Abstract
P22phox is a ubiquitous protein encoded by the CYBA gene located on the long arm of chromosome 16 at position 24, containing six exons and spanning 8.5 kb. P22phox is a critical component of the superoxide-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs). It is associated with NOX2 to form cytochrome b558 expressed mainly in phagocytes and responsible for the killing of microorganisms when bacterial and fungal infections occur. CYBA mutations lead to one of the autosomal recessive forms of chronic granulomatous disease (AR220CGD) clinically characterized by recurrent and severe infections in early childhood. However, p22phox is also the partner of NOX1, NOX3 and NOX4, but not NOX5, which are analogs of NOX2, the first identified member of the NOX family. P22phox–NOX complexes have emerged as one of the most relevant sources of reactive oxygen species (ROS) in tissues and cells, and are associated with several diseases such as cardiovascular and cerebrovascular diseases. The p22phox-deficient mouse strain nmf333 has made it possible to highlight the role of p22phox in the control of inner ear balance in association with NOX3. However, the relevance of p22phox for NOX3 function remains uncertain because AR220CGD patients do not suffer from vestibular dysfunction. Finally, a large number of genetic variations of CYBA have been reported, among them the C242T polymorphism, which has been extensively studied in association with coronary artery and heart diseases, but conflicting results continue to be reported.
Graphical abstract
1. Introduction
The p22phox protein was identified first in 1987 during the purification of cytochrome b−245mv from human neutrophil (Parkos et al., 1987). A few years before, this low-potential cytochrome b, also called cytochrome b558 because of its spectral properties, was demonstrated as the major component of the microbicidal nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex in phagocytes (Segal et al., 1981) (Harper et al., 1984). Cytochrome b558, the redox element of the NADPH oxidase complex, is a membrane heterodimer composed of two subunits: p22phox (also called the alpha subunit or the light chain of cytochrome b558) and gp91phox (renamed NOX2 in the 2000s), also referred to as the beta or heavy chain or large subunit. The significance of the NADPH oxydase system in the killing of ingested pathogens during phagocytosis is evidenced by chronic granulomatous disease (CGD). This disease, caused by a defective oxidase complex in phagocytes, is characterized by recurrent and life-threatening infections, most often in childhood (van den Berg et al., 2009). The absence of p22phox in the granulocytes of patients suffering from X-linked CGD, the major form of CGD caused by mutation in CYBB encoding NOX2, highlighted its functional importance (Dinauer et al., 1987; Teahan et al., 1987; Parkos et al., 1989). It was shown that p22phox was closely associated with NOX2 and played an important structural role in the cytochrome b558 synthesis process because the two subunits stabilized each other (Yu et al., 1997). Finally, the structure of CYBA, the p22phox-encoding gene, its chromosomal location and its involvement in one of the autosomal recessive forms of CGD (AR220CGD) were reported in 1990 (Bu-Ghanim et al., 1990; Dinauer et al., 1990). During the 2000s the discovery of NOX2 analogs emphasized the role of p22phox. In the last decade, the NADPH oxidase (NOX enzymes) family has emerged as one of the most relevant sources of reactive oxygen species (ROS) in tissues and cells (Sumimoto, 2008; Lambeth and Neish, 2014). Today, the central role played by NOXs (especially NOX1, NOX2 and NOX4) in cardiovascular diseases, such as hypertension, diabetes, renal disease, heart failure, atherosclerosis and cerebrovascular diseases, is widely recognized (Bedard and Krause, 2007; Krause et al., 2012). This family comprises five members – NOX1, NOX2, NOX3, NOX4 and NOX5 membrane proteins – which, except for NOX5, have p22phox as a membrane partner. The pathophysiological impact of its absence or dysfunction in AR220CGD patients remains an open question regarding the expression and the role of other NOXs. However, below we will focus on an animal model of a mutated p22phox protein that helped decipher the role of p22phox and NOX3 (Nakano et al., 2007; Nakano et al., 2008).
The second important role of p22phox was discovered by Leto et al. in 1994 (Leto et al., 1994). They demonstrated that p22phox interacted at the organizer subunit of the NADPH oxidase complex and p47phox during the activation process of NOX2. Finally, unlike NOX2, in CYBA numerous polymorphisms have often been described to be associated with vascular diseases such as hypertension, coronary heart disease, cerebrovascular diseases, diabetes, atherosclerosis and renal disease. Since excellent reviews have reported this point, we will only focus on recent data related to the pathological impact of these polymorphisms.
2. CYBA gene and p22phox transcript
The human p22phox gene, called CYBA (OMIM number 233690), is located on the long arm of chromosome 16 at position 24 (16q24: 88,643,288 to 88,651,084, OMIM 608508), and contains six exons and spans 8.5 kb (Fig. 1). Mutations in CYBA result in one of the autosomal recessive forms of CGD (AR220CGD) (Roos et al., 2010a). This point will be covered by section 4, “Mutations in CYBA and severity of AR22CGD.” By screening a cDNA library constructed from human promyelocytic leukemia cells, Parkos et al. isolated a cDNA corresponding to the light chain of cytochrome b558 (Parkos et al., 1988). The p22phox cDNA was also cloned in rat vascular smooth muscle cells (VSMCs) and showed that the rat gene is homologous to both the human and mouse genes (Fukui et al., 1995). P22phox human mRNA is 0.8 kb, has a constitutive expression in a variety of cell types and is not related to the NOX2 transcript expression, suggesting that both subunits have an independent transcription process (Parkos et al., 1988; Cheng et al., 2001). However, concomitant p22phox and NOX2 expression is a prerequisite for correct synthesis of cytochrome b558, the redox center of the NADPH oxidase complex. This point will be discussed in section 3.
Fig. 1.
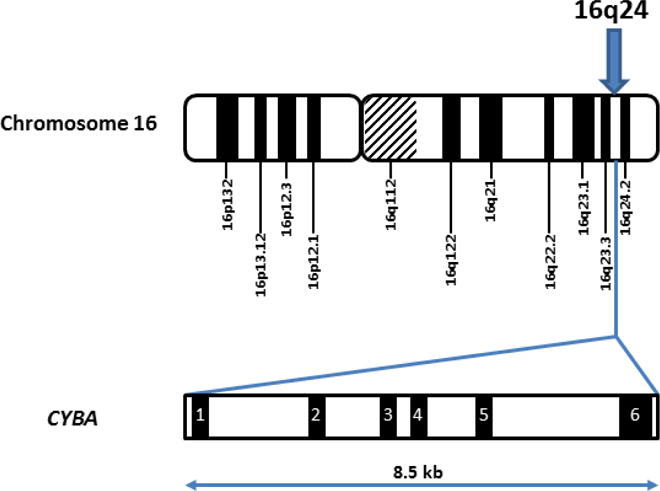
CYBA gene (OMIM number 233690) located on the long arm of chromosome 16 at position 24 (16q24: 88,643,288 to 88,651,084, OMIM 608508) contains 6 exons and spans 8.5 kb.
3 P22phox structure and physiological role
P22phox is a transmembrane protein that contains 195 amino acids and has a molecular mass of 22.0 kDa. It associates with NOX2 and with NOX1, NOX3 and NOX4 in a 1:1 complex and has a ubiquitous expression (Bedard and Krause, 2007). In neutrophils p22phox is associated with NOX2 to form the redox element of the NADPH oxidase complex, cytochrome b558. The activation and regulatory processes of NADPH oxidases have been extensively studied in phagocytes. In resting cells, the dormant NADPH oxidase complex is dissociated and becomes activated during phagocytosis to produce superoxide. Its activity is subtly controlled by a dynamic process involving assembly of several cytosolic proteins (p40phox, p47phox, p67phox, Rac1/2) around cytochrome b558 (Fig. 2). This assembly initiates the electron transfer from NADPH to oxygen via a FAD molecule and two hemes, ultimately resulting in the production of oxidizing species involved in the destruction of phagocytosed pathogens. Phosphorylation events trigger the assembly process of NADPH oxidase and its activity. NOX2, p22phox, p47phox and p67phox seem to be phosphorylated at the same time as NADPH oxidase activation (El-Benna et al., 2009; Raad et al., 2009; Lewis et al, 2010; Dang et al., 2011).
