Abstract
Purpose
To determine the efficacy of hepatic artery embolization (HAE) as a therapy for gastrointestinal stromal tumor (GIST) in patients who are refractory to imatinib and sunitinib.
Methods
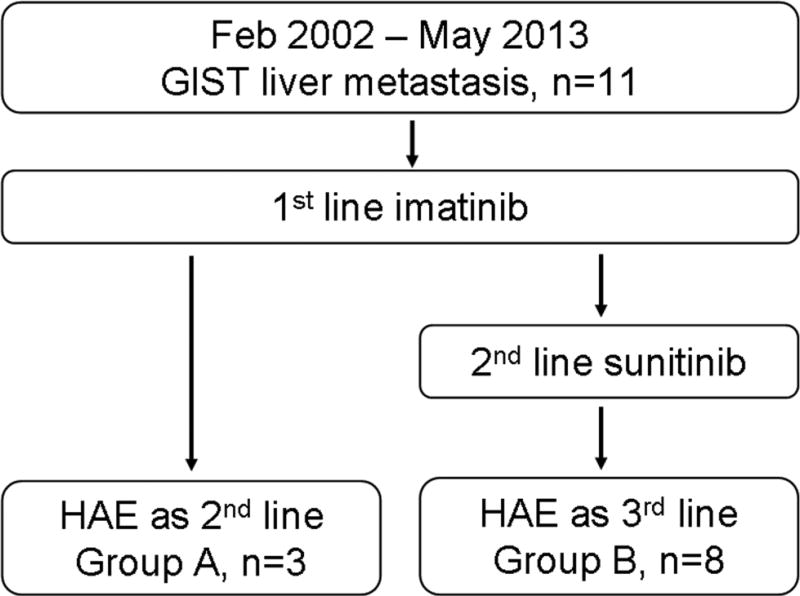
After institutional review board approval, a retrospective review revealed 11 patients with GIST metastatic to the liver who underwent 15 HAEs between February 2002 and May 2013.These patients were stratified into 2 groups according to the previous treatment: A) those treated with HAE as second-line treatment after failing first-line imatinib (n=3) and B) those treated with HAE as third-line therapy after failing first-line imatinib and second-line sunitinib (n=8). Initial therapeutic response, overall survival (OS), progression-free survival (PFS), and safety were evaluated.
Results
Initial therapeutic response rates at 3 month after HAE were 27.3% (95% confidence interval: CI, 6.0%–61.0%) by Response Evaluation Criteria in Solid Tumor (RECIST) version 1.0 and 45.5% (95% CI, 16.7%–76.6%) by modified RECIST (mRECIST). The median OS and PFS after HAE were 14.9 months and 3.9 months in group A, and 23.8 months and 3.4 months in group B, respectively. No procedure-related mortality or major complication was observed.
Conclusions
HAE is an effective and well-tolerated therapeutic option for GIST liver metastases. Although larger studies are necessary, HAE should be considered as an alternative or adjuvant to third-line or even second-line systemic treatment.
Keywords: hepatic artery embolization, gastrointestinal stromal tumor, liver, imatinib, sunitinib
INTRODUCTION
The liver is the most common site of metastasis from gastrointestinal stromal tumor (GIST), and 20%–60% of metastases occur in the liver [1,2]. Moreover, the extent of involvement of liver by metastases is a major determinant of patients’ survival [2].
The current standard of care for metastatic GIST (mGIST) recommends imatinib as the 1st-line therapy [3]. Imatinib therapy is limited by primary resistance to the drug in approximately 10%–15% of patients [4]; moreover, 80% of patients eventually develop disease progression driven by secondary-resistance mutations located in additional c-kit exons [5]. Sunitinib is considered to be the standard 2nd-line therapy after the failure of imatinib [6–9]. The primary resistance rate of sunitinib is more than 50%, with a lower survival benefit [6–9]. Therefore, another therapeutic option to manage mGIST patients who are refractory to imatinib and sunitinib has been anticipated.
Hepatic artery embolization (HAE) and transarterial chemoembolization (TACE) are useful therapeutic options in the treatment of hypervascular liver tumors, and some studies have shown favorable results using TACE for GIST liver metastases [10–12]; nonetheless, there are few studies that evaluate the efficacy of HAE [13], and none that evaluated the role of HAE after the use of modern systemic treatment regimens. Thus, the clinical role of HAE in the treatment of GIST liver metastases remains unclear. In this study, we evaluated the efficacy and clinical role of HAE therapy in mGIST patients refractory to imatinib and sunitinib.
