Abstract
Alcoholism is a relapsing disorder with limited treatment options, in part due to our limited understanding of the disease etiology. We have recently shown that increased ethanol-seeking in a behavioral model of relapse in a rat model of alcoholism was associated with increased oligodendrogenesis which was positively correlated with platelet/endothelial cell adhesion molecule (PECAM-1) expression in the medial prefrontal cortex (mPFC). The current study investigated whether newly born oligodendrocytes form close physical associations with endothelial cells expressing PECAM-1 and whether these changes were accompanied by altered blood-brain barrier (BBB) integrity. Colableling and confocal analysis demonstrate that newly born oligodendroglia were always located in close physical proximity to PECAM-1 in the mPFC of rats that were ethanol dependent and demonstrated high propensity for relapse. Notably, the endothelial proximity of new oligodendrocytes was associated with reduced expression of endothelial barrier antigen (SMI-71), a marker for BBB integrity. Furthermore, voluntary wheel running during abstinence enhanced SMI-71 expression in endothelial cells, indicating protection against abstinence-induced reduction in BBB integrity. Taken together, these results suggest that ethanol experience and abstinence disrupts homeostasis in the oligo-vascular niche in the mPFC. Reversing these mechanisms may hold the key to reducing propensity for relapse in individuals with moderate to severe alcohol use disorder.
Keywords: BrdU, PECAM-1, Blood-brain barrier, SMI-71, ethanol, self-administration
Introduction
Chronic intermittent ethanol vapor inhalation (CIE) procedure is a widely accepted model for moderate to severe alcohol use disorder (AUD; (Gilpin et al., 2008; Vendruscolo & Roberts, 2014). This model is invaluable for identifying the circuitry and neurochemistry underlying the reward-deficient and stress-surfeit characteristics of addiction (Koob & Volkow, 2010); however the addiction pathology extends beyond adaptations in neurocircuitry into processes such as inflammation and gliosis (Coller & Hutchinson, 2012; Somkuwar et al., 2014). In fact, neuronal damage in AUD has been ascribed, in part, to the concerted feed-forward interaction between blood-brain barrier (BBB) damage and neuroinflammation (Haorah et al., 2007; Privratsky et al., 2010; Alikunju et al., 2011). Thus, understanding ethanol-induced disruptions in function of cerebral endothelium and other non-neuronal cells may help elucidate AUD neuropathology.
The current study explores the role of the oligovascular niche (Arai & Lo, 2009) in AUD. Disrupted oligodendroglial homeostasis in the medial prefrontal cortex (mPFC) was associated with escalated ethanol intake in CIE rats (Richardson et al., 2009; Somkuwar et al., 2015). Specifically, high ethanol intake was shown to reduce proliferation of stem-like cells in several areas of the adult rodent brain, including mPFC (Nixon & Crews, 2002; Crews & Nixon, 2009; Richardson et al., 2009; Hansson et al., 2010). During early ethanol abstinence (3-7 days), compensatory hyperproliferation of stem cells was observed in several brain regions (Nixon & Crews, 2004; Hansson et al., 2010), including the mPFC (Somkuwar et al., 2015). These newly born neural cells in the mPFC survived and differentiated, primarily (>85%) into new oligodendrocytes (Somkuwar et al., 2015). This increased/rebound oligodendrogenesis was associated with increased ethanol drinking (Somkuwar et al., 2015), increased ethanol seeking and decreased neuronal activation as measured by Fos expression in the mPFC after prolonged abstinence (Somkuwar et al., 2016). Voluntary wheel running (WR) during prolonged abstinence did not alter ethanol drinking, but inhibited ethanol seeking, increased mPFC neuronal activation and rescued mPFC oligodendroglial homeostasis (Somkuwar et al., 2016). Since oligodendroglial turnover and maturation depends on cerebrovascular health (Arai & Lo, 2009; Miyamoto et al., 2014; Maki et al., 2015), it was not surprising that a cerebral endothelial marker, platelet/endothelial cell adhesion molecule-1 (PECAM-1 or CD-31), was also increased in association with the increased ethanol seeking, and these alterations were reduced by wheel running (Somkuwar et al., 2016). However, it is unclear whether the oligodendroglial hyperproliferation is connected functionally to compromised cerebrovascular health in this model of AUD.
We hypothesized that (1) protracted abstinence from ethanol enhances oligodendrocytes within close proximity to vasculature, (2) the enhanced oligodendroglial-vascular proximity is associated with reduced BBB integrity, and (3) wheel running during abstinence will prevent these alterations. The mPFC tissue from rats previously found to exhibit enhanced oligodendrogenesis and increased ethanol seeking after prolonged abstinence (Somkuwar et al., 2016) was used for the current study. The relative localization of PECAM-1 and the newly born oligodendrocyte was conducted to understand the anatomical relation between the damaged endothelium and oligodendrogenesis. BBB integrity was investigated using immunohistochemical analysis of endothelial barrier antigen (EBA or SMI-71). For example, studies conducted in adult rats in models of neuroinflammation and spinal cord injury have demonstrated that SMI-71 expression in the brain and spinal cord negatively correlated with infiltrated evans blue (EB) dye, suggesting that a reduction in SMI-71 expression shows enhanced BBB permeability and reduced BBB integrity (Matsushita et al., 2015; Park et al., 2015). Animal models of dyskinesia in adult rats also demonstrate a negative association between albumin immunostaining in the neuropil (measure of dysfunctional BBB) and SMI-71 immunostaining in the brain, supporting the previous findings that SMI-71 expression decreases with enhanced dysfunctional BBB (Westin et al., 2006). Furthermore, a direct support for SMI-71 as a measure for vascular damage, and hence BBB integrity, comes from toxin-induced studies in adult rats, where microvascular damage induced by clostridium perfringens type D epsilon toxin reduced SMI-71 immunostaining (Finnie et al., 2014). SMI-71 is expressed on the luminal side of endothelial cells only in areas with a selectively permeable BBB (Sternberger & Sternberger, 1987). Under pathological conditions that disrupt BBB function and increase BBB permeability, such as cerebral ischemia (Pelz et al., 2013), rarefaction of SMI-71 expression is expected.