Fig. 2. Molecular mechanisms of NADPH oxidase complex activation.
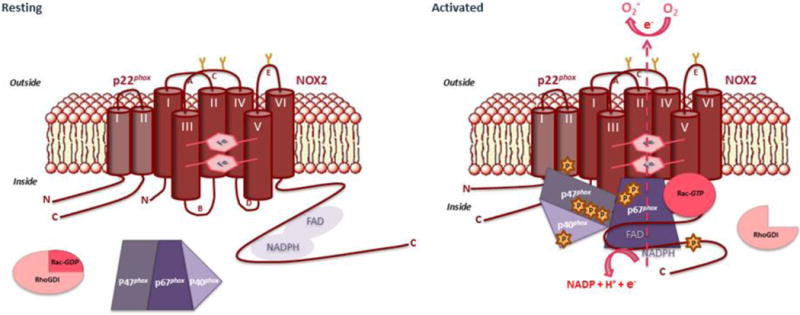
The NADPH oxidase complex of phagocytes is dissociated in resting cells. Cytochome b558 composed of Nox2 and p22phox is localized in the plasma membrane and the cytosolic factors p47phox, p67phox and p40phox form a complex in the cytoplasm. The small GTP-binding protein Rac associates with Rho-GDI in its inactive GDP form is in the cytosol. Upon activation, signaling events cause phosphorylations and conformational changes of the NADPH oxidase subunits leading to their assembly and activation. Activated Rac-GTP translocates, anchors in the membrane and binds to the NADPH oxidase complex. The fully assembled NADPH oxidase complex is able to trigger electron transfer from NADPH to FAD and hemes to reduce molecular oxygen into superoxide anions.
The main physiological role of p22phox is to contribute to the maturation and stabilization of the heterodimer that it forms with NOX enzymes (NOX1–4). Neutrophils of CGD patients with cytochrome b558 mutations lack both NOX2 and p22phox regardless of which subunit is affected by mutations, confirming the stabilization role of both subunits (CYBB or CYBA encoding NOX2 and p22phox, respectively) (Parkos et al., 1989; Porter et al, 1994). The synthesis process of cytochrome b558 in phagocytes is a complex mechanism. Previous studies showed that in phagocytes NOX2 is cotranslationally glycosylated and first detected as a high mannose 65-kDa (p65) monomer in the endoplasmic reticulum (ER) even in the absence of p22phox. However, full maturation of NOX2 requires sequential incorporation of hemes into p65, heterodimerization with p22phox occurring in the late ER step and final N-glycosylation in the Golgi, which drives the transport of the complex to the plasma membrane and into specific granules in neutrophils (Yu et al., 1998; Yu et al., 1999; DeLeo et al., 2000). In primary cells, the heterodimerization and enzymatic function of NOX2 as well as NOX1, 3 and 4 require the presence of p22phox; NOX5 is an exception (Bedard and Krause, 2007). Association of NOXs with p22phox in the late endoplasmic reticulum also seems to be a prerequisite for the localization of the heterodimer to specific membrane compartments such as perinuclear vesicles for NOX4 and plasma membranes for NOX1, 2 and 3 (Ambasta et al., 2004; Martyn et al., 2006; Nakano et al., 2007; von Lohneysen et al., 2010). The importance of some sequences of p22phox for its interaction with NOXs has been highlighted. Mutational analysis demonstrated distinct interactions of p22phox with NOX1, NOX3 and NOX4 (von Lohneysen et al., 2008). For example, the p22phox Tyr121 to His mutant is able to form a functional complex with NOX4 but not with NOX1 and NOX3 (Fig. 3). In addition, some regions of NOX2 that interact with p22phox have recently been suggested using special X−CGD mutations modeled in the PLB-985 cell line (Beaumel et al., 2014).
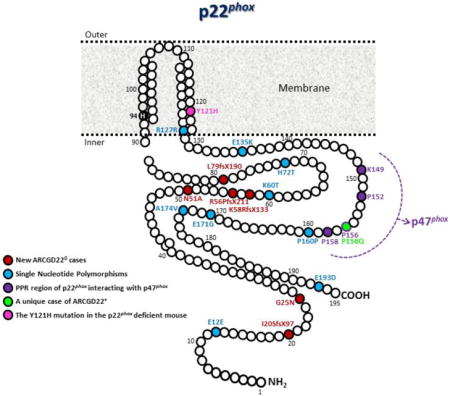
Fig. 3. Schematic representation of the two transmembrane-spanning model of the p22phox protein.

The positions of the nine SNPs in the p22phox protein sequence are indicated in blue, while the six new mutations in the encoding region of p22phox leading to AR-CGD220 cases are in red. The missense mutation P156Q leading to an AR-CGD22+ case is in green, while the missense mutation Y121H found in the nmf333 mouse is in pink. Histidine 94 is located in the first transmembrane passage. Its role as a heme-binding residue was highly debated. The polyproline rich region (K149 to E162 sequence) of p22phox containing a consensus motif PxxP that interacted with the SH3 (SRC homology 3) domains of p47phox is colored in purple.
The hydropathic profile of p22phox deduced from the gene sequence is compatible with at least two (possibly three or four) transmembrane passages, with different p22phox models proposed. P22phox was modeled with three transmembrane passages (Groemping and Rittinger, 2005; Meijles et al., 2012). However, the most probable are the two- or four-transmembrane spanning models because they are compatible with a cytosolic location of both the N- and C-terminal tail of p22phox (Imajoh-Ohmi et al., 1992; Burritt et al., 1998; Dahan et al., 2002). Studies using specific antibodies directed against p22phox supported a model anchored by two membrane-embedded domains in which the N- and C-terminal domains extend into the cytoplasm (Fig. 3) (Taylor et al., 2004; Taylor et al., 2006). In 1992 Leto et al. identified a polyproline-rich region (PRR) (K149 to E162 sequence) of p22phox containing a consensus motif PxxP that interacted with the SH3 (SRC homology 3) domains of p47phox. “Peptide walking” experiments confirmed the importance of this sequence in the assembly of phagocytic NADPH oxidase during activation (Dahan et al., 2002). In addition, deletion mutagenesis of p22phox highlighted that the N-terminal 11 residues of p22phox were essential for maturation of cytochrome b558 and oxidase activity, whereas deletion of 54 residues of the C-terminus of p22phox preserves the maturation but not the oxidase activity, as expected by the removal of the PRR domain required for recruitment of p47phox (Zhu et al., 2006). Then the complex between the tandem SH3 domains of p47phox and the PRR-rich sequence of p22phox (especially Pro152, Pro156 and Arg158) was resolved using crystallization and physical methods (Groemping et al., 2003; Ogura et al., 2006). This PRR-rich sequence of p22phox also interacts with the cytosolic organizer NOXO1 homologs to p47phox expressed in nonphagocytic cells during the activation of NADPH oxidases (NOX1, NOX2 and NOX3) except for NOX4, which is constitutively expressed (Sumimoto, 2008). It is also interesting to note that phosphorylation of Thr147, close to the PRR region of p22phox, enhances NADPH oxidase activity by promoting p47phox binding in phagocytes (Lewis et al., 2010). In addition, p21-activated kinases (Pak), a Rac2 effector during the oxidase activation process, seems to bind directly to p22phox, leading to favoring p47phox phosphorylation in the assembling NADPH oxidase complex (Lewis et al, 2010).