METHODS
Patients
Under an institutional review board (IRB) waiver, a retrospective review of all patients who underwent HAE between February 2002 and May 2013 was performed to identify patients treated for GIST metastasis.
Eleven patients with characteristics summarized in Table 1 were included. There were 6 males (54.5%) and 5 females (45.5%), with a mean age of 58.0±11.6 (range, 41–76) years. The primary site of GIST was the stomach in 8 patients (72.7%), ileum in 1 patient (9.1%), duodenum in 1 patient (9.1%), and retroperitoneum in 1 patient (9.1%). The histological diagnosis of GIST was established according to the World Health Organization Classification of Tumors by expert pathologists [14]. In all patients, HAE was performed because the control of liver metastasis was considered mandatory to prolong patient survival. Decision to perform HAE was made by multidisciplinary team including surgeons, oncologists, and interventional radiologists.
Table 1.
Patient backgrounds and tumor characteristics
| Parameter | Overall | Group A | Group B | p alue |
|---|---|---|---|---|
| Patient No. | 11 | 3 | 8 | |
| Age (years) | 58.0 ± 11.6 | 61.7 ± 12.7 | 59.9 ± 13.0 | 0.54 |
| ≦60 | 7 (63.6) | 1 (33.3) | 6 (75.0) | 0.49 |
| >60 | 4 (36.4) | 2 (66.7) | 2 (25.0) | |
| Gender | >0.99 | |||
| Male | 6 (54.5) | 2 (66.7) | 4 (50.0) | |
| Female | 5 (45.5) | 1 (33.3) | 4 (50.0) | |
| Primary site | 0.49 | |||
| Stomach | 8 (72.7) | 3 (100) | 5 (62.5) | |
| Other | 3 (27.3) | 0 | 3 (37.5) | |
| Ileum | 1 (9.1) | 0 | 1 (12.5) | |
| Duodenum | 1 (9.1) | 0 | 1 (12.5) | |
| Retroperitoneum | 1 (9.1) | 0 | 1 (12.5) | |
| Maximum diameter of liver metastasis (cm) | >0.99 | |||
| ≦5 | 5 (45.5) | 1 (33.3) | 4 (50.0) | |
| >5 | 6 (54.5) | 2 (66.7) | 4 (50.0) | |
| Tumor number | >0.99 | |||
| ≦5 | 4 (36.4) | 1 (33.3) | 3 (37.5) | |
| >5 | 7 (63.6) | 2 (66.7) | 5 (62.5) | |
| Extrahepatic lesion | >0.99 | |||
| Abscent | 6 (54.5) | 2 (66.7) | 4 (50.0) | |
| Present | 5 (45.5) | 1 (33.3) | 4 (50.0) | |
| Peritoneum, lung, rectum | 1 (9.1) | 1 (9.1) | 0 | |
| Peritoneum | 1 (9.1) | 0 | 1 (9.1) | |
| Lung | 1 (9.1) | 0 | 1 (9.1) | |
| Stomach | 1 (9.1) | 0 | 1 (9.1) | |
| Leg | 1 (9.1) | 0 | 1 (9.1) |
Note.-Numbers in parentheses are percentages.
All patients received imatinib therapy as a first-line treatment of liver metastasis. These 11 patients were divided into 2 groups according to the previous treatment (Figure 1). Three patients (27.3%, group A) received HAE as the second-line therapy after the failure of first-line imatinib, because liver was only the site of metastasis at that time (n=2), and it was considered as the major determinant of survival (n=1). The other 8 patients (72.7%, group B) received HAE as the third-line treatment after the failure of both first-line imatinib and second-line sunitinib. All patients underwent routine physical examinations, laboratory tests, and imaging studies such as chest radiography, abdominal pre-contrast and contrast enhanced dynamic computed tomography (CT) or magnetic resonance (MR) imaging before HAE (Figure 2).
Figure 1.
Flow chart of patients included in this study. GIST: gastrointestinal stromal tumor, HAE: hepatic arterial embolization.