Material/Methods
Prefrontal cortical tissue from forty-nine adult male Wistar rats (Charles River) were used for the study. All experimental procedures were carried out in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996), and were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute.
Effects of history of ethanol experience and access to running wheel during ethanol abstinence on the oligo-vascular homeostasis in the mPFC was investigated to provide potential mechanistic link between the behavioral protection against ethanol seeking and the neuro-adaptive effects of wheel running during abstinence (Somkuwar et al., 2016). The experimental design is presented schematically (Figure 1). Briefly, adult male Wistar rats were trained to self-administer ethanol (10% v/v in water) using operant conditioning. The rats were divided into two groups; one received ethanol vapors in a 14hON/10hOFF CIE schedule (CIE-ED; n=17) for a period of seven weeks to develop moderate to severe AUD (Gilpin et al., 2008). The other group did not receive ethanol vapor, however, self-administered ethanol (ED; n=20). Ethanol self-administration in these rats was reported previously; while CIE-ED escalated ethanol drinking, ED maintained low ethanol intake over weeks (Somkuwar et al., 2016). After week 7, all ethanol access was removed and ethanol abstinence was maintained under either standard housing conditions (CIE-ED; n=8 and ED; n=8) or with ad libitum home-cage wheel access (CIE-ED-WR; n=9 and ED-WR; n=12). An additional cohort of age-matched rats with regular housing conditions (ethanol and wheel naïve; Naive, n=6), and with wheel access (WR, n=6) were maintained for the same duration as the rats with ethanol history. After 3 days of abstinence, all rats were administered an exogenous marker of cell division, 5′-bromo-2-deoxyuridine (BrdU; 150 mg/kg, i.p.) to label proliferating cells (Dayer et al., 2003; Mandyam et al., 2007; Taupin, 2007). After 20 days of abstinence, ED, CIE-ED, ED-WR and CIE-ED-WR rats were subjected to one session of ethanol self-administration, six sessions of extinction training, and one session of cued-context induced reinstatement of ethanol-seeking behavior. For extinction sessions, operant boxes different from those used for self-administration were used and the house-light and white noise were turned on, and no cue-lights were available following lever presses. Finally lever response did not result in the delivery of ethanol. Both lever responses were recorded. After 6 days of extinction, rats were subjected to one session of cued-context reinstatement of ethanol seeking. Specifically, rats were introduced to operant chambers under conditions identical to training and maintenance (no house-light, no white noise; operant boxes used for self-administration). Active lever responses resulted in the presentation of the cue-light for 4 sec, but did not result in the delivery of ethanol. Both active and inactive lever responses were recorded. The self-administration behavior and wheel running activity for these rats have been published previously (Somkuwar et al., 2016). Two-hours after the reinstatement session, all rats were euthanized and their brain were post-fixed in 4% paraformaldehyde for immunohistochemistry.
Figure 1.
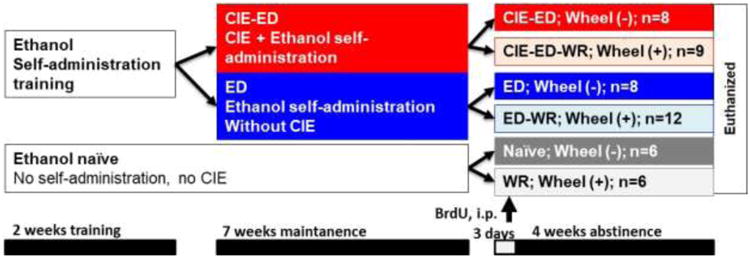
Schematic of experimental design indicating the experimental groups, the number of animals in each group, time line for each part of the experiment and time point for BrdU injection.
Brain tissue was sliced in 40μm sections along the coronal plane on a freezing microtome. Every ninth section through the PFC (+3.7 to +2.5 mm from bregma; 4 sections per rat) was mounted on Superfrost® Plus slides and dried overnight and used for BrdU analysis. Quantitative immunohistochemical assay performed using a previously published optical fractionator method (Kim et al., 2015); the sections were counter-stained with Vector Fast Red (a nuclear stain). The quantitative data for BrdU has been previously reported (Somkuwar et al., 2016). To determine the phenotype of BrdU labeled cells colabeling of BrdU (Abcam) was performed with the transcription factor Olig2 (gift from Dr. Charles Stiles), followed by biotinylated secondary and fluorescent labeling via tyