Abstract
Objectives
Large numbers of elderly patients in the United States receive no treatment for esophageal cancer, despite evidence that multimodality treatment can increase survival. Our goal is to identify factors that may contribute to lack of treatment.
Materials and Methods
Using Surveillance Epidemiology and End Results (SEER)-Medicare Linked Database (2001–2009), we identified regional esophageal cancer patients ≥65 y/o., Treatment was defined as receiving any medical or surgical therapy for esophageal cancer. Logistic regression analysis was performed to identify factors associated with failure to receive treatment. Overall survival (OS) was analyzed using the Kaplan-Meier method and Cox proportional hazard model.
Results
There were 5 072 patients (median age, 75 years; IQR, 71–81 years). Majority were treated with definitive chemoradiation (48.49%). Factors associated with lack of treatment included West geographic region and ≥80 y/o. Patients who received therapy had better OS (log-rank, p < 0.001). Compared with treated patients, non-treated patients had worse adjusted OS (HR, 1.43; 95% CI, 1.33–1.55; p < 0.001).
Conclusions
Elderly patients with locally advanced esophageal cancer who received treatment had improved 5-year survival compared with patients without treatment. Disparities in utilization of treatment are associated with regional and socioeconomic factors, not presence of comorbidities.
Keywords: esophageal cancer, treatment, survival, disparities in care, elderly
INTRODUCTION
Esophageal cancer is the eighth most common cancer worldwide1 with an estimated 16 980 new cases diagnosed in 2015 in the United States alone.2 Despite increased use of multimodality therapies, prognosis for this disease remains dismal with a reported 5-year overall survival (OS) rate of 17.9%. Treatment for esophageal cancer is based on stage at diagnosis. While surgery remains the primary treatment for early-stage cancers, locally advanced disease generally requires multimodality therapy. Well-designed clinical trials have demonstrated that therapeutic protocols optimized for localized and regional stages of this lethal disease can improve patient survival.3 However, despite availability of level 1 evidence to guide the medical and surgical care for these patients, there exists significant inconsistency in delivery of best possible treatments for patients with potentially curable, locally advanced esophageal cancer.
Many factors that lead to disparities in esophageal cancer management have been identified—race, socioeconomic status, type of hospital where diagnosis was made, and geographical variation in delivery of therapy.4–7 With regards to the elderly population specifically, although there are conflicting reports on outcomes after treatment for esophageal cancer, a number of studies have demonstrated that advanced age does not increase risk for mortality after esophagectomy.8, 9 Additionally, there are other studies that have demonstrated that elderly patients can be treated safely with neoadjuvant therapy, despite potentially experiencing an increase in side effects.10, 11 Regardless of this data, there remains a significant bias among healthcare providers against providing “aggressive therapy” to elderly patients who may likely benefit from it.
Although multimodality treatment has been shown to increase survival in this group, a large number of elderly patients in the United States do not receive treatment following diagnosis of esophageal cancer. Since esophageal cancer is most frequently diagnosed in patients between 65 and 74 years of age, we chose to use the Surveillance Epidemiology and End Results (SEER)-Medicare Linked Database to identify the existing national practice patterns for treatment of elderly patients with locally advanced esophageal cancer. Additionally, we sought to examine factors that may contribute to disparities in lack of treatment received by this group of patients.
MATERIALS AND METHODS
Data source and study population
This was a retrospective cohort study using the SEER-Medicare Linked Database from January 1, 2001 to December 31, 2009. The SEER program consists of Medicare beneficiaries diagnosed with cancer and currently collects data on cancer diagnoses and therapies from 18 regional cancer registries; this covers approximately 30% of the U.S. population.12 Our study population consisted of patients diagnosed with esophageal cancer, which included patients diagnosed with gastric cancer of the cardia since these patients followed esophageal treatment guidelines. Our study was limited to patients with histologically-confirmed cancer diagnoses who were continuously enrolled in Medicare Parts A and B in the year prior to diagnosis; diagnoses from death certificate or via autopsy reports were not included in our study. Additionally, we narrowed our focus to patients diagnosed with regionalized adenocarcinoma or squamous cell carcinoma (SCC).
Variables
Treatment was defined as receiving any recommended therapy for esophageal cancer—which includes chemotherapy, radiation, surgery (esophagectomy, gastrectomy, or excision/destruction), or a combination of one or more of these interventions. We considered and evaluated 18 possible treatment paths (Appendix 1). Patients who received treatment were compared with those who received no treatment on baseline demographic and clinical characteristics. Demographic characteristics included year of diagnosis, SEER-region (grouped into 4 geographic regions: Northeast [NE], Midwest [MW], South [S], and West [W]; Figure 1), age, sex, race (white, black, or other [Asian, Hispanic, or Native American]), marital status (single, married, divorced, or widowed), median household income, and at least 12 years of education (defined using United States Census Bureau zip code data and categorized into quartiles). Income and education were used as a proxy for socioeconomic status. Clinical characteristics consisted of Charlson Comorbidity Index (Deyo adaptation excluding malignancy),13 diagnosis of Barrett’s esophagus (International Classification of Diseases, 9th edition14 [ICD-9] code 530.85), histology (ICD-Oncology [ICD-O], 3rd edition, codes for adenocarcinoma or SCC), tumor location (ICD-O code for lower third/cardia/abdominal, esophagus NOS, overlapping, upper third/cervical, or middle third/thoracic) and differentiation (well/moderate, poor/undifferentiated, or unknown).