The role of histidine 94 in the predominantly hydrophobic region of p22phox (Fig. 3) as a heme-binding residue, has been hotly debated. First it was proposed that both subunits of cytochrome b558 contained hemes (Quinn et al., 1992). Then spectral properties of recombinant NOX2 and p22phox expressed separately or together in the Cos7 cell line demonstrated that NOX2 was the only heme-binding subunit of the NADPH oxidase (Yu et al., 1998). Indeed, mutagenesis of p22phox His94 demonstrated that when substituted with leucine, tyrosine or methionine, cytochrome b558 expression, heme binding and NADPH oxidase function were unaffected, ruling out a possible functional role of this residue. However, the His94 to Arg mutation found in an AR220CGD patient resulted in intrinsic p22phox instability and secondarily instability of NOX2, leading to deficient NADPH oxidase activity (Rae et al., 2000). Finally, His101, His115, His209 and His222 of NOX2 were identified as critical for heme binding and biosynthetic maturation of cytochrome b558 (Biberstine-Kinkade et al., 2001).
4. Mutations in CYBA and severity of AR22CGD
CGD is a rare inherited disorder in which phagocytic cells are unable to kill pathogens during an infection. It is a genetically heterogeneous disease with all ethnic groups equally affected. The molecular basis of CGD is characterized by two types of transmission and four main genetic forms. The major genetic form of CGD is X-linked CGD caused by mutations in the CYBB gene encoding NOX2. X-CGD accounts for about 70% of the total cases reported to date (Roos et al., 2010b). The other forms of CGD are autosomal recessive (AR), characterized by mutations in CYBA, NCF1 and NCF2 encoding p22phox, p47phox and p67phox, respectively (Roos et al., 2010a). Whereas AR670CGD is extremely rare (less than 6% of cases), AR470CGD occurs with high frequency (about 25% of CGD cases) due to the presence of two NCF1 pseudogenes carrying the main mutation. Like mutations in NCF2, mutations in the CYBA gene encoding p22phox are extremely rare (about 6% of total CGD cases diagnosed worldwide) and lead to AR220CGD. However, in countries such as Turkey, Tunisia, Morocco and Jordan AR inheritance can be the predominant form because of the high rate of consanguinity (El Kares et al., 2006; Bakri et al., 2009; Koker et al., 2013; Bousfiha et al., 2014).
An update of the promoter region of CYBA contains TATA and CCAC boxes and Sp1, γ-interferon and nuclear factor κB sites (Moreno et al., 2003). The last update of CYBA mutations reported in 2010 by Roos et al. showed 55 different mutations (Roos et al., 2010a). It accounted for 173 identified alleles in 96 patients worldwide. Fifty-one mutations led to an AR220CGD type, meaning that there was no p22phox expression, whereas for three mutations, this expression was not measured. This highlights that all the residues in the p22phox sequence are important for the structural stability of p22phox. Indeed, mutations are quite uniformly distributed in the coding sequence of p22phox listed in the database at. The only missense mutation, Pro156Gln (Fig. 3), leading to the sole A22+CGD, is located in the potential cytosolic C-terminal tail of p22phox (Leusen et al., 1994). This mutation in the PRR of p22phox disrupted the interaction between p22phox and p47phox, confirming the importance of this domain in oxidase activation in neutrophils. In addition, Sumimoto et al. demonstrated the direct interaction of p22phox with p47phox using the yeast two-hybrid system approach (Sumimoto et al., 1996)]. Most of them are missense mutations or small deletions (34.6 and 29.1%, respectively) (Roos et al., 2010a). Splice site, nonsense and insertions account for 20, 12.7 and 3.6% of total mutations in alleles, respectively. Only one mutation is a large deletion (>10 kb) that removed all but the extreme 5′ coding region of the gene (Dinauer et al., 1990). Since 2010 some eight new mutations in CYBA have been published (Table 1 and Fig. 3) (Teimourian et al., 2008; Koker et al., 2009; Jakobsen et al., 2012; Koker et al., 2013; Xu et al., 2014a).
Table 1.
New published CYBA mutations leading to AR220 CGD (since the last update of Roos et al. 2010)
| Nucleotide change | Mutation type | Amino acid change | CGD type | Reference |
|---|---|---|---|---|
| c.58+4_7delAGTG | Deletion | p.ILe20SerfsX97 | 220 | Köker et al., 2013 |
| c.58+2T>G | splice site? | ND | 220 | Köker et al., 2009 |
| c.74G>A | Missense | p.Gly25Asp | 220 | Köker et al., 2009 |
| C.152T>G | Missense | p.Leu51Ala | 220 | Xu et al., 2014 |
| c. 166_167insC | Insertion | p.Arg56ProfsX211 | 220 | Köker et al., 2013 |
| c.l74delG | Deletion | p.Lys58ArgfsX133 | 220 | Teimourian et al., 2008 |
| c.246_273de128bp | Deletion | p.Leu79fsX190 | 220 | Xu et al., 2014 |
| Deletion of exon 6 | splice site? | ND | 220 | Jakobsen et al., 2012 |
Given the rarity of the AR22 CGD forms, clear information on the severity of this type of CGD is difficult to establish. Kuhns et al. demonstrated a relationship between the presence of a residual ROS production and the survival of CGD patients (Kuhns et al., 2010). In the seven patients with p22phox deficiency studied, the range of ROI production varied widely, but the highest production was seen in patients with missense mutations. The study of 89 patients in a Turkish cohort showed that a residual NADPH oxidase activity can be measured by dihydrorhodamine (DHR) fluorescence in four CGD patients (AR22RCGD) with missense mutations in p22phox (p.Gly24Arg, p.Ala124Val and p.Ala125Thr), but the relationship with the severity of the disease was not clear (Koker et al., 2013). Probably in this case there was slight p22phox and NOX2 expression supporting this residual oxidase activity. However, the Kaplan-Meier graph showing the survival rate of CGD patients demonstrated that the AR220CGD forms were as severe as X-linked CGD. The severe course of the disease and the young age at diagnosis of the AR22CGD patients in the Iranian and Jordanian cohorts confirmed this result (Teimourian et al., 2008; Wolach et al., 2008; Bakri et al., 2009). In these late cases, the CYBA mutations result in the absence of p22phox and NOX2 (indirectly) expression and disable cytochrome b558, the redox element of the NADPH oxidase complex. Therefore, these mutations behave similarly to severe CYBB mutations in case of XCGD.
5. Single nucleotide polymorphisms and associated pathologies
Oxidative stress plays a key role in the pathophysiological mechanisms of numerous diseases. It is defined as an imbalance between the production of reactive oxygen and nitrogen species (NO) and detoxification by appropriate cellular systems (Nathan and Cunningham-Bussel, 2013). Excessive ROS generation by NOX enzymes is linked to a range of diseases including cardiovascular diseases such as atherosclerosis, hypertension, diabetes, neurodegenerative disease and ischemia/reperfusion injury (Bedard and Krause, 2007). Increased expression of p22phox in lymphoblast cell lines was shown to be related to increased ROS production in hypertension (Pettit et al., 2002). NOX1, NOX2 and NOX4, which require p22phox to be functional, are important contributors of ROS in tissues, most particularly vascular cells. Thus the variability of ROS production by NOXs could influence the risk of such diseases. However, increased oxidative stress by p22phox overexpression has not been functionally characterized or attributed to a particular NOX family member. Contrary to CYBB, CYBA supports a relatively large number of polymorphisms that could influence the level of ROS generation (Table 2). Thus the genetic variability of the CYBA gene has made it an attractive candidate to explain the influence of CYBA variant expression on complex disease states. However, the functionality of polymorphisms greatly depends on their location within the gene. Some polymorphisms lead to amino acid changes (Fig. 3), others are silent or located in the promoter regions (Table 2).
Table 2.