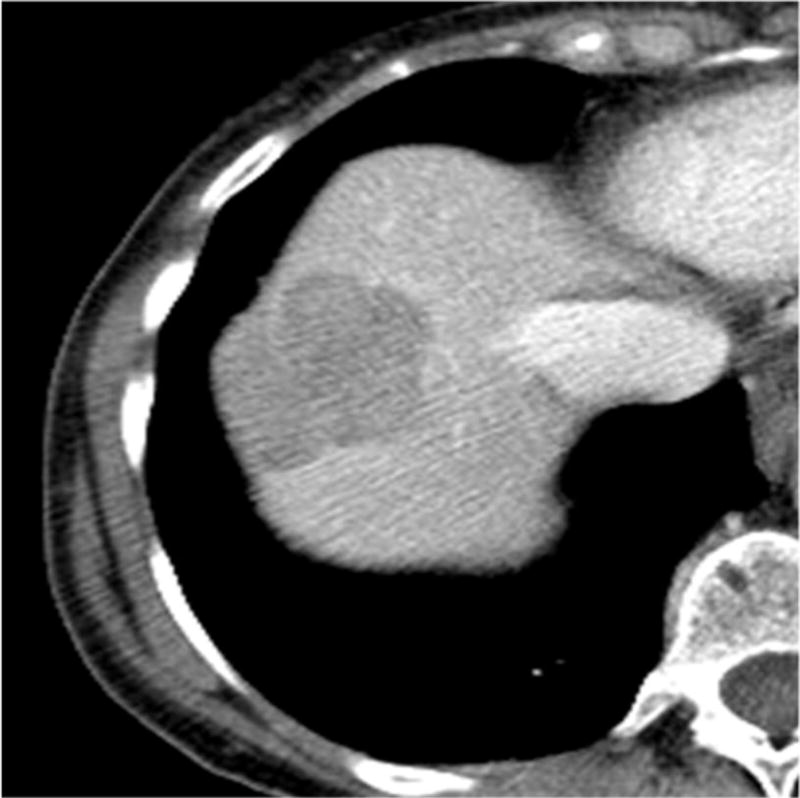
Figure 2.
Images of GIST metastatic to the liver in a 76-year-old man. (a) Axial contrast-enhanced CT scan shows a liver metastasis with maximum diameter of 3.9 cm in the anterior segment of the liver. (b) 3 month after HAE, tumor size decreased and be replaced to non-enhancing area.
Hepatic Artery Embolization
Routine hepatic angiography was performed via the common femoral artery with a 4- or 5-french angiographic catheter to identify hepatic arterial anatomy, lesion location, and feeding arteries. Feeding arteries that supplied the target lesion(s) were catheterized as selectively as possible with the use of microcatheters. Embolization was performed by using Embosphere® Microspheres (40–120 or 100–300 um; Biosphere Medical, Rockland, MA), Bead Block® (100–300 um; Biocompatibles, Farnham, UK), or Embozene® Microspheres (100 um; Celonova BioSciences, San Antonio, TX) per user preference. The endpoint of embolization was complete stasis of antegrade blood flow in the feeding arteries. In 5 patients, after initial embolization with one of these agents, polyvinyl alcohol (PVA) (100 um) was used to achieve complete stasis. If multiple lesions were present in the liver, and it was not possible to perform the embolization selectively, lobar embolization was performed. For patients with bilobar tumors, staged procedures were performed within 3 months to avoid increased toxicity from whole-liver treatment at one sitting. A total of 15 HAEs were performed (mean, 1.4; range, 1±3).
Follow-up
Follow-up included routine physical examinations, laboratory examinations, and imaging studies. Contrast-enhanced multi-phase CT or MR imaging were performed at 1 and 3 months after HAE and subsequently every 3–6 months after the procedure.
Assessment
In this study, initial therapeutic response, overall survival (OS), progression-free survival (PFS), and safety in each treatment group were evaluated.
Initial therapeutic response was evaluated based on Response Evaluation Criteria in Solid Tumor (RECIST) version 1.0 and modified RECIST (mRECIST) [15,16], at 1 and 3 months after HAE by 2 radiologists (H.T. and F.C.). OS time was defined as the time from the first HAE to the date of death, or to the date of last-follow-up for patients who were alive at the time of writing this manuscript. PFS was calculated from the date of first HAE to the date of disease progression, date of death, or date of last follow-up for patients without disease progression. Patients’ medical records were reviewed for up to 30 days after each embolization to capture complications. Complications were graded according to Society of Interventional Radiology (SIR) guidelines [17]. A major complication is defined as an event that required therapy, increased level of care requiring hospital admission or substantially lengthened hospital stay, caused permanent damage or death. All other complications were considered minor.