Figure 1.
Census regions and divisions of the United States.
(https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf)
Statistical analysis
Baseline patient demographics and clinical factors were compared between the treatment and no-treatment groups using Student’s t-test for continuous variables and Pearson’s Chi-square test for categorical variables. Multivariable logistic regression analysis was performed to identify factors associated with lack of treatment among elderly esophageal cancer patients. First, exploratory data analysis was performed using univariate logistic regression. The initial model included all covariates with associations in exploratory analysis (p < 0.25), as recommended by Hosmer and Lemeshow.15 Additionally, all covariates of clinical importance were included, regardless of statistical significance. The model was then refined based on clinical importance of covariates and their impact on overall fit, as assessed by likelihood ratio tests. As a result, the final logistic regression model for lack of treatment was adjusted for the following covariates: year of diagnosis, SEER region, age, sex, race, marital status, median household income, at least 12 years of education, Charlson comorbidity index, diagnosis of Barrett’s esophagus, histology, and differentiation. Overall survival was defined as time (in months) from diagnosis to death or last follow-up date. Survival analysis was performed using the Kaplan-Meier method comparing survival curves with the log-rank test. Multivariable analysis was performed to examine the association between treatment and OS while adjusting for other covariates using the Cox proportional hazard model. Statistical significance was indicated by p-value <0.05. All data analyses and management were performed using Stata/MP version 14 (StataCorp LP, College Station, TX, USA).
RESULTS
A total of 5 072 regionalized esophageal cancer patients met our study inclusion criteria. Treatment among these patients was fairly underutilized as 1 762 (34.74%) patients received no treatment of any kind. Among those receiving treatment, the first choice was definitive chemoradiation (48.49%), followed by surgery (16.13%), radiation alone (12.78%), chemotherapy alone (11.9%), surgery + adjuvant (5.32%), and induction + surgery +/− adjuvant (5.38%) (Figure 2). A higher than expected number of patients had not undergone surgery for the following reasons: not recommended (73.36%), contraindicated (9.91%), refused (4.78%), death before treatment (0.68%), and unknown (11.28%). Median age was 75 years (Interquartile range [IQR], 71–78 years), the vast majority of patients were white (90.65%) and male (79.26%), and more than half of patients were married (66.82%) (Table 1). Most of tumors were located in the lower third/cardia/abdominal site (86.87%) and only 8.87% of patients were diagnosed with SCC. Patients who left their cancer untreated tended to be older (median, 77 years of age vs 75 years of age; p < 0.001), female (23.84% vs 19.09%; p < 0.001), unmarried, non-white, and more likely to reside in the West region of the United States (63.39% vs 43.32%, p < 0.001). Additionally, patients who did not receive treatment had lower median income and lower education. However, there were no significant differences observed in histology, tumor location, and differentiation between both groups of patients.
Figure 2.
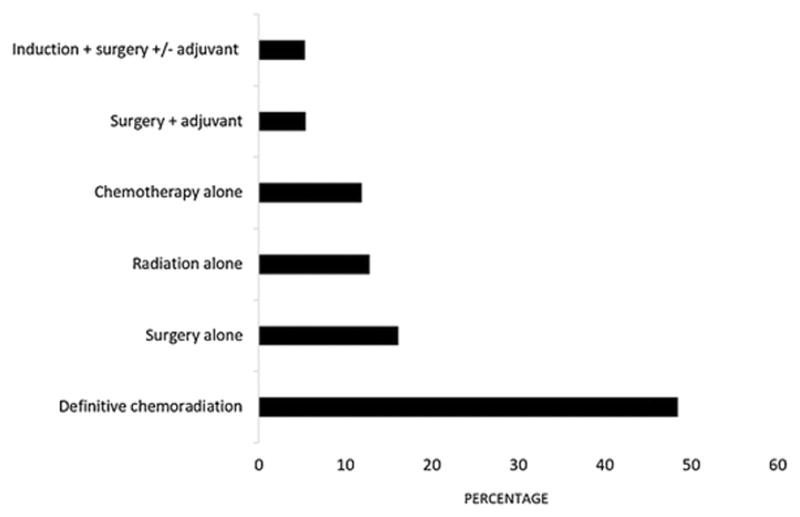
Treatment groups for elderly patients diagnosed with regional esophageal cancer.
TABLE 1.