Human CYBA polymorphism update
| SNP | Reference | Location | effect | Reference |
|---|---|---|---|---|
| minus 930A>G | rs9932581 | Promoter | Activation by C/EBP | Moreno et al., 2003 |
| minus 852G>C | NA | Promoter | NA | Doi et al., 2005; Moreno et al., 2007 |
| minus 6751>A | rs16966671 | Promoter | Activated by HIF-1-α | Moreno et al., 2007 |
| minus 536C>T | rs13306296 | Promoter | NA | Moreno et al., 2007 |
| C.59-37A/G | rs3794624 | Intron 1 | NA | Rae et al., 2000; Patente et al., 2015 |
| c.36A>G# | rs8053867 | exon 1 | p.Glu12Glu | Bedard et al., 2009 |
| C.179A/C | NA | exon 3 | p.Lys60Thr | Rae et al., 2000 |
| c.214T>C* (or also called C242T) | rs4673 | exon 2 | p.His72Tyr | Dinauer et al., 1990; Bedard et al., 2009 |
| c.288-138ins50 | NA | exon 5 | NA | El Kares et al., 2006 |
| c.381T>C* | rs17354689 | exon 6 | p.Arg127arg | Bedard et al., 2009 |
| c.403G>A | NA | exon 6 | p.Glu135Lys | Rae et al., 2000 |
| c.480G>A# | NA | exon 6 | p.Pro160Pro | De Boer et al., 1992; Rae et al., 2000: Bedard et al., 2009 |
| c.512A>G# | ss107795100 | exon 6 | p.Glu171Gly | Bedard et al., 2009; |
| c.521T>C* (or also called C549T) | rs1049254 | exon 6 | p.Ala174Val | Dinauer et al., 2000; Bedard et al., 2009 |
| c.579G>T# | ss107795101 | exon 6 | p.Glu193Asp | Bedard et al., 2009 |
| c612A>G* (+24 of 3′ UT region (or | rs1049255 | 3′UTR | NA | De Boer et al., 1992; Bedard et al., 2009 |
The first and most widely studied is the C242T polymorphism, identified in 1990 by Dinauer et al., located in exon 4 at position 214 from the ATG and resulting in a nonconservative His72 substitution for a Tyr (Dinauer et al., 1990) (Fig. 3). Inoue et al. first found that the T allele of the C242 polymorphism might have a protective effect against coronary artery disease (Inoue et al., 1998). A functional effect of the C242T variant on the ROS level in human phagocytes, as in vascular cells (most often a decrease of ROS associated with the T allele), has been extensively reported (San Jose et al., 2008; Moreno and Zalba, 2010). In a study conducted in a cohort of 530 consecutive patients selected based on angiographic evidence of coronary atherosclerosis, Arca et al. demonstrated that the T242 allele was a predictor of lower risk of recurrence of cardiovascular events and was associated with reduced systemic oxidative stress (Arca et al., 2008). However, invariant local conformation in the p22phox p.Tyr72His polymorphism suggested by mass spectral analysis in human purified cytochrome b558 suggested maturational differences as the source of the wide variation of ROS production rather than a functional impact (Taylor et al., 2011). Despite evidence of the effect of this polymorphism on ROS generation at the cellular level, the association of the CYBA C242T polymorphism with cardiovascular diseases has been widely reported, although with conflicting results (Moreno and Zalba, 2010).
Some polymorphisms reported in Table 2 are located in the promoter of the CYBA gene. For example, the G allele of the −930A>G variant seems to interact with the CCAAT/enhancer-binding protein δ (C/EBP) and promotes higher transcription levels of p22phox than the A allele (Moreno et al., 2003). Again, controversial results have been published regarding the association of the GG genotype with hypertension (Moreno and Zalba, 2010). Single nucleotide polymorphism (SNP) analysis may explain the discrepancies among CYBA association studies.
A global approach such as haplotype analysis is probably a better approach to understand the impact of CYBA genetic variability on diseases (Gardemann et al., 1999; Moreno et al., 2007). A study conducted in lymphoblastoid cell lines obtained from 50 unrelated individuals demonstrated a substantial genetic variability of p22phox with three common and four rare single-nucleotide variants (* and # in Table 2, respectively) (Bedard et al., 2009). Based on the three common SNPs, seven haplogroups were defined and one haplogroup containing all three major SNPs (c.214T>C, c.521T>C and c.24G>A) showed markedly reduced ROS generation compared to others. A recent study of allelic variations in the CYBA gene highlighted that the G allele of the −930A>G variant was related to kidney complications in patients with type 1 diabetes (Patente et al., 2015). As diseases such as coronary heart diseases and diabetes are multivariate diseases, accurate phenotyping should be essential. Indeed gene–gene or gene–environment interactions are important factors to take into account. CYBA variants together with polymorphism analysis of lipid metabolism or stress oxidant pathway genes are of great interest (Nikitin et al., 2010; Katakami et al., 2014; Franko et al., 2015). However, for future investigations regarding the effect of these polymorphisms, it is crucial that the number of patients under study provide sufficient statistical power. In addition, genetic studies that include control of external factors should be extremely informative.
Finally, beginning in 2010 nine Chinese meta-analyses of the C242T polymorphism were published in relation with coronary artery disease (CAD) (Fang et al., 2010; Wu et al., 2013; Liang et al., 2014; Xu et al., 2014b; Hu et al., 2015), hypertension (Qin et al., 2013), atherosclerosis or diabetes and its complications (Li et al., 2015) and ischemic cerebrovascular diseases (Gu et al., 2013; Li et al., 2013). Three of them were associated with the analysis of A930G or A640G polymorphisms (Qin et al., 2013; Liang et al., 2014; Xu et al., 2014b). The results of these meta-analyses are summarized in Table 3. The first evidence is that the impact of C242T on CAD is not clear because five meta-analyses gave controversial results. The first one published in 2010 demonstrated that the T allele carried an increased risk of CAD among the Asian population only, but not in the total or Caucasian population (Fang et al., 2010). A few years later, Wu et al. confirmed the association of the T allele with a risk of CA in the total population, a moderate risk in the Caucasian population but no risk in the Asian population (Wu et al., 2013). However, the last three articles on the C242T polymorphism suggested that the T allele was protective against CAD in the Asian population (Xu et al., 2014b; Hu et al., 2015). The A640G polymorphism seems also to be associated with a lower risk of CAD in the Asian population (Liang et al., 2014; Xu et al., 2014b). These few examples are given to illustrate that the results from meta-analyses should be taken with caution. Several factors could influence the results: the search strategy, the identification of relevant studies (publication bias), the statistical analysis including sufficient sampling, the prevalence of the studied polymorphism in the studied population [minor allele frequency (MAF)], and the type of population (e.g., whether or not the study was population-based). The results of these meta-analyses need to be confirmed with larger samples. In addition, meta-analysis based on a genome-wide association study data will be of great interest in the future.
Table 3.