Statistical Analysis
Comparison between each group was conducted using Mann-Whitney’s U-test for continuous variables and Fisher’s exact test for categorical variables. The cumulative OS and PFS curves were generated using the Kaplan-Meier method. A P value <0.05 was considered statistically significant. All statistical analyses were performed with software (SAS, release 9.1; SAS Institute, Cary, NC).
RESULTS
Initial therapeutic response
The initial therapeutic response rates were, respectively, 27.3% (95% confidence interval: CI, 6.0%–61.6%) based on RECIST and 45.5% (95% CI, 16.7%–76.6%) based on mRECIST (Table 2). When initial therapeutic responses were stratified according to each patient group, response rates of group A and B were, respectively, 33.3% (95% CI, 0.8%–90.6%) and 25.0% (95% CI, 3.2%–65.1%) based on RECIST, and 33.3% (95% CI, 0.8%–90.6%) and 50.0% (95% CI, 15.7%–84.3%) based on mRECIST. There were no significant difference of response rates between the two groups (P>0.99).
Table 2.
Tumor Response According to RECIST and mRECIST Criteria
| Criteria | CR | PR | SD | PD | NE | OR (95% CI) |
|---|---|---|---|---|---|---|
| Overall (n=11) | ||||||
| RECIST | 0 | 3 | 5 | 3 | 0 | 27.3% (6.0–61.0%) |
| mRECIST | 0 | 5 | 3 | 3 | 0 | 45.5% (16.7–76.6%) |
| Group A (n=3) | ||||||
| RECIST | 0 | 1 | 1 | 1 | 0 | 33.3% (0.8–90.6%) |
| mRECIST | 0 | 1 | 1 | 1 | 0 | 33.3% (0.8–90.6%) |
| Group B (n=8) | ||||||
| RECIST | 0 | 2 | 4 | 2 | 0 | 25.0% (3.2–65.1%) |
| mRECIST | 0 | 4 | 2 | 2 | 0 | 50.0% (15.7–84.3%) |
RECIST: Response Evaluation Criteria in Solid Tumors, mRECIST: modified Response Evaluation Criteria in Solid Tumors, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, NE: not evaluable, OR: objective response, CI: confidence interval
Overall survival
During the mean follow-up period of 17.9±6.9 months (range, 7.5–28.1 months), 10 patients (90.9%) died. Causes of death were cancer progression in all patients. The cumulative 6-month and 1-year OS rates were, respectively, 100% and 66.7% (95% CI, 5.4%–94.5%) in group A, and 100% and 75.0% (95% CI, 31.5%–93.1%) in group B. The median OS was 14.9 months in group A and 23.8 months in group B.
Progression-free survival
Tumor progression was found in the liver in 10 patients (90.9%) and pelvis in 1 patient (9.1%) after HAE. Cumulative 6-month and 1-year PFS rates were, respectively, 33.3% (95% CI, 0.9%–77.4%) and 0% in group A, and 37.5% (95% CI, 8.7%–67.4%) and 12.5% (95% CI, 0.7%–42.3%) in group B. Median PFS times were 3.9 months in group A and 3.4 months in group B, respectively. When PFS times were calculated by focusing on the progression of liver disease, median PFS times were 3.9 months in group A and 5.8 months in group B, respectively.
Complications
Most patients had elements of self-limited post-embolization syndrome that lasted for a median of 3 days (range: 1–7 days), including pain, fever, nausea/vomiting, and fatigue as is expected after HAE [18,19]. There was no procedure-related mortality or major complications after HAE.