Demographic and clinical characteristics of older patients diagnosed with regional esophageal cancer, SEER-MEDICARE 2001–2009
| Characteristic | Total N = 5 072 |
No Treatment 1 762 (34.74) |
Treatment 3 310 (65.26) |
p |
|---|---|---|---|---|
| Year, n (%) | <0.001 | |||
| 2001–2003 | 1556 (30.68) | 621 (35.24) | 935 (28.25) | |
| 2004–2006 | 1723 (33.97) | 569 (32.29) | 1154 (34.86) | |
| 2007–2009 | 1793 (35.35) | 572 (32.46) | 1221 (36.89) | |
| SEER region, n (%) | <0.001 | |||
| NE | 1071 (21.12) | 256 (14.53) | 815 (25.33) | |
| MW | 611 (12.05) | 130 (7.38) | 481 (14.53) | |
| S | 839 (16.54) | 259 (14.7) | 580 (17.52) | |
| W | 2551 (50.3) | 1117 (63.39) | 1434 (43.32) | |
| Age, y, n (%) | <0.001 | |||
| 65–69 | 920 (18.14) | 246 (13.96) | 674 (20.36) | |
| 70–74 | 1372 (27.05) | 421 (23.89) | 951 (28.73) | |
| 75–79 | 1325 (26.12) | 465 (26.39) | 860 (25.98) | |
| ≥80 | 1455 (28.69) | 630 (35.75) | 825 (24.92) | |
| Sex, n (%) | <0.001 | |||
| Female | 1052 (20.74) | 420 (23.84) | 632 (19.09) | |
| Male | 4020 (79.26) | 1342 (76.14) | 2678 (80.91) | |
| Race, n (%) | 0.008 | |||
| White | 4598 (90.65) | 1567 (88.93) | 3031 (91.57) | |
| Black | 210 (4.14) | 84 (4.77) | 126 (3.81) | |
| Othera | 264 (5.21) | 111 (6.3) | 153 (4.62) | |
| Marital statusb, n (%) | <0.001 | |||
| Single (never married) | 328 (6.68) | 125 (7.37) | 203 (6.32) | |
| Married | 3280 (66.82) | 1048 (61.6) | 2232 (69.49) | |
| Divorced | 354 (7.21) | 132 (7.78) | 222 (6.91) | |
| Widowed | 947 (19.29) | 392 (23.1) | 555 (17.28) | |
| Median household incomec, n (%) | 0.025 | |||
| Q1 | 1262 (24.88) | 456 (25.88) | 806 (24.35) | |
| Q2 | 1274 (25.12) | 460 (26.11) | 814 (24.59) | |
| Q3 | 1265 (24.94) | 449 (25.48) | 816 (24.65) | |
| Q4 | 1271 (25.06) | 397 (22.53) | 874 (26.4) | |
| At least 12 y of educationc, n (%) | <0.001 | |||
| Q1 | 1199 (25.00) | 485 (28.96) | 714 (22.88) | |
| Q2 | 1194 (24.9) | 446 (26.63) | 748 (23.97) | |
| Q3 | 1204 (25.1) | 373 (22.27) | 831 (26.46) | |
| Q4 | 1199 (25.0) | 371 (22.15) | 828 (26.53) | |
| Charlson comorbidity index, n (%) | <0.001 | |||
| 0 | 3107 (61.26) | 1309 (74.29) | 1798 (54.32) | |
| 1 | 1092 (21.53) | 241 (13.68) | 851 (25.71) | |
| ≥2 | 873 (17.21) | 212 (12.03) | 661 (19.97) | |
| Barrett’s Esophagus, n (%) | 835 (16.46) | 85 (4.82) | 750 (22.66) | <0.001 |
| Histology, n (%) | 0.053 | |||
| Adenocarcinoma | 4622 (91.13) | 1587 (90.07) | 3035 (91.69) | |
| Squamous cell carcinoma | 450 (8.87) | 175 (9.93) | 275 (8.31) | |
| Tumor location, n (%) | 0.305 | |||
| Lower third/Cardia NOS/Abdominal | 4406 (86.87) | 1508 (85.58) | 2898 (87.55) | |
| Esophagus NOS | 148 (2.92) | 54 (3.06) | 94 (2.84) | |
| Overlapping | 112 (2.21) | 45 (2.55) | 67 (2.02) | |
| Upper Third/Cervical | 105 (2.07) | 37 (2.10) | 68 (2.05) | |
| Middle Third/Thoracic | 301 (5.93) | 118 (6.70) | 183 (5.53) | |
| Differentiation, n (%) | 0.067 | |||
| Well/moderate | 1694 (33.4) | 552 (31.33) | 1142 (34.5) | |
| Poor/undifferentiated | 2753 (54.28) | 991 (56.24) | 1762 (53.23) | |
| Unknown | 625 (12.32) | 219 (12.43) | 406 (12.27) | |
| Distance to nearest NCI, n (%) | <0.001 | |||
| <25 miles | 2467 (48.96) | 932 (53.04) | 1535 (46.77) | |
| 25–49.9 miles | 1028 (20.4) | 349 (19.86) | 679 (20.69) | |
| ≥50 miles | 1544 (30.64) | 476 (27.09) | 1068 (32.54) |
SEER, Surveillance, Epidemiology, and End Results; IQR, Interquartile Range; NCI, National Cancer Institute.
Asian, Hispanic and Native American
Unknown: 388 patients.
Defined using Census zip code data and categorized into quartiles.