Data directory of meta-analysis of CYBA polymorphisms
| Polymorphism | Diseases | Database | Articles eligibled versus screened | Number of patients | Number of controls | Ethmicity | Statistical method | Result | Reference |
|---|---|---|---|---|---|---|---|---|---|
| C242T and A930G | Hypertension | Pubmed, Embase, CNKI, CBM, Chongqing VIP, Wang Fang* | 13/256 | 2644 for C242T; 2003 for A930G | 1967 for C242T, 2434 for A930G | Asianand Caucasian | Dominant and allelic models | A930G associated with hypertension; C242T is not no ethnicity differences | Qin et al., 2013 |
| C242T | Ischamic stroke | Pubmed, Embase, CNKI, CBM, Chongqing VIP, Wang Fang | 8/87 | 2387 | 2498 | Asian and Caucasian | Dominant, overd ominant. codominant and allelic models | C242T is not associated with ischemic stroke except among the hospital-based studies; no ethnicity differences | Gu et al., 2013 |
| C242T | Ischamic cerebrovascular disease | Pubmed, Embase, Web of Science* | 6/94 | 1948 | 2357 | Asian and Caucasian | Dominant, recessive, additive and allelic models | C242T is not associated with ischemic cerebrovascular disease; no ethnicity differences | Li et al., 2013 |
| C242T | Coronary Arten Disease (CAD) | Pubmed, CNKI, CBM, Chongqing VIP, Web of Science | 15.16 | 6273 | 5045 | Asian and Caucasian | Dominant, codominant and recessra models | T allele easy an increased risk of CAD among asian populatiot onhy | Fang et al, 2010 |
| C242T | Coronary Artery Disease (CAD) | Hand search, Pubmed, Embase, CNKI, CBM, Wang Fang* | 21/150 | 9279 | 9349 | Asian and Caucasian | Dominant, allelic. recessive models; homozygote comparison | Tallele cany an increased risk of CAD; C242T has heterogeneous effect on CAD across different etnicities (moderate among Caucasian. lack significance among Asians) | Wu et al., 2013 |
| C242T and A640G | Coronary Artery Disease (CAD) | Pubmed, EMBASE, Web of Science. Wang Fang* | 21/63 | 8845 far C242T; 2094 for A640G | 6855 for C242T; 1102 for A640G | Asian and Caucasian | Dominant. recessive. and allelic models | Tallele carry a decreased risk of CAD among Asian population and the A640G polymorphism is associated with decreased risk of CAD | Xu et al., 2014 |
| A640G | Coronary heart disease (CHD) | Pubmed, Embase, CNKI, CBM, Chongqing VIP, Wang Fang* | 8/179 | 3904 | 3498 | Asian and Caucasian | Dominant, recessive, codominant 1, codominant 2 and allalic models | A640G is associated with CHD among the total population and Caucasians only (codominant 2) | Liang et al., 2014 |
| C242T and A C242T | Acute comnary syndrome (ACS) | Pubmed, EBSCI O and EMBASE* | 10/271 | 6102 | 8669 | Asian and Caucasian | Dominant, recessivs models; allele and homozygote comparisons | Tallele is a protectee factor against developping ACS in Asian population | Hu et al., 2015 |
| C242T and A C242T | Type 2 diabete and compliations | Pubmed, EMBASE, CNKI, Web of Science | 11/72 | 1661 with T2DM including 1063 with DN and 3S6 with CA | 1265 with T2DM including 1026 with DN and 273 with CA | Asian in majority | Dominant, recessive, additive and allelic models | C242T polymorphism is associated with increased risk of T2DM, DN but not CA | Li et al., 2015 |
According to the Preferred Reporting items for Systematic reviews and Meta-Analyses (PRISMA) guideline,
According to the Meta-analysis of observational Studies in Epidemiology (MOOSE) guideline, CBM; Chinese Biological Medical Literature Database, CNKI; Chinese National Knowledge infrastructure database, VIP; Database of Chinese scientific and technical periodicals, T2DM; Type 2 diabetes mellitus, DN; diabetic nephropathy, CA; carotid atherosclerosis
6. AR220CGD cellular and mice models: clinical significance
Since p22phox has a ubiquitous distribution, the only cell model that does not express it is AR220CGD hematopoietic cells isolated from CGD patients. In the past, Epstein-Barr virus (EBV) immortalized B lymphocytes from AR220CGD patients were used to evaluate gene therapy approaches (Maly et al., 1993). Then cytochrome b558 synthesis was also studied in AR220CGD EBV-B lymphocytes to delimitate the respective role of NOX2 and p22phox (Porter et al., 1994). However, maturation of NOX2 in the myeloid cell line is not exactly comparable to what occurs in the nonphagocytic cell line (Batot et al., 1998). Recently, we produced AR220CGD neutrophils and macrophages from induced pluripotent stem cells dedifferentiated from CGD patients’ fibroblasts (Brault et al., 2014). This model is very helpful not only as a pathophysiological model of this CGD type, but also to develop new therapeutic approaches such as gene therapy (Zou et al., 2011).
In 2008 Nakano et al. described the molecular and phenotypic characterization of a p22phox-deficient mouse strain called nmf333 generated by ethylnitrosourea-induced mutagenesis at the Jackson laboratory (Nakano et al., 2008). A Tyr121His missense mutation was found in CYBA in the second predicted transmembrane helix of p22phox (Fig. 3). The p22phox deficiency leads to clinical and biological characteristics of CGD in the nmf333 mouse as well as to a severe balance disorder. Since the site of p22phox expression was in the inner ear, they proposed that p22phox be involved in the control of vestibular organogenesis. In addition, NOX3, for which p22phox plays a critical role in its structural maturation and subcellular targeting, was also proposed as the main NADPH oxidase of the inner ear. This was demonstrated by the functional impact of NOX3 mutations in head-tilt mice with vestibular defects (Banfi et al., 2004; Paffenholz et al., 2004; Nakano et al., 2007). Yet the in vivo relevance of p22phox for NOX3 function remains uncertain because AR220CGD patients do not suffer from vestibular dysfunction (personal data).
Mori et al. reported that in Matsumoto Eosinophilia Shinshu (MES) rats a loss-of-function mutation in CYBA (deletion of the authentic 5′ splice donor in intron 4 and alternative utilization of the cryptic GpT splice donor sequence located 51 bp downstream) was responsible for spontaneous and severe blood eosinophilia without apparent parasitic infections, allergies or neoplastic disorders (Mori et al., 2009). These rats suffered from a balance defect due to a leak of otoconia in the inner ear, like nmf333 mice. However, MES rats retained normal innate immune defense against Staphylococcus aureus infection probably because of hypereosinophilia. They succeeded in normalizing eosinophilia, NADPH oxidase in leukocytes and a balance defect by introducing a normal CYBA transgene. However, the mechanisms by which a CYBA mutation results in eosinophilia remain unknown.
7. Conclusion
Since the initial discovery of a 22-kDa protein (p22phox) associated with a 90-kDa (gp91phox or NOX2) during the purification of cytochrome b558 from human granulocytes (Parkos et al., 1987), major advances have been made in understanding the structure and the function of this protein. One of the highlights that have given rise to increased interest in p22phox function is the discovery of NOX2 analogs such as NOX1, NOX3 and NOX4 involved in numerous pathophysiological processes and possibly associated with p22phox to be functional. However, until now it has not been clear that NOX1, NOX3 and NOX4 can be expressed in tissues in absence of p22phox. Thus the exact role of p22phox in other cells than neutrophils remains to be clarified. In addition, uncertainty remains regarding the structuration of the protein embedded in membranes in absence of crystallographic data. The role of p22phox phosphorylation and the precise sites of interaction with NOXs are also open questions. Finally, the role of common genetic polymorphisms within the promoter and exonic sequences of CYBA, mostly in cardiovascular diseases, continues to be debated. Further studies with a global approach including sufficient sampling will be of great interest to understand the relationship between the genetic background and the patients’ clinical expression.
Highlights.
P22phox is a ubiquitous protein encoded by the CYBA gene located on chromosome 16.
P22phox is a critical component of the superoxide-generating NADPH oxidases.
Mutations in CYBA lead to the rare form of chronic granulomatous disease AR220CGD.
P22phox-deficient mice suffer from vestibular dysfunction.
Polymorphisms in CYBA seem to be associated with coronary artery and heart diseases.
Acknowledgments
This review and the corresponding Gene Wiki article were written as part of the Gene Wiki Review series – a series resulting from collaboration between the journal Gene and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of Gene. The corresponding Gene Wiki entry for this review can be found here: (https://en.wikipedia.org/wiki/Cytochrome_b-245,alpha_polypeptide). The work was supported by grants from Interreg France-Suisse (Programme de Cooperation Territoriale Européenne, FEDER, 2013–2015). It was also supported by grants from Université Grenoble Alpes (AGIR program), the Medical School and the Delegation for Clinical Research and Innovations, Pôle Recherche, CHU Grenoble (DRCI, Rementips project), Grenoble, France. The authors would like to thank Dr. Julie Brault, Cecile Martel, Michele Mollin and Sylvain Beaumel for their work at the CDiReC. Special thanks are extended to Linda Northrup for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The author declares no conflict of interest.