DISCUSSION
Results of our study show that HAE may provide promising survival results in patients with GIST liver metastasis after the failure of imatinib and sunitinib. Currently, regorafenib is the only drug approved by the Food and Drug Administration (FDA) in the United States, in cases where both imatinib and sunitinib failed or caused severe side effects, but the beneficial impact of regorafenib is limited [20]. In a phase 3 study, median PFS time of patients who received regorafenib after the failure of 1st line imatinib and 2nd line sunitinib was reported to be 4.8 months [21] (Table 3). The median OS and PFS of other new drugs have been reported to be 8.2–11.8 months and 1.8–5.2 months [21–23] (Table 3), respectively. The use of HAE in the treatment of GIST has been previously described however its use in the face of recent chemotherapeutic agents has not been assessed [13]. To our knowledge, this is the first report of HAE in the management of GIST hepatic metastasis progressive while on imatinib and sunitinib. Given the favorable OS and PFS results in this study (median OS time, 23.8 months: median PFS time, 3.4 months), HAE could be an alternative or adjuvant therapeutic option for the treatment of GIST liver metastasis in patients with liver progression, while on first-line imatinib and second-line sunitinib. This of course need to be validated in larger studies..
Table 3.
Literature review of patients survival after 1st line imatinib and 2nd line sunitinib failure
| Author | Year | Patient No | Treatment | RR (RECIST) |
median OS (month) |
median PFS (month) |
|---|---|---|---|---|---|---|
| Demetri et al.20] | 2013 | 133 | Regorafenib | 4.5% | … | 4.8 |
| Italiano et al.21] | 2012 | 40 | Imatinib rechallenge | … | 7.5 | 2.9 |
| Park et al.22] | 2012 | 31 | Sorafenib | 12.9% | 9.7 | 4.9 |
| Italiano et al.21] | 2012 | 55 | Sorafenib | … | 10.7 | 4.9 |
| Italiano et al.21] | 2012 | 67 | Nilotinib | … | 11.8 | 4.1 |
| Reichardt et al.23] | 2012 | 165 | Nilotinib | 0.6% | 11.1 | 4.0 |
| Current study | 8 | HAE | 25.0% | 23.8 | 3.4 |
RR: response rate, RECIST: Response Evaluation Criteria in Solid Tumor, OS: overall survival, PFS: progression free survival, HAE: hepatic artery embolization
In three patients who received HAE as second-line treatment after the failure of first-line imatinib the median OS and PFS times were 14.9 months and 3.9 months, respectively. This is comparable to the previous studies (14.1–25.0 months and 5.3–8.4 months) in which sunitinib was used as second-line treatment after the failure of first-line imatinib [6–9] (Table 4). These results suggest that HAE can be considered even as a second-line treatment, if liver metastases are considered as the determinant of survival, a common scenario in patients with liver only or liver dominant disease.
Table 4.
Literature review of patients survival after 1st line imatinib failure
| Author | Year | Patient No | Treatment | RR (RECIST) |
median OS (month) |
median PFS (month) |
|---|---|---|---|---|---|---|
| Demetri et al.6] | 2012 | 243 | Sunitinib | 6.6% | 17.0 | 5.3 |
| Chen et al.7] | 2011 | 23 | Sunitinib | 26.1% | 14.1 | 8.4 |
| George et al.8] | 2009 | 60 | Sunitinib | 13.3% | 25.0 | 7.9 |
| Demetri et al.9] | 2006 | 207 | Sunitinib | 6.8% | … | 5.6 |
| Current study | 3 | HAE | 33.3% | 14.9 | 3.9 |
RR: response rate, RECIST: Response Evaluation Criteria in Solid Tumor, OS: overall survival, PFS: progression free survival, HAE: hepatic artery embolization
It is interesting that OS of patients who received HAE after both first- and second line treatments (group B) did better than those who received after first-line treatment alone (group A). One explanation is that Group B had better baseline demographic than Group A, including small tumor size and less tumor number, leading to better survival, although no significant differences were observed.
Traditionally, TACE had been the preferred transarterial treatment for unresectable liver metastases of GIST [10–12]. Response rates of TACE for the treatment of GIST liver metastases have been reported to be 14.1%–54.5 % by RECIST [10–12]. GIST is known to be a chemotherapy-resistant tumor, and the effects of chemotherapeutic agents used for TACE are unproven. The antitumor effects of this procedure could be primarily due to the ischemic effect of embolization. Indeed, the response rate in this study was, 27.3% by RECIST and 45.5% by mRECIST, comparable to the results previously reported with TACE [10–12]. No patients developed major complications after HAE in this study, whereas up to 12% of moderate to severe adverse effects had been reported after TACE [10]. Given that therapeutic responses are similar between TACE and HAE, and lack of chemotherapy related adverse events, HAE therapy could be considered as a first-line therapy in the management of GIST liver metastasis.