Several factors, such as SEER region, increased age, marital status, education, and diagnosis of Barrett’s esophagus, remained significant in the adjusted logistic regression model that we used to identify factors associated with lack of treatment for esophageal cancer for elderly patients. For example, while administration of treatment varied across all SEER regions, patients from the South and West regions were more likely to not be treated compared with those in the Northeast region (NE: ref; S: OR = 1.49, 95% CI = 1.17–1.9, p < 0.001; W: OR = 2.59, 2.14–3.13, p < 0.001) (Table 2). Not surprisingly, the odds of not receiving treatment increased with age (65–69 years: ref; 75–79 years: OR = 1.56, 1.27–1.93, p < 0.001; ≥80 years: OR = 2.3, 1.86–2.84, p < 0.001) and lower education quartile (Q4: ref; Q1: OR = 1.73, 1.34–2.23, p < 0.001; Q2: OR = 1.59, 1.27–2.0, p < 0.001). The odds for lack of treatment also increased for widows (Married: ref; Widowed: OR = 1.26, 0.05–1.51, p = 0.014). Conversely, patients diagnosed with Barrett’s esophagus had decreased odds of leaving their esophageal cancer untreated (OR = 0.19, 0.14–0.24, p < 0.001). Of all, the factors associated most strongly with lack of treatment were West geographic region and ≥80 years of age.
TABLE 2.
Factors associated with lack of treatment for older patients diagnosed with regional esophageal cancer, SEER-MEDICARE 2001–2009
| Factor | OR (95% CI) | p |
|---|---|---|
| Year | ||
| 2007–2009 | Reference | |
| 2001–2003 | 1.17 (0.99–1.37) | 0.063 |
| 2004–2006 | 1.02 (0.87–1.2) | 0.777 |
| SEER region | ||
| NE | Reference | |
| MW | 0.92 (0.7–1.21) | 0.55 |
| S | 1.49 (1.17–1.9) | 0.001 |
| W | 2.59 (2.14–3.13) | <0.001 |
| Age, y | ||
| 65–69 | Reference | |
| 70–74 | 1.18 (0.95–1.45) | 0.131 |
| 75–79 | 1.56 (1.27–1.93) | <0.001 |
| ≥80 | 2.30 (1.86–2.84) | <0.001 |
| Sex | ||
| Female | Reference | |
| Male | 0.92 (0.77–1.09) | 0.324 |
| Race | ||
| White | Reference | |
| Black | 1.27 (0.9–1.78) | 0.168 |
| Othera | 0.88 (0.66–1.17) | 0.377 |
| Marital statusb | ||
| Married | Reference | |
| Single (never married) | 1.24 (0.96–1.62) | 0.105 |
| Divorced | 1.25 (0.96–1.62) | 0.102 |
| Widowed | 1.26 (1.05–1.51) | 0.014 |
| Median household incomec | ||
| Q4 | Reference | |
| Q3 | 1.05 (0.85–1.31) | 0.634 |
| Q2 | 0.87 (0.68–1.11) | 0.259 |
| Q1 | 0.84 (0.64–1.11) | 0.218 |
| At least 12 y of educationc | ||
| Q4 | Reference | |
| Q3 | 1.19 (0.97–1.46) | 0.096 |
| Q2 | 1.59 (1.27–2.00) | <0.001 |
| Q1 | 1.73 (1.34–2.23) | <0.001 |
| Charlson comorbidity index | ||
| 0 | Reference | |
| 1 | 0.37 (0.31–0.44) | <0.001 |
| ≥2 | 0.4 (0.33–0.49) | <0.001 |
| Barrett’s Esophagus | 0.19 (0.14–0.24) | <0.001 |
| Histology | ||
| Adenocarcinoma | Reference | 0.398 |
| Squamous cell carcinoma | 0.9 (0.72–1.14) | |
| Differentiation | ||
| Well/moderate | Reference | |
| Poor/undifferentiated | 1.06 (0.92–1.22) | 0.453 |
| Unknown | 1.04 (0.83–1.3) | 0.722 |
SEER, Surveillance, Epidemiology, and End Results; OR, Odd Ratio; CI, Confidence Interval.
Asian, Hispanic, and Native American.
Unknown: 388 patients.
Defined using United States Census Bureau zip code data and categorized into quartiles.
Elderly patients diagnosed with esophageal cancer who had received any type of recommended treatment had significantly better 5-year OS compared with patients who did not receive any treatment (log-rank, p < 0.001) (Figure 3). The 5-year OS was 13.79% (95% CI, 12.68–14.94%), while 5-year OS stratified by receipt of treatment was 14.92% (95% CI, 13.55–16.36%) for treated patients and 11.07% (95% CI, 9.3–13.01%) for untreated patients. Median survival time is listed in Table 3. In both unadjusted and adjusted Cox proportional hazard regression analyses, lack of treatment was associated with worse OS compared with receiving treatment (adjusted HR = 1.43, 95% CI 1.33–1.55, p < 0.001) (Table 4).
Figure 3.