References
- Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–41. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- Arca M, Conti B, Montali A, Pignatelli P, Campagna F, Barilla F, Tanzilli G, Verna R, Vestri A, Gaudio C, Violi F. C242T polymorphism of NADPH oxidase p22phox and recurrence of cardiovascular events in coronary artery disease. Arterioscler Thromb Vasc Biol. 2008;28:752–7. doi: 10.1161/ATVBAHA.107.154823. [DOI] [PubMed] [Google Scholar]
- Bakri FG, Martel C, Khuri-Bulos N, Mahafzah A, El-Khateeb MS, Al-Wahadneh AM, Hayajneh WA, Hamamy HA, Maquet E, Molin M, Stasia MJ. First report of clinical, functional, and molecular investigation of chronic granulomatous disease in nine Jordanian families. J Clin Immunol. 2009;29:215–30. doi: 10.1007/s10875-008-9243-y. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–72. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Batot G, Paclet MH, Doussiere J, Vergnaud S, Martel C, Vignais PV, Morel F. Biochemical and immunochemical properties of B lymphocyte cytochrome b558. Biochim Biophys Acta. 1998;1406:188–202. doi: 10.1016/s0925-4439(98)00004-0. [DOI] [PubMed] [Google Scholar]
- Beaumel S, Grunwald D, Fieschi F, Stasia MJ. Identification of NOX2 regions for normal biosynthesis of cytochrome b558 in phagocytes highlighting essential residues for p22phox binding. Biochem J. 2014;464:425–37. doi: 10.1042/BJ20140555. [DOI] [PubMed] [Google Scholar]
- Bedard K, Attar H, Bonnefont J, Jaquet V, Borel C, Plastre O, Stasia MJ, Antonarakis SE, Krause KH. Three common polymorphisms in the CYBA gene form a haplotype associated with decreased ROS generation. Hum Mutat. 2009;30:1123–33. doi: 10.1002/humu.21029. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Biberstine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Nauseef WM, Dinauer MC. Heme-ligating histidines in flavocytochrome b(558): identification of specific histidines in gp91(phox) J Biol Chem. 2001;276:31105–12. doi: 10.1074/jbc.M103327200. [DOI] [PubMed] [Google Scholar]
- Bousfiha AA, Jeddane L, El Hafidi N, Benajiba N, Rada N, El Bakkouri J, Kili A, Benmiloud S, Benhsaien I, Faiz I, Maataoui O, Aadam Z, Aglaguel A, Baba LA, Jouhadi Z, Abilkassem R, Bouskraoui M, Hida M, Najib J, Alj HS, Ailal F, Moroccan Society for Primary, I First report on the Moroccan registry of primary immunodeficiencies: 15 years of experience (1998–2012) J Clin Immunol. 2014;34:459–68. doi: 10.1007/s10875-014-0005-8. [DOI] [PubMed] [Google Scholar]
- Brault J, Goutagny E, Telugu N, Shao K, Baquie M, Satre V, Coutton C, Grunwald D, Brion JP, Barlogis V, Stephan JL, Plantaz D, Hescheler J, Krause KH, Saric T, Stasia MJ. Optimized Generation of Functional Neutrophils and Macrophages from Patient-Specific Induced Pluripotent Stem Cells: Ex Vivo Models of X(0)-Linked, AR22(0)- and AR47(0)- Chronic Granulomatous Diseases. Biores Open Access. 2014;3:311–26. doi: 10.1089/biores.2014.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu-Ghanim HN, Casimir CM, Povey S, Segal AW. The alpha subunit of cytochrome b-245 mapped to chromosome 16. Genomics. 1990;8:568–70. doi: 10.1016/0888-7543(90)90045-v. [DOI] [PubMed] [Google Scholar]
- Burritt JB, Busse SC, Gizachew D, Siemsen DW, Quinn MT, Bond CW, Dratz EA, Jesaitis AJ. Antibody imprint of a membrane protein surface. Phagocyte flavocytochrome b. J Biol Chem. 1998;273:24847–52. doi: 10.1074/jbc.273.38.24847. [DOI] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–40. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Dahan I, Issaeva I, Gorzalczany Y, Sigal N, Hirshberg M, Pick E. Mapping of functional domains in the p22(phox) subunit of flavocytochrome b(559) participating in the assembly of the NADPH oxidase complex by “peptide walking”. J Biol Chem. 2002;277:8421–32. doi: 10.1074/jbc.M109778200. [DOI] [PubMed] [Google Scholar]
- Dang PM, Raad H, Derkawi RA, Boussetta T, Paclet MH, Belambri SA, Makni-Maalej K, Kroviarski Y, Morel F, Gougerot-Pocidalo MA, El-Benna J. The NADPH oxidase cytosolic component p67phox is constitutively phosphorylated in human neutrophils: Regulation by a protein tyrosine kinase, MEK1/2 and phosphatases 1/2A. Biochem Pharmacol. 2011;82:1145–52. doi: 10.1016/j.bcp.2011.07.070. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, Nauseef WM. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem. 2000;275:13986–93. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- Dinauer MC, Orkin SH, Brown R, Jesaitis AJ, Parkos CA. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature. 1987;327:717–20. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Dinauer MC, Pierce EA, Bruns GA, Curnutte JT, Orkin SH. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990;86:1729–37. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–25. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kares R, Barbouche MR, Elloumi-Zghal H, Bejaoui M, Chemli J, Mellouli F, Tebib N, Abdelmoula MS, Boukthir S, Fitouri Z, M’Rad S, Bouslama K, Touiri H, Abdelhak S, Dellagi MK. Genetic and mutational heterogeneity of autosomal recessive chronic granulomatous disease in Tunisia. J Hum Genet. 2006;51:887–95. doi: 10.1007/s10038-006-0039-8. [DOI] [PubMed] [Google Scholar]
- Fang S, Wang L, Jia C. Association of p22phox gene C242T polymorphism with coronary artery disease: a meta-analysis. Thromb Res. 2010;125:e197–201. doi: 10.1016/j.thromres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Franko B, Benhamou PY, Genty C, Jouve T, Nasse L, Rzeoecki V, Semeraro P, Stasia MJ, Zaoui P. RAGE and CYBA polymorphisms are associated with microalbuminuria and end-stage renal disease onset in a cohort of type 1 diabetes mellitus patients over a 20-year follow-up. Acta Diabetol. 2015 doi: 10.1007/s00592-015-0820-2. [DOI] [PubMed] [Google Scholar]
- Fukui T, Lassegue B, Kai H, Alexander RW, Griendling KK. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta. 1995;1231:215–9. doi: 10.1016/0005-2728(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Gardemann A, Mages P, Katz N, Tillmanns H, Haberbosch W. The p22 phox A640G gene polymorphism but not the C242T gene variation is associated with coronary heart disease in younger individuals. Atherosclerosis. 1999;145:315–23. doi: 10.1016/s0021-9150(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–55. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–16. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Su L, Liang B, Tang N, Long J, Tan J, Chen Q, Xie J, Wu G, Yan Y, Huang G, Zu X. Association between the C242T polymorphism of p22phox gene and ischemic stroke: a meta-analysis. J Neurol Sci. 2013;330:100–10. doi: 10.1016/j.jns.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Harper AM, Dunne MJ, Segal AW. Purification of cytochrome b-245 from human neutrophils. Biochem J. 1984;219:519–27. doi: 10.1042/bj2190519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Huang MY, Hu XY, Xie XJ, Xiang MX, Liu XB, Wang JA. Meta-analysis of C242T polymorphism in CYBA genes: risk of acute coronary syndrome is lower in Asians but not in Caucasians. J Zhejiang Univ Sci B. 2015;16:370–9. doi: 10.1631/jzus.B1400241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajoh-Ohmi S, Tokita K, Ochiai H, Nakamura M, Kanegasaki S. Topology of cytochrome b558 in neutrophil membrane analyzed by anti-peptide antibodies and proteolysis. J Biol Chem. 1992;267:180–4. [PubMed] [Google Scholar]
