH. Cardiac disease. In: Datta S, Gay SM, Livingston J, editors. Anesthetic and Obstetric Management of High Risk Pregnancy. St. Louis: Mosby Year Book, Inc; 1991. p. 210. [Google Scholar]
- 24.Dajani AS, Bisno AL, Chung KJ, Durack DT, Freed M, Gerber MA, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. 1990;264:2919–22. [PubMed] [Google Scholar]
- 25.Moore RA. Anesthesia for pediatric congenital heart patient for non-cardiac surgery. Anesthesiol Rev. 1981;8:23. [Google Scholar]
- 26.Modig J. Influence of regional anesthesia, local anesthetics, and sympathicomimetics on the pathophysiology of deep vein thrombosis. Acta Chir Scand Suppl. 1989;550:119–24. [PubMed] [Google Scholar]
- 27.Task force on guidelines for PA catheterization. Anesthesiology. 1993;78:380–96. [PubMed] [Google Scholar]
- 28.Oxorn D, Edelist G, Smith MS. An introduction to transoesophageal echocardiography: II. Clinical applications. Can J Anaesth. 1996;43:278–94. doi: 10.1007/BF03011745. [DOI] [PubMed] [Google Scholar]
- 29.Wong V, Cheng CH, Chan KC. Fetal and neonatal outcome of exposure to anticoagulants during pregnancy. Am J Med Genet. 1993;45:17–21. doi: 10.1002/ajmg.1320450107. [DOI] [PubMed] [Google Scholar]
- 30.Born D, Martinez EE, Almeida PA, Santos DV, Carvalho AC, Moron AF, et al. Pregnancy in patients with prosthetic heart valves: The effects of anticoagulation on mother, fetus, and neonate. Am Heart J. 1992;124:413–7. doi: 10.1016/0002-8703(92)90606-v. [DOI] [PubMed] [Google Scholar]
- 31.Toglia MR, Weg JG. Venous thromboembolism during pregnancy. N Engl J Med. 1996;335:108–14. doi: 10.1056/NEJM199607113350207. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg JS, Hirsh J. Use of antithrombotic agents during pregnancy. Chest. 1995;108(4 Suppl):305S–11S. doi: 10.1378/chest.108.4_supplement.305s. [DOI] [PubMed] [Google Scholar]
- 33.Sanson BJ, Lensing AW, Prins MH, Ginsberg JS, Barkagan ZS, Lavenne-Pardonge E, et al. Safety of low-molecular-weight heparin in pregnancy: A systematic review. Thromb Haemost. 1999;81:668–72. [PubMed] [Google Scholar]
- 34.Lee CN, Wu CC, Lin PY, Hsieh FJ, Chen HY. Pregnancy following cardiac prosthetic valve replacement. Obstet Gynecol. 1994;83:353–6. [PubMed] [Google Scholar]
- 35.Badduke BR, Jamieson WR, Miyagishima RT, Munro AI, Gerein AN, MacNab J, et al. Pregnancy and childbearing in a population with biologic valvular prostheses. J Thorac Cardiovasc Surg. 1991;102:179–86. [PubMed] [Google Scholar]
- 36.Sbarouni E, Oakley CM. Outcome of pregnancy in women with valve prostheses. Br Heart J. 1994;71:196–201. doi: 10.1136/hrt.71.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbour LA, Pickard J. Controversies in thromboembolic disease during pregnancy: A critical review. Obstet Gynecol. 1995;86(4 Pt 1):621–33. [PubMed] [Google Scholar]
- 38.Ramamurthy S, Talwar KK, Saxena A, Juneja R, Takkar D. Prosthetic mitral valve thrombosis in pregnancy successfully treated with streptokinase. Am Heart J. 1994;127:446–8. doi: 10.1016/0002-8703(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 39.Furui T, Kurauchi O, Oguchi H, Nomura S, Mizutani S, Tomoda Y. Pregnancy and successful delivery in a patient with triple heart valve prosthesis. Int J Gynaecol Obstet. 1993;41:89–92. doi: 10.1016/0020-7292(93)90160-x. [DOI] [PubMed] [Google Scholar]