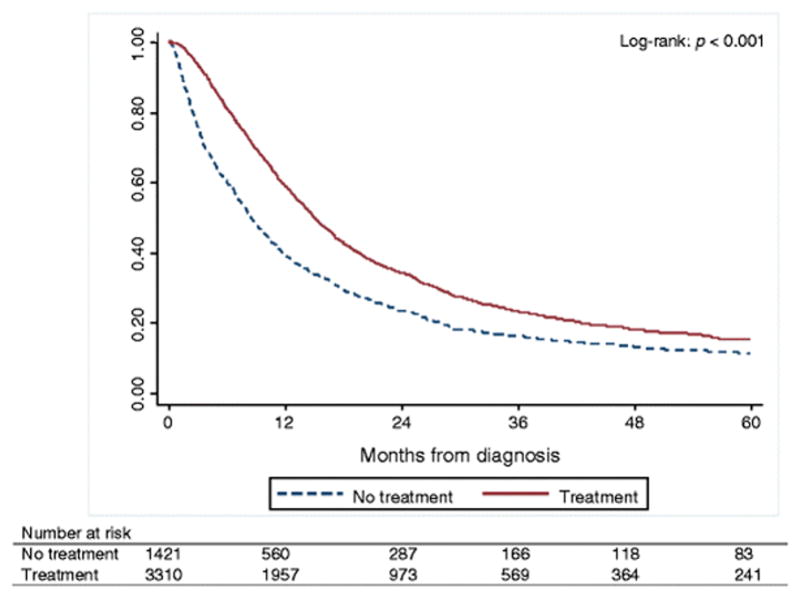
Kaplan-Meier curves of 5-year overall survival rates for older, regional esophageal cancer patients who have received treatment compared with those who have not received treatment.
*341 patients were excluded from survival analysis due to no reliable follow-up on them.
TABLE 3.
Overall survival for older patients diagnosed with regional esophageal cancer, SEER-MEDICARE 2001–2009
| Survival type | Median survival (IQR), months |
|---|---|
| Overall survival | 13.15 (5.98–29.26) |
| Treatment group | 14.99 (7.63–32.91) |
| No treatment group | 8.48 (3.19–22.06) |
IQR, Interquartile range.
TABLE 4.
Overall survival for older patients diagnosed with regional esophageal cancer, SEER-MEDICARE 2001–2009
| Survival type | Unadjusted HR (95% CI) | p | Adjusted HR1 (95% CI) | p |
|---|---|---|---|---|
| Treatment group | Reference | Reference | ||
| No treatment group | 1.47 (1.37–1.57) | <0.001 | 1.43 (1.33–1.55) | <0.001 |
HR, hazard ratio; CI, confidence interval.
Adjusted for year of diagnosis, SEER region, age, sex, race, marital status, income, education, Charlson comorbidity index, Barrett’s esophagus, histology, tumor location, and grade.
DISCUSSION
Trimodality therapy, which is considered standard of care for patients diagnosed with regional esophageal cancer, was used with very few (<6%) patients in our study; in fact, nearly 35% of patients with potentially curable esophageal cancer received no treatment for their disease. Regional and socioeconomic disparities, increasing age, and marital and educational status were associated with lack of treatment. It is important to note that patients who had undergone treatment—even treatments with no curative intent—had improved overall and cancer-specific 5-year survival rates.
Our results are supported by previous findings and illustrate a general national trend in the management of older patients affected by this very aggressive cancer. Paulson et al. reported that esophagectomy is underused significantly in the United States, with only 34% of the 2 386 SEER-Medicare patients with stage I, II, and III disease having undergone esophagectomies in their study.16 As in our current study, Paulson and colleagues found the racial and socioeconomic disparities were associated with lack of treatment—only 19% of non-white patients and 28% of patients living in areas with higher poverty rates had undergone surgery. These factors may be associated with decreased access to specialized treatment centers, thereby resulting in under-utilization of surgical therapy. In a study that compared national SEER registry data to both regional data from the Healthcare Association of New York State registry and single-institution data from the University of Rochester Medical Center (URMC), which specializes in the treatment of esophageal cancer,17 a significantly higher percentage of patients with esophageal cancer had undergone resection at URMC (68%) compared with the state of New York (42%) and in the United States in general (36%). When looking only at stage I–III disease, rate of resection at URMC was 88% compared with 44% in SEER registry patients.