- Inoue N, Kawashima S, Kanazawa K, Yamada S, Akita H, Yokoyama M. Polymorphism of the NADH/NADPH oxidase p22 phox gene in patients with coronary artery disease. Circulation. 1998;97:135–7. doi: 10.1161/01.cir.97.2.135. [DOI] [PubMed] [Google Scholar]
- Jakobsen MA, Katzenstein TL, Valerius NH, Roos D, Fisker N, Mogensen TH, Jensen PO, Barington T. Genetical analysis of all Danish patients diagnosed with chronic granulomatous disease. Scand J Immunol. 2012;76:505–11. doi: 10.1111/j.1365-3083.2012.02771.x. [DOI] [PubMed] [Google Scholar]
- Katakami N, Kaneto H, Matsuoka TA, Takahara M, Osonoi T, Saitou M, Kawai K, Ishibashi F, Kashiwagi A, Kawamori R, Shimomura I, Yamasaki Y. Accumulation of oxidative stress-related gene polymorphisms and the risk of coronary heart disease events in patients with type 2 diabetes–an 8-year prospective study. Atherosclerosis. 2014;235:408–14. doi: 10.1016/j.atherosclerosis.2014.05.936. [DOI] [PubMed] [Google Scholar]
- Koker MY, Camcioglu Y, van Leeuwen K, Kilic SS, Barlan I, Yilmaz M, Metin A, de Boer M, Avcilar H, Patiroglu T, Yildiran A, Yegin O, Tezcan I, Sanal O, Roos D. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. 2013;132:1156–1163 e5. doi: 10.1016/j.jaci.2013.05.039. [DOI] [PubMed] [Google Scholar]
- Koker MY, van Leeuwen K, de Boer M, Celmeli F, Metin A, Ozgur TT, Tezcan I, Sanal O, Roos D. Six different CYBA mutations including three novel mutations in ten families from Turkey, resulting in autosomal recessive chronic granulomatous disease. Eur J Clin Invest. 2009;39:311–9. doi: 10.1111/j.1365-2362.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- Krause KH, Lambeth D, Kronke M. NOX enzymes as drug targets. Cell Mol Life Sci. 2012;69:2279–82. doi: 10.1007/s00018-012-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, Zarember KA, Malech HL, Holland SM, Gallin JI. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–10. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–45. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- Leto TL, Adams AG, de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc Natl Acad Sci U S A. 1994;91:10650–4. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusen JH, Bolscher BG, Hilarius PM, Weening RS, Kaulfersch W, Seger RA, Roos D, Verhoeven AJ. 156Pro–>Gln substitution in the light chain of cytochrome b558 of the human NADPH oxidase (p22-phox) leads to defective translocation of the cytosolic proteins p47-phox and p67-phox. J Exp Med. 1994;180:2329–34. doi: 10.1084/jem.180.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EM, Sergeant S, Ledford B, Stull N, Dinauer MC, McPhail LC. Phosphorylation of p22phox on threonine 147 enhances NADPH oxidase activity by promoting p47phox binding. J Biol Chem. 2010;285:2959–67. doi: 10.1074/jbc.M109.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BH, Zhang LL, Zhang BB, Yin YW, Dai LM, Pi Y, Guo L, Gao CY, Fang CQ, Wang JZ, Li JC. Association between NADPH oxidase p22(phox) C242T polymorphism and ischemic cerebrovascular disease: a meta-analysis. PLoS One. 2013;8:e56478. doi: 10.1371/journal.pone.0056478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Qiu T, Qin C. NADPH oxidase p22phox C242T polymorphism and ischemic cerebrovascular disease: an updated meta-analysis. Med Sci Monit. 2015;21:231–8. doi: 10.12659/MSM.892253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Wei Q, Shen T, Su L, Yan Y, Wu G, Lu J, Gu L. The A640G polymorphism in the NAD(P)H oxidase p22phox gene (CYBA) is associated with risk reduction of coronary heart disease: a meta-analysis. Clin Biochem. 2014;47:409–16. doi: 10.1016/j.clinbiochem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Maly FE, Schuerer-Maly CC, Quilliam L, Cochrane CG, Newburger PE, Curnutte JT, Gifford M, Dinauer MC. Restitution of superoxide generation in autosomal cytochrome-negative chronic granulomatous disease (A22(0) CGD)-derived B lymphocyte cell lines by transfection with p22phax cDNA. J Exp Med. 1993;178:2047–53. doi: 10.1084/jem.178.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Meijles DN, Howlin BJ, Li JM. Consensus in silico computational modelling of the p22phox subunit of the NADPH oxidase. Comput Biol Chem. 2012;39:6–13. doi: 10.1016/j.compbiolchem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Moreno MU, San Jose G, Fortuno A, Beloqui O, Redon J, Chaves FJ, Corella D, Diez J, Zalba G. A novel CYBA variant, the −675A/T polymorphism, is associated with essential hypertension. J Hypertens. 2007;25:1620–6. doi: 10.1097/HJH.0b013e3281ac211d. [DOI] [PubMed] [Google Scholar]
- Moreno MU, San Jose G, Orbe J, Paramo JA, Beloqui O, Diez J, Zalba G. Preliminary characterisation of the promoter of the human p22(phox) gene: identification of a new polymorphism associated with hypertension. FEBS Lett. 2003;542:27–31. doi: 10.1016/s0014-5793(03)00331-4. [DOI] [PubMed] [Google Scholar]
- Moreno MU, Zalba G. CYBA gene variants as biomarkers for coronary artery disease. Drug News Perspect. 2010;23:316–24. doi: 10.1358/dnp.2010.23.5.1437711. [DOI] [PubMed] [Google Scholar]
- Mori M, Li G, Hashimoto M, Nishio A, Tomozawa H, Suzuki N, Usami S, Higuchi K, Matsumoto K. Pivotal Advance: Eosinophilia in the MES rat strain is caused by a loss-of-function mutation in the gene for cytochrome b(-245), alpha polypeptide (Cyba) J Leukoc Biol. 2009;86:473–8. doi: 10.1189/jlb.1108715. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, Nauseef WM. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem J. 2007;403:97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Longo-Guess CM, Bergstrom DE, Nauseef WM, Jones SM, Banfi B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–85. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–61. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin AG, Chistiakov DA, Minushkina LO, Zateyshchikov DA, Nosikov VV. Association of the CYBA, PPARGC1A, PPARG3, and PPARD gene variants with coronary artery disease and metabolic risk factors of coronary atherosclerosis in a Russian population. Heart Vessels. 2010;25:229–36. doi: 10.1007/s00380-009-1159-9. [DOI] [PubMed] [Google Scholar]
- Ogura K, Nobuhisa I, Yuzawa S, Takeya R, Torikai S, Saikawa K, Sumimoto H, Inagaki F. NMR solution structure of the tandem Src homology 3 domains of p47phox complexed with a p22phox-derived proline-rich peptide. J Biol Chem. 2006;281:3660–8. doi: 10.1074/jbc.M505193200. [DOI] [PubMed] [Google Scholar]
- Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann U, Marquardt A, Bareiss A, Laufs J, Russ A, Stumm G, Schimenti JC, Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–91. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos CA, Allen RA, Cochrane CG, Jesaitis AJ. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987;80:732–42. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos CA, Dinauer MC, Jesaitis AJ, Orkin SH, Curnutte JT. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989;73:1416–20. [PubMed] [Google Scholar]