Our study has some limitations; namely, the small patient number and retrospective nature without any control arm. Despite these limitations, our encouraging results suggest the need for further comparative studies of HAE with standard systemic therapy for GIST liver metastases.
In conclusion, HAE is an effective and well-tolerated therapeutic option for GIST liver metastases. Although larger studies are necessary, our study suggests that HAE may be considered as an alternative or adjuvant to third-line or possibly even second-line systemic treatment and should be the hepatic arterial therapy of choice.
Supplementary Material
Figure 3.
Survival curve after HAE in the treatment of liver metastases of GIST. The cumulative 6-month and 1-year OS rates were, respectively, 100% and 66.7% (95% CI, 5.4–94.5%) in group A, and 100% and 75.0% (95% CI, 31.5–93.1%) in group B. The median OS times were 14.9 months in group A and 23.8 months in group B, respectively. The cumulative 6-month and 1-year PFS rates were, respectively, 33.3% (95% CI, 0.9–77.4%) and 0% in group A, and 37.5% (95% CI, 8.7–67.4%) and 12.5% (95% CI, 0.7–42.3%) in group B. The median PFS times were 3.9 months in group A and 3.4 months in group B, respectively. HAE: hepatic arterial embolization, OS: overall survival, PFS: progression-free survival.
Footnotes
No author has any conflict of interest related to this study.
References
- 1.Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117:289–93. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 2.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–8. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caram MV, Schuetze SM. Advanced or metastatic gastrointestinal stromal tumors: systemic treatment options. J Surg Oncol. 2011;104:888–95. doi: 10.1002/jso.21930. [DOI] [PubMed] [Google Scholar]
- 4.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247–53. doi: 10.1200/JCO.2009.24.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–90. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, Garrett CR, Schöffski P, Shah MH, Verweij J, Leyvraz S, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18:3170–9. doi: 10.1158/1078-0432.CCR-11-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YY, Yeh CN, Cheng CT, Chen TW, Rau KM, Jan YY. Sunitinib for Taiwanese patients with gastrointestinal stromal tumor after imatinib treatment failure or intolerance. World J Gastroenterol. 2011;17:2113–9. doi: 10.3748/wjg.v17.i16.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959–68. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Gupta S, Trent JC, Vauthey JN, Krishnamurthy S, Ensor J, et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer. 2006;107:2833–41. doi: 10.1002/cncr.22336. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi K, Szklaruk J, Trent JC, Ensor J, Ahrar K, Wallace MJ, et al. Hepatic arterial embolization and chemoembolization for imatinib-resistant gastrointestinal stromal tumors. Am J Clin Oncol. 2009;32:574–81. doi: 10.1097/COC.0b013e31819cca35. [DOI] [PubMed] [Google Scholar]
- 12.Cao G, Li J, Shen L, Zhu X. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol. 2012;18:6134–40. doi: 10.3748/wjg.v18.i42.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maluccio MA, Covey AM, Schubert J, Brody LA, Sofocleous CT, Getrajdman GI, DeMatteo R, Brown KT. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer. 2006;1077:1617–23. doi: 10.1002/cncr.22191. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. IARC press; Lyon: 2000. [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:199–202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 18.Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2009;20:S219–S226. doi: 10.1016/j.jvir.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Sofocleous CT, Petre EN, Gonen M, et al. Factors affecting periprocedural morbidity and mortality and long-term patient survival after arterial embolization of hepatic neuroendocrine metastases. J Vasc Interv Radiol. 2014;25:22–30. doi: 10.1016/j.jvir.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. GRID study investigators. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Italiano A, Cioffi A, Coco P, Maki RG, Schöffski P, Rutkowski P, et al. Patterns of care, prognosis, and survival in patients with metastatic gastrointestinal stromal tumors (GIST) refractory to first-line imatinib and second-line sunitinib. Ann Surg Oncol. 2012;19:1551–9. doi: 10.1245/s10434-011-2120-6. [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Ryu MH, Ryoo BY, Im SA, Kwon HC, Lee SS, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30:2377–83. doi: 10.1007/s10637-012-9795-9. [DOI] [PubMed] [Google Scholar]
- 23.Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23:1680–7. doi: 10.1093/annonc/mdr598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.