Underutilization of surgery may be related to high rates of mortality and morbidity that have been reported with esophagectomies. This is particularly relevant in elderly patients who may have limited life expectancy. Herein, we have demonstrated that neoadjuvant concurrent chemoradiotherapy followed by surgery is rarely administered to patients with locally advanced disease, despite the knowledge that trimodality treatment improves survival of these patients.18 Instead, we have found that the most common type of treatment was definitive chemoradiation. One factor that has potentially influenced treatment decisions may be existence of 2 randomized trials that showed no improvement with the addition of surgery after chemoradiation.19, 20 However, it is important to note that these trials showed very high perioperative mortality (10%), which was much higher than the mortality rates (0.5–4.0%) reported recently by highly specialized and high-volume centers.21–25 Taking into account these important discrepancies, the 2007 National Comprehensive Cancer Network (NCCN) esophageal cancer guidelines state that surgical mortality was too high in these trials, thus making it difficult to compare survival using trimodality therapy versus definitive chemoradiation.26 Concerns about side effects may also influence the decision to limit treatment for elderly patients, although several recent studies have shown that multimodality therapy can be tolerated by elderly patients. For example, Sylvie et al. reported recently that in their study they evaluated 174 patients ≥70 years of age who had undergone neoadjuvant chemotherapy followed by surgery and compared their outcomes with those of patients <70 years of age. They found that elderly patients had pathological complete remission and survival rates comparable with younger patients, even when less intensive chemotherapy regimens were required. Postoperative mortality and morbidity was also comparable among the younger and older groups, thus suggesting that age alone is not associated with worse outcome after multimodality therapy. In fact, advances in perioperative care, centralization of care to high-volume centers, and introduction of minimally invasive techniques have led to a significant decrease in postoperative morbidity after esophagectomy, which makes this option a valid possibility, even for elderly patients.27–30 Furthermore, the introduction of newer, less toxic induction chemotherapy regimens and safer radiotherapy delivery methods18 have been shown to be better tolerated and not associated with increased postoperative morbidity.31
It is puzzling that such a large number of patients in our study received no treatment of any kind. We believe that this lack of treatment could be attributed to a nihilistic attitude towards esophageal cancer, patient misinformation regarding modern treatment options, and lack of physician expertise—these are all factors that can be associated with levels of income and education. In fact, several studies have shown that socioeconomic status is closely associated in determining treatment options for cancer patients.32,33 Patients of low socioeconomic status are more likely to have more advanced tumors at time of diagnosis, more comorbidities, and to be less educated about cancer and treatment options. Even in countries where healthcare is equally accessible to the entire population, low socioeconomic status has been shown to be associated with lower probabilities for curative treatments and lower survival rates.6 Although previous studies have shown that hospital type at time of diagnosis influences treatment type, we did not find any association between proximity to a National Cancer Institute-designated cancer center and treatment allocation; however, we did find regional variations in treatment patterns. These variations may reflect differences in treatment practices amongst physicians, as well as patient break beliefs regarding aggressiveness of care and access to different resources. The treatment rate seems to have increased over time, thereby suggesting possibly an improvement in access to care and awareness among patients and physicians. Continued efforts to further educate health care providers and patients regarding overall improved outcomes with this disease as well as enhanced understanding of all treatment options available is critical to change the traditionally hopeless view brought on by a diagnosis of esophageal cancer.
Our study does have several limitations. First, Medicare is an administrative billing dataset. It has been well documented that claims-based databases, such as Medicare, are constructed primarily for reimbursement rather than research purposes and are susceptible to errors due to missing or inaccurately entered codes. However, given that coding errors and missing data should be randomly distributed across all categories, this would seem unlikely to alter the validity of our findings. A second limitation associated with use of administrative data is potential confounding by treatment indication. Although we tried controlling for regionalized stage, we used the Summary Staging classification rather than the American Joint Committee on Cancer classification since the latter was not reliably applied prior to 2004. Summary Staging is a single digit system with only 8 categories: in situ, local, regional to lymph nodes, regional by direct extension, both regional lymph nodes and regional extension, regional not otherwise specified, distant, and unknown. Although less detailed, this method of staging has shown longitudinal stability for population-based cancer registries and has been used in numerous studies. Clinical stage is also not as reliable as pathological stage and patients may have been under-staged or over-staged in our study, which may have potentially biased our results. Additionally, since we created so many different treatment groups, we were unable to verify if individual patients may have had incomplete treatment.
A final limitation of our study was the use of a study cohort that was restricted to patients >65 years of age; this could have potentially limited the external validity of our results across the entire age range of esophageal cancer patients. However, we chose to use the SEER-Medicare Linked Database since esophageal cancer is a disease that is predominant in the elderly population (median age, 67 years), thus making this an ideal source of data that captures a sample that is representative of the majority of affected patients in the United States. Additionally, we noted that these data have been used extensively to study many other cancers, with results that are generalizable to younger patients.
CONCLUSION
In conclusion, a distressingly small percentage of patients in our study received accurate treatment for potentially curable locally advanced esophageal cancer. Predictors of no treatment for esophageal patients included region, socioeconomic status, and age. Treatment was associated with higher overall and cancer-specific survival rates. Better awareness of esophageal cancer and its treatment options for physicians and patients, improved access to care, and recognition of socioeconomic barriers may improve survival rates for patients diagnosed with this deadly disease.
Acknowledgments
The authors thank Daren Jackson, PhD for his contribution in editing the paper.
Financial support: Mr. Edwin Lewis provided generous support for Dr. Lidor’s Johns Hopkins Department of Surgery Research Fund.