- Parkos CA, Dinauer MC, Walker LE, Allen RA, Jesaitis AJ, Orkin SH. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A. 1988;85:3319–23. doi: 10.1073/pnas.85.10.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patente TA, Mohammedi K, Bellili-Munoz N, Driss F, Sanchez M, Fumeron F, Roussel R, Hadjadj S, Correa-Giannella ML, Marre M, Velho G. Allelic variations in the CYBA gene of NADPH oxidase and risk of kidney complications in patients with type 1 diabetes. Free Radic Biol Med. 2015;86:16–24. doi: 10.1016/j.freeradbiomed.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Pettit AI, Wong RK, Lee V, Jennings S, Quinn PA, Ng LL. Increased free radical production in hypertension due to increased expression of the NADPH oxidase subunit p22(phox) in lymphoblast cell lines. J Hypertens. 2002;20:677–83. doi: 10.1097/00004872-200204000-00025. [DOI] [PubMed] [Google Scholar]
- Porter CD, Parkar MH, Verhoeven AJ, Levinsky RJ, Collins MK, Kinnon C. p22-phox-deficient chronic granulomatous disease: reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91-phox. Blood. 1994;84:2767–75. [PubMed] [Google Scholar]
- Qin YW, Peng J, Liang BY, Su L, Chen Q, Xie JJ, Gu L. The A930G polymorphism ofP22phox (CYBA) gene but not C242T variation is associated with hypertension: a meta-analysis. PLoS One. 2013;8:e82465. doi: 10.1371/journal.pone.0082465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn MT, Mullen ML, Jesaitis AJ. Human neutrophil cytochrome b contains multiple hemes. Evidence for heme associated with both subunits. J Biol Chem. 1992;267:7303–9. [PubMed] [Google Scholar]
- Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. Faseb J. 2009;23:1011–22. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Noack D, Heyworth PG, Ellis BA, Curnutte JT, Cross AR. Molecular analysis of 9 new families with chronic granulomatous disease caused by mutations in CYBA, the gene encoding p22(phox) Blood. 2000;96:1106–12. [PubMed] [Google Scholar]
- Roos D, Kuhns DB, Maddalena A, Bustamante J, Kannengiesser C, de Boer M, van Leeuwen K, Koker MY, Wolach B, Roesler J, Malech HL, Holland SM, Gallin JI, Stasia MJ. Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update) Blood Cells Mol Dis. 2010a;44:291–9. doi: 10.1016/j.bcmd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, Avcin T, de Boer M, Bustamante J, Condino-Neto A, Di Matteo G, He J, Hill HR, Holland SM, Kannengiesser C, Koker MY, Kondratenko I, van Leeuwen K, Malech HL, Marodi L, Nunoi H, Stasia MJ, Ventura AM, Witwer CT, Wolach B, Gallin JI. Hematologically important mutations: X-linked chronic granulomatous disease (third update) Blood Cells Mol Dis. 2010b;45:246–65. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Jose G, Fortuno A, Beloqui O, Diez J, Zalba G. NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin Sci (Lond) 2008;114:173–82. doi: 10.1042/CS20070130. [DOI] [PubMed] [Google Scholar]
- Segal AW, Harper A, Garcia R, Jones OT, Cross AR. The nature and function of the microbicidal oxidase system of neutrophils. Bull Eur Physiopathol Respir. 1981;17(Suppl):187–91. [PubMed] [Google Scholar]
- Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–77. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Hata K, Mizuki K, Ito T, Kage Y, Sakaki Y, Fukumaki Y, Nakamura M, Takeshige K. Assembly and activation of the phagocyte NADPH oxidase. Specific interaction of the N-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J Biol Chem. 1996;271:22152–8. doi: 10.1074/jbc.271.36.22152. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Baniulis D, Burritt JB, Gripentrog JM, Lord CI, Riesselman MH, Maaty WS, Bothner BP, Angel TE, Dratz EA, Linton GF, Malech HL, Jesaitis AJ. Analysis of human phagocyte flavocytochrome b(558) by mass spectrometry. J Biol Chem. 2006;281:37045–56. doi: 10.1074/jbc.M607354200. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Burritt JB, Baniulis D, Foubert TR, Lord CI, Dinauer MC, Parkos CA, Jesaitis AJ. Site-specific inhibitors of NADPH oxidase activity and structural probes of flavocytochrome b: characterization of six monoclonal antibodies to the p22phox subunit. J Immunol. 2004;173:7349–57. doi: 10.4049/jimmunol.173.12.7349. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Dratz EA, Jesaitis AJ. Invariant local conformation in p22phox p.Y72H polymorphisms suggested by mass spectral analysis of crosslinked human neutrophil flavocytochrome b. Biochimie. 2011;93:1502–9. doi: 10.1016/j.biochi.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahan C, Rowe P, Parker P, Totty N, Segal AW. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. Nature. 1987;327:720–1. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Teimourian S, Zomorodian E, Badalzadeh M, Pouya A, Kannengiesser C, Mansouri D, Cheraghi T, Parvaneh N. Characterization of six novel mutations in CYBA: the gene causing autosomal recessive chronic granulomatous disease. Br J Haematol. 2008;141:848–51. doi: 10.1111/j.1365-2141.2008.07148.x. [DOI] [PubMed] [Google Scholar]
- van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Espanol T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem. 2008;283:35273–82. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lohneysen K, Noack D, Wood MR, Friedman JS, Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol. 2010;30:961–75. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolach B, Gavrieli R, de Boer M, Gottesman G, Ben-Ari J, Rottem M, Schlesinger Y, Grisaru-Soen G, Etzioni A, Roos D. Chronic granulomatous disease in Israel: clinical, functional and molecular studies of 38 patients. Clin Immunol. 2008;129:103–14. doi: 10.1016/j.clim.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Wu Z, Lou Y, Jin W, Liu Y, Lu L, Chen Q, Xie Y, Lu G. Relationship of the p22phox (CYBA) gene polymorphism C242T with risk of coronary artery disease: a meta-analysis. PLoS One. 2013;8:e70885. doi: 10.1371/journal.pone.0070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Tian W, Li SJ, Zhang LY, Liu W, Zhao Y, Zhang ZY, Tang XM, Wang M, Wu DQ, Shi JS, Ding Y, Zhao XD, Yang XQ, Jiang LP. Clinical and molecular features of 38 children with chronic granulomatous disease in mainland china. J Clin Immunol. 2014a;34:633–41. doi: 10.1007/s10875-014-0061-0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Yuan F, Shen X, Wen H, Li W, Cheng B, Wu J. Polymorphisms of C242T and A640G in CYBA gene and the risk of coronary artery disease: a meta-analysis. PLoS One. 2014b;9:e84251. doi: 10.1371/journal.pone.0084251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, DeLeo FR, Biberstine-Kinkade KJ, Renee J, Nauseef WM, Dinauer MC. Biosynthesis of flavocytochrome b558. gp91(phox) is synthesized as a 65-kDa precursor (p65) in the endoplasmic reticulum. J Biol Chem. 1999;274:4364–9. doi: 10.1074/jbc.274.7.4364. [DOI] [PubMed] [Google Scholar]
- Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A. 1998;95:7993–8. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhen L, Dinauer MC. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J Biol Chem. 1997;272:27288–94. doi: 10.1074/jbc.272.43.27288. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Marchal CC, Casbon AJ, Stull N, von Lohneysen K, Knaus UG, Jesaitis AJ, McCormick S, Nauseef WM, Dinauer MC. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J Biol Chem. 2006;281:30336–46. doi: 10.1074/jbc.M607191200. [DOI] [PubMed] [Google Scholar]
- Zou J, Sweeney CL, Chou BK, Choi U, Pan J, Wang H, Dowey SN, Cheng L, Malech HL. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood. 2011;117:5561–72. doi: 10.1182/blood-2010-12-328161. [DOI] [PMC free article] [PubMed] [Google Scholar]