- 40.Clark SL. Monitoring and anaesthetic management of parturients with mitral stenosis. Can J Anaesth. 1987;34:654. doi: 10.1007/BF03010530. [DOI] [PubMed] [Google Scholar]
- 41.Batson MA, Longmire S, Csontos E. Alfentanil for urgent caesarean section in a patient with severe mitral stenosis and pulmonary hypertension. Can J Anaesth. 1990;37:685–8. doi: 10.1007/BF03006492. [DOI] [PubMed] [Google Scholar]
- 42.Hemmings GT, Whalley DG, O'Connor PJ, Benjamin A, Dunn C. Invasive monitoring and anaesthetic management of a parturient with mitral stenosis. Can J Anaesth. 1987;34:182–5. doi: 10.1007/BF03015343. [DOI] [PubMed] [Google Scholar]
- 43.Ngan Kee WD, Shen J, Chiu AT, Lok I, Khaw KS. Combined spinal-epidural analgesia in the management of labouring parturients with mitral stenosis. Anaesth Intensive Care. 1999;27:523–6. doi: 10.1177/0310057X9902700516. [DOI] [PubMed] [Google Scholar]
- 44.Ziskind Z, Etchin A, Frenkel Y, Mashiach S, Lusky A, Goor DA, et al. Epidural anesthesia with the Trendelenburg position for cesarean section with or without a cardiac surgical procedure in patients with severe mitral stenosis: A hemodynamic study. J Cardiothorac Anesth. 1990;4:354–9. doi: 10.1016/0888-6296(90)90045-h. [DOI] [PubMed] [Google Scholar]
- 45.Easterling TR, Chadwick HS, Otto CM, Benedetti TJ. Aortic stenosis in pregnancy. Obstet Gynecol. 1988;72:113–8. [PubMed] [Google Scholar]
- 46.Lao TT, Sermer M, MaGee L, Farine D, Colman JM. Congenital aortic stenosis and pregnancy – A reappraisal. Am J Obstet Gynecol. 1993;169:540–5. doi: 10.1016/0002-9378(93)90616-q. [DOI] [PubMed] [Google Scholar]
- 47.McIvor RA. Percutaneous balloon aortic valvuloplasty during pregnancy. Int J Cardiol. 1991;32:1–3. doi: 10.1016/0167-5273(91)90037-p. [DOI] [PubMed] [Google Scholar]
- 48.Choi HJ, Chui L, Hurd JM, Tremper KK. Epidural anesthesia for a woman with severe aortic stenosis undergoing a cesarean section. Anesthesiol Rev. 1992;19:61. [Google Scholar]
- 49.Brian JE, Jr, Seifen AB, Clark RB, Robertson DM, Quirk JG. Aortic stenosis, cesarean delivery, and epidural anesthesia. J Clin Anesth. 1993;5:154–7. doi: 10.1016/0952-8180(93)90145-5. [DOI] [PubMed] [Google Scholar]
- 50.Colclough GW, Ackerman WE, 3rd, Walmsley PM, Hessel EA. Epidural anesthesia for a parturient with critical aortic stenosis. J Clin Anesth. 1995;7:264–5. doi: 10.1016/0952-8180(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 51.Atanassoff PG, Schmid ER, Jenni R, Arbenz U, Alon E, Pasch T. Epidural anesthesia for a cesarean section in a patient with pulmonary atresia and ventricular septal defect. J Clin Anesth. 1991;3:399–402. doi: 10.1016/0952-8180(91)90184-o. [DOI] [PubMed] [Google Scholar]
- 52.Douglas MJ, Farquharson DF, Ross PL, Renwick JE. Cardiovascular collapse following an overdose of prostaglandin F2 alpha: A case report. Can J Anaesth. 1989;36:466–9. doi: 10.1007/BF03005350. [DOI] [PubMed] [Google Scholar]
- 53.Redfern N, Bower S, Bullock RE, Hull CJ. Alfentanil for caesarean section complicated by severe aortic stenosis. A case report. Br J Anaesth. 1988;60:477. doi: 10.1093/bja/59.10.1309. [DOI] [PubMed] [Google Scholar]