APPENDIX. TREATMENT GROUPS
| Surgery | Surgery only | Surgery followed by adjuvant therapy (no chemotherapy and/or radiation in 90 days before surgery, chemotherapy and/or radiation in 90 days after surgery) | ||||
| Radiation | Radiation only | Subsequent chemotherapy/radiation (chemotherapy starting within 90 days of starting radiation, no surgery) | Radiation induction alone followed by surgery (radiation in 90 days before surgery, no treatment after surgery) | Radiation induction alone followed by surgery and then by adjuvant therapy (radiation in 90 days before surgery, chemotherapy and/or radiation in 90 days after surgery) | Subsequent chemotherapy/radiation followed by surgery (chemotherapy within 90 days of starting radiation, surgery within 90 days of starting radiation, no treatment after surgery) | Subsequent chemotherapy/radiation followed by surgery and then by adjuvant therapy (chemotherapy within 90 days of starting radiation, surgery within 90 days of starting radiation, chemotherapy and/or radiation within 90 days of surgery) |
| Chemotherapy | Chemotherapy only | Subsequent chemotherapy/radiation (radiation starting within 90 days of starting chemotherapy, no surgery) | Chemotherapy induction alone followed by surgery (Chemotherapy in 90 days before surgery, no treatment after surgery) | Chemotherapy induction alone followed by surgery and then by adjuvant therapy (Chemotherapy in 90 days before surgery, chemotherapy and/or radiation in 90 days after surgery) | Subsequent chemotherapy/radiation followed by surgery (radiation within 90 days of starting chemotherapy, surgery within 90 days of starting chemotherapy, no treatment after surgery) | Subsequent chemotherapy/radiation followed by surgery and then by adjuvant therapy (radiation within 90 days of starting chemotherapy, surgery within 90 days of starting chemotherapy, chemotherapy and/or radiation within 90 days of surgery) |
| Concurrent Chemoradiation | Concurrent chemoradiation and no surgery within 90 days of starting chemotherapy/radiation | Concurrent chemoradiation and no surgery within 90 days of starting chemotherapy/radiation | Concurrent chemoradiation followed by surgery within 90 days of starting chemotherapy/rad, nothing after surgery | Concurrent chemoradiation followed by surgery and then by adjuvant therapy (surgery within 90 days of starting chemotherapy/radiation and chemotherapy/radiation within 90 days of surgery) |
Footnotes
Author contributions:
Conception or design of the work; or the acquisition, analysis, or interpretation of data: DM, MS, ALB, AOL
Drafting the work or revisiting it critically for important intellectual content: DM, MS, ALB, AOL
Final approval of the version to be published: DM, MS, ALB, AOL
Statement: We used the SEER-Medicare Linked Database in our study. Interpretation and reporting of these data was the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in creating the SEER-Medicare Linked Database.
Disclosures: The authors of this study have no relevant conflicts of interest to disclose.
Meeting presentation: Plenary presentation at 2016 Digestive Disease Week (May 21–24, 2016) at the San Diego Convention Center in San Diego, CA.
References
- 1.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. [2016, January 23];SEER Stat Fact Sheets: Esophageal Cancer, [online] 2013 Available: http://seer.cancer.gov/statfacts/html/esoph.html.
- 3.van Heijl M, van Lanschot JJ, Koppert LB, van Berge Henegouwen MI, Muller K, Steyerberg EW, van Dekken H, Wijnhoven BPL, Tilanus HW, Richel DJ, Busch ORC, Bartelsman JF, Koning CCE, Offerhaus GJ, van der Gaast A. Neoadjuvant chemoradiation followed by surgery versus surgery alone for patients with adenocarcinoma or squamous cell carcinoma of the esophagus (CROSS) BMC Surg. 2008;8:21. doi: 10.1186/1471-2482-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeter M, van Steenbergen LN, Lemmens VE, Rutten HJ, Roukemja JA, Wijnhoven BP, Nieuwenhuijzen GA. Hospital of diagnosis and probability to receive a curative treatment for oesophageal cancer. Eur J Surg Oncol. 2014;40:1338–1345. doi: 10.1016/j.ejso.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Revels SL, Morris AM, Reddy RM, Akateh C, Wong SL. Racial disparities in esophageal cancer outcomes. Ann Surg Oncol. 2013;20:1136–1141. doi: 10.1245/s10434-012-2807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bus P, Aarts MJ, Lemmens VE, van Oijen MG, Creemers GJ, Nieuwenhuijzen GA, van Baal JW, Siersema PD. The effect of socioeconomic status on staging and treatment decisions in esophageal cancer. J Clin Gastroenterol. 2012;46:833–839. doi: 10.1097/MCG.0b013e31824e8ff8. [DOI] [PubMed] [Google Scholar]
- 7.Taioli E, Wolf AS, Camacho-Rivera M, Kaufman A, Lee DS, Bhora F, Flores RM. Racial disparities in esophageal cancer survival after surgery. J Surg Oncol. 2016;113:659–664. doi: 10.1002/jso.24203. [DOI] [PubMed] [Google Scholar]
- 8.Markar SR, Low DE. Physiology, not chronology, dictates outcomes after esophagectomy for esophageal cancer: outcomes in patients 80 years and older. Ann Surg Oncol. 2013;20:1020–1026. doi: 10.1245/s10434-012-2703-x. [DOI] [PubMed] [Google Scholar]
- 9.Pultrum BB, Bosch DJ, Nijsten MW, Rodgers MG, Groen H, Slaets JP, Plukker JT. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol. 2010;17:1572–1580. doi: 10.1245/s10434-010-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylvie L, Silvia S, Salah-Eddin AB, Markus F, Florian L, Peter TP, Bernhard H, Martin A, Alexander N. Impact of age on the feasibility and efficacy of neoadjuvant chemotherapy in patients with locally advanced oesophagogastric cancer. Eur J Cancer. 2015;51:1918–1926. doi: 10.1016/j.ejca.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzen S, Pauligk C, Homann N, Schmalenberg H, Jäger E, Al-Batran SE. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br J Cancer. 2013;108:519–526. doi: 10.1038/bjc.2012.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute. [2016, January 23];Overview of the SEER Program, [online] 2013 Available: http:\\seer.cancer.gov/about/overview.html.