- 54.Sharma SK, Gambling DR, Gajraj NM, Truong C, Sidawi EJ. Anesthetic management of a parturient with mixed mitral valve disease and uncontrolled atrial fibrillation. Int J Obstet Anesth. 1994;3:157–62. doi: 10.1016/0959-289x(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 55.Lynch C, 3rd, Rizor RF. Anesthetic management and monitoring of a parturient with mitral and aortic valvular disease. Anesth Analg. 1982;61:788–92. [PubMed] [Google Scholar]
- 56.Shin YK, King JC. Combined mitral and aortic stenosis in a parturient: Epidural anesthesia for labor and delivery. Anesth Analg. 1993;76:682–3. doi: 10.1213/00000539-199303000-00074. [DOI] [PubMed] [Google Scholar]
- 57.Mangano DT. Anesthesia for the pregnant cardiac patient. In: Shnider SM, Levinson G, editors. Anesthesia for Obstetrics. 3rd ed. Williams & Wilkins; 1993. p. 485. [Google Scholar]
- 58.Mortensen JD, Ellsworth HS. Pregnancy before and after surgical correction of left-to-right cardiovascular shunts. Obstet Gynecol. 1967;29:241–3. [PubMed] [Google Scholar]
- 59.Patton DE, Lee W, Cotton DB, Miller J, Carpenter RJ, Jr, Huhta J, et al. Cyanotic maternal heart disease in pregnancy. Obstet Gynecol Surv. 1990;45:594–600. doi: 10.1097/00006254-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Burn J. 'The next lady has a heart defect'. Br J Obstet Gynaecol. 1987;94:97–9. doi: 10.1111/j.1471-0528.1987.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 61.Slomka F, Salmeron S, Zetlaoui P, Cohen H, Simonneau G, Samii K. Primary pulmonary hypertension and pregnancy: Anesthetic management for delivery. Anesthesiology. 1988;69:959–61. doi: 10.1097/00000542-198812000-00028. [DOI] [PubMed] [Google Scholar]
- 62.Robinson DE, Leicht CH. Epidural analgesia with low-dose bupivacaine and fentanyl for labor and delivery in a parturient with severe pulmonary hypertension. Anesthesiology. 1988;68:285–8. doi: 10.1097/00000542-198802000-00020. [DOI] [PubMed] [Google Scholar]
- 63.Kasai H, Gohda Y, Sasaki K, Kemmotsu O. Anesthetic management of caesarean section in a patient with primary pulmonary hypertension. Masui. 1988;37:476–82. [PubMed] [Google Scholar]
- 64.Spinnato JA, Kraynack BJ, Cooper MW. Eisenmenger's syndrome in pregnancy: Epidural anesthesia for elective cesarean section. N Engl J Med. 1981;304:1215–7. doi: 10.1056/NEJM198105143042007. [DOI] [PubMed] [Google Scholar]
- 65.Tibaldi G, Marchi L, Huscher M, Forlini G. Anesthesia for cesarean section in a pregnant woman with Eisenmenger's syndrome. Description of a clinical case. Minerva Ginecol. 1988;40:145–6. [PubMed] [Google Scholar]
- 66.Monnery L, Nanson J, Charlton G. Primary pulmonary hypertension in pregnancy; a role for novel vasodilators. Br J Anaesth. 2001;87:295–8. doi: 10.1093/bja/87.2.295. [DOI] [PubMed] [Google Scholar]
- 67.Baum VC. The adult patient with congenital heart disease. J Cardiothorac Vasc Anesth. 1996;10:261–82. doi: 10.1016/s1053-0770(96)80251-5. [DOI] [PubMed] [Google Scholar]
- 68.Weiss BM, Atanassoff PG. Cyanotic congenital heart disease and pregnancy: Natural selection, pulmonary hypertension, and anesthesia. J Clin Anesth. 1993;5:332–41. doi: 10.1016/0952-8180(93)90130-7. [DOI] [PubMed] [Google Scholar]
- 69.Robinson S. Pulmonary artery catheters in Eisenmenger's syndrome: Many risks, few benefits. Anesthesiology. 1983;58:588–90. doi: 10.1097/00000542-198306000-00031. [DOI] [PubMed] [Google Scholar]
- 70.Roberts SL, Chestnut DH. Anesthesia for the obstetric patient with cardiac disease. Clin Obstet Gynecol. 1987;30:601–10. doi: 10.1097/00003081-198709000-00014. [DOI] [PubMed] [Google Scholar]
- 71.Spielman FJ. Anaesthetic management of the obstetric patient with cardiac disease. Clin Anaesthesiol. 1986;4:247. [Google Scholar]
- 72.Mostafa SM. Spinal anaesthesia for caesarean section. Management of a parturient with severe cardiovascular disease. Br J Anaesth. 1984;56:1275–7. doi: 10.1093/bja/56.11.1275. [DOI] [PubMed] [Google Scholar]
- 73.Prince GD. Spinal anaesthesia and cardiac disease. Br J Anaesth. 1986;58:683–4. doi: 10.1093/bja/58.6.683. [DOI] [PubMed] [Google Scholar]
- 74.Ransom DM, Leicht CH. Continuous spinal analgesia with sufentanil for labor and delivery in a parturient with severe pulmonary stenosis. Anesth Analg. 1995;80:418–21. doi: 10.1097/00000539-199502000-00038. [DOI] [PubMed] [Google Scholar]
- 75.Ahmad S, Hawes D, Dooley S, Faure E, Brunner EA. Intrathecal morphine in a parturient with a single ventricle. Anesthesiology. 1981;54:515–7. doi: 10.1097/00000542-198106000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Abboud TK, Raya J, Noueihed R, Daniel J. Intrathecal morphine for relief of labor pain in a parturient with severe pulmonary hypertension. Anesthesiology. 1983;59:477–9. doi: 10.1097/00000542-198311000-00025. [DOI] [PubMed] [Google Scholar]
- 77.Pollack KL, Chestnut DH, Wenstrom KD. Anesthetic management of a parturient with Eisenmenger's syndrome. Anesth Analg. 1990;70:212–5. doi: 10.1213/00000539-199002000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Leyse R, Ofstun M, Dillard DH, Merendino KA. Congenital aortic stenosis in pregnancy, corrected by extracorporeal circulation. JAMA. 1961;32:1009. doi: 10.1001/jama.1961.03040250035011. [DOI] [PubMed] [Google Scholar]
- 79.Rossouw GJ, Knott-Craig CJ, Barnard PM, Macgregor LA, Van Zyl WP. Intracardiac operation in seven pregnant women. Ann Thorac Surg. 1993;55:1172–4. doi: 10.1016/0003-4975(93)90027-f. [DOI] [PubMed] [Google Scholar]
- 80.Ben-Ami M, Battino S, Rosenfeld T, Marin G, Shalev E. Aortic valve replacement during pregnancy. A case report and review of the literature. Acta Obstet Gynecol Scand. 1990;69:651–3. doi: 10.3109/00016349009028713. [DOI] [PubMed] [Google Scholar]
- 81.Becker RM. Intracardiac surgery in pregnant women. Ann Thorac Surg. 1983;36:453–8. doi: 10.1016/s0003-4975(10)60486-9. [DOI] [PubMed] [Google Scholar]
- 82.Eilen B, Kaiser IH, Becker RM, Cohen MN. Aortic valve replacement in the third trimester of pregnancy: Case report and review of the literature. Obstet Gynecol. 1981;57:119–21. [PubMed] [Google Scholar]
- 83.Paulus DA, Layon AJ, Mayfield WR, D'Amico R, Taylor WJ, James CF. Intrauterine pregnancy and aortic valve replacement. J Clin Anesth. 1995;7:338–46. doi: 10.1016/0952-8180(95)00011-6. [DOI] [PubMed] [Google Scholar]