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Medicode (Firm) ICD-9-CM: International classification of diseases, 9th revision, clinical modification. Salt Lake City, Utah: Medicode; 1996. [Google Scholar]
- 15.Hosmer D, Lemeshow S. Applied Logistic Regression. 2. New York: Wiley; 2000. [Google Scholar]
- 16.Paulson EC, Ra J, Armstrong K, Wirtalla C, Spitz F, Kelz RR. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143:1198–1203. doi: 10.1001/archsurg.143.12.1198. [DOI] [PubMed] [Google Scholar]
- 17.Dubecz A, Sepesi B, Salvador R, Polomsky M, Watson TJ, Raymond DP, Jones CE, Litle VR, Wisnivesky JP, Peters JH. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg. 2010;211:754–761. doi: 10.1016/j.jamcollsurg.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 18.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 19.Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binguet C. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 20.Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, Klump B, Budach W, Teichmann R, Schmitt M, Schmitt G, Franke C, Wilke H. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 21.Portale G, Hagen JA, Peters JH, Chan LS, DeMeester SR, Gandamihardja TA, DeMeester TR. Modern 5-year survival of resectable esophageal adenocarcinoma: single institution experience with 263 patients. J Am Coll Surg. 2006;202:588–596. doi: 10.1016/j.jamcollsurg.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 23.Rentz J, Bull D, Harpole D, Bailey S, Neumayer L, Pappas T, Krasnicka B, Henderson W, Daley J, Khuri S. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114–1120. doi: 10.1067/mtc.2003.315. [DOI] [PubMed] [Google Scholar]
- 24.Berry MF, Atkins BZ, Tong BC, Harpole DH, D’Amico TA, Onaitis MW. A comprehensive evaluation for aspiration after esophagectomy reduces the incidence of postoperative pneumonia. J Thorac Cardiovasc Surg. 2010;140:1266–1271. doi: 10.1016/j.jtcvs.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363–372. doi: 10.1097/SLA.0b013e31814697f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. [2016, January 23];NCCN Guidelines for Treatment of Cancer by Site: Esophageal Cancer, [online] 2007 Available: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 27.Markar SR, Bodnar A, Rosales J, Song G, Low DE. The impact of neoadjuvant chemoradiotherapy on perioperative outcomes, tumor pathology, and survival in clinical stage II and III esophageal cancer. Ann Surg Oncol. 2013;20:3935–3941. doi: 10.1245/s10434-013-3137-9. [DOI] [PubMed] [Google Scholar]
- 28.Kinugasa S, Tachibana M, Yoshimura H, Dhar DK, Shibakita M, Ohno S, Kubota H, Masunaga R, Nagasue N. Esophageal resection in elderly esophageal carcinoma patients: improvement in postoperative complications. Ann Thorac Surg. 2001;71:414–418. doi: 10.1016/s0003-4975(00)02333-x. [DOI] [PubMed] [Google Scholar]
- 29.Ellis FH, Jr, Williamson WA, Heatley GJ. Cancer of the esophagus and cardia: does age influence treatment selection and surgical outcomes? J Am Coll Surg. 1998;187:345–351. doi: 10.1016/s1072-7515(98)00195-1. [DOI] [PubMed] [Google Scholar]
- 30.Ruol A, Portale G, Zaninotto G, Cagol M, Cavallin F, Castoro C, Sileni VC, Alfieri R, Rampado S, Ancona E. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg. 2007;133:1186–1192. doi: 10.1016/j.jtcvs.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 31.Molena D, Mungo B, Stem M, Lidor A. Does neo-adjuvant therapy for esophageal cancer increase postoperative morbidity or mortality? Dis Esophagus. 2015;28(7):644–51. doi: 10.1111/dote.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung MC, Koniaris LG, Yang R, Zhuge Y, Mackinnon JA, Byrne MM, Franceschi D. Do all patients with carcinoma of the esophagus benefit from treatment at teaching facilities? J Surg Oncol. 2010;102:18–26. doi: 10.1002/jso.21509. [DOI] [PubMed] [Google Scholar]
- 33.van Vliet EP, Eijkemans MJ, Steyerberg EW, Kuipers EJ, Tilanus HW, van der Gaast A, Siersema PD. The role of socio-economic status in the decision making on diagnosis and treatment of oesophageal cancer in The Netherlands. Br J Cancer. 2006;95:1180–1185. doi: 10.1038/sj.bjc.6603374. [DOI] [PMC free article] [PubMed] [Google Scholar]