- 84.Bernal JM, Miralles PJ. Cardiac surgery with cardiopulmonary bypass during pregnancy. Obstet Gynecol Surv. 1986;41:1–6. doi: 10.1097/00006254-198601000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Shah AM, Ikram S, Kulatilake EN, Pearson JF, Hall RJ. Emergency mitral valve replacement immediately following caesarean section. Eur Heart J. 1992;13:847–9. doi: 10.1093/oxfordjournals.eurheartj.a060268. [DOI] [PubMed] [Google Scholar]
- 86.Lamarra M, Azzu AA, Kulatilake EN. Cardiopulmonary bypass in the early puerperium: Possible new role for aprotinin. Ann Thorac Surg. 1992;54:361–3. doi: 10.1016/0003-4975(92)91403-v. [DOI] [PubMed] [Google Scholar]
- 87.Holzman RS, Nargozian CD, Marnach R, McMillan CO. Epidural anesthesia in patients with palliated cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 1992;6:340–3. doi: 10.1016/1053-0770(92)90155-z. [DOI] [PubMed] [Google Scholar]
- 88.Whittemore R, Hobbins JC, Engle MA. Pregnancy and its outcome in women with and without surgical treatment of congenital heart disease. Am J Cardiol. 1982;50:641–51. doi: 10.1016/0002-9149(82)90334-4. [DOI] [PubMed] [Google Scholar]
- 89.Zavisca FG, Johnson MD, Holubec JT, Kao YJ, Racz GB. General anesthesia for cesarean section in a parturient with a single ventricle and pulmonary atresia. J Clin Anesth. 1993;5:315–20. doi: 10.1016/0952-8180(93)90126-y. [DOI] [PubMed] [Google Scholar]
- 90.Carp H, Jayaram A, Vadhera R, Nichols M, Morton M. Epidural anesthesia for cesarean delivery and vaginal birth after maternal Fontan repair: Report of two cases. Anesth Analg. 1994;78:1190–2. doi: 10.1213/00000539-199406000-00032. [DOI] [PubMed] [Google Scholar]
- 91.Cohen AM, Mulvein J. Obstetric anaesthetic management in a patient with the Fontan circulation. Br J Anaesth. 1994;73:252–5. doi: 10.1093/bja/73.2.252. [DOI] [PubMed] [Google Scholar]
- 92.Monteiro RS, Dob DP, Cauldwell MR, Gatzoulis MA. Anaesthetic management of parturients with univentricular congenital heart disease and the Fontan operation. Int J Obstet Anesth. 2016;28:83–91. doi: 10.1016/j.ijoa.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 93.de Leval MR. Right heart bypass operations. In: Stark J, de Leval MR, editors. Surgery for Congenital Heart Defects. Philadelphia: WB Saunders; 1994. p. 565. [Google Scholar]
- 94.Fairley CJ, Clarke JT. Use of esmolol in a parturient with hypertrophic obstructive cardiomyopathy. Br J Anaesth. 1995;75:801–4. doi: 10.1093/bja/75.6.801. [DOI] [PubMed] [Google Scholar]
- 95.Ducey JP, Knape KG. Maternal esmolol administration resulting in fetal distress and cesarean section in a term pregnancy. Anesthesiology. 1992;77:829–32. doi: 10.1097/00000542-199210000-00034. [DOI] [PubMed] [Google Scholar]
- 96.Loubser P, Suh K, Cohen S. Adverse effects of spinal anesthesia in a patient with idiopathic hypertrophic subaortic stenosis. Anesthesiology. 1984;60:228–30. doi: 10.1097/00000542-198403000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Boccio RV, Chung JH, Harrison DM. Anesthetic management of cesarean section in a patient with idiopathic hypertrophic subaortic stenosis. Anesthesiology. 1986;65:663–5. doi: 10.1097/00000542-198612000-00017. [DOI] [PubMed] [Google Scholar]
- 98.Tessler MJ, Hudson R, Naugler-Colville M, Biehl DR. Pulmonary oedema in two parturients with hypertrophic obstructive cardiomyopathy (HOCM) Can J Anaesth. 1990;37(4 Pt 1):469–73. doi: 10.1007/BF03005629. [DOI] [PubMed] [Google Scholar]
- 99.Nwosu EC, Burke MF. Cardiomyopathy of pregnancy. Br J Obstet Gynaecol. 1993;100:1145–7. doi: 10.1111/j.1471-0528.1993.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 100.Yacoub A, Martel MJ. Pregnancy in a patient with primary dilated cardiomyopathy. Obset Gynecol. 2002;99:928. doi: 10.1016/s0029-7844(01)01745-8. [DOI] [PubMed] [Google Scholar]
- 101.Brown G, O'Leary M, Douglas I, Herkes R. Perioperative management of a case of severe peripartum cardiomyopathy. Anaesth Intensive Care. 1992;20:80–3. doi: 10.1177/0310057X9202000117. [DOI] [PubMed] [Google Scholar]
- 102.Breen TW, Janzen JA. Pulmonary hypertension and cardiomyopathy: Anaesthetic management for caesarean section. Can J Anaesth. 1991;38:895–9. doi: 10.1007/BF03036969. [DOI] [PubMed] [Google Scholar]
- 103.Kluger MT, Bersten AD. Multi-organ failure in peripartum cardiomyopathy. Anaesth Intensive Care. 1991;19:450–3. doi: 10.1177/0310057X9101900327. [DOI] [PubMed] [Google Scholar]
- 104.Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94:311–6. doi: 10.1016/s0029-7844(99)00293-8. [DOI] [PubMed] [Google Scholar]
- 105.Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL. Peripartum myocarditis and cardiomyopathy. Circulation. 1990;81:922–8. doi: 10.1161/01.cir.81.3.922. [DOI] [PubMed] [Google Scholar]
- 106.Beards SC, Freebairn RC, Lipman J. Successful use of continuous veno-venous haemofiltration to treat profound fluid retention in severe peripartum cardiomyopathy. Anaesthesia. 1993;48:1065–7. doi: 10.1111/j.1365-2044.1993.tb07528.x. [DOI] [PubMed] [Google Scholar]
- 107.Gambling DR, Flanagan ML, Huckell VF, Lucas SB, Kim JH. Anaesthetic management and non-invasive monitoring for caesarean section in a patient with cardiomyopathy. Can J Anaesth. 1987;34:505–8. doi: 10.1007/BF03014358. [DOI] [PubMed] [Google Scholar]
- 108.McIndoe AK, Hammond EJ, Babington PC. Peripartum cardiomyopathy presenting as a cardiac arrest at induction of anaesthesia for emergency caesarean section. Br J Anaesth. 1995;75:97–101. doi: 10.1093/bja/75.1.97. [DOI] [PubMed] [Google Scholar]
- 109.Löwenstein BR, Vain NW, Perrone SV, Wright DR, Boullón FJ, Favaloro RG. Successful pregnancy and vaginal delivery after heart transplantation. Am J Obstet Gynecol. 1988;158(3 Pt 1):589–90. doi: 10.1016/0002-9378(88)90035-x. [DOI] [PubMed] [Google Scholar]
- 110.Camann WR, Goldman GA, Johnson MD, Moore J, Greene M. Cesarean delivery in a patient with a transplanted heart. Anesthesiology. 1989;71:618–20. doi: 10.1097/00000542-198910000-00027. [DOI] [PubMed] [Google Scholar]
- 111.Kossoy LR, Herbert CM, 3rd, Wentz AC. Management of heart transplant recipients: Guidelines for the obstetrician-gynecologist. Am J Obstet Gynecol. 1988;159:490–9. doi: 10.1016/s0002-9378(88)80116-9. [DOI] [PubMed] [Google Scholar]
- 112.Deeg HJ, Kennedy MS, Sanders JE, Thomas ED, Storb R. Successful pregnancy after marrow transplantation for severe aplastic anemia and immunosuppression with cyclosporine. JAMA. 1983;250:647. [PubMed] [Google Scholar]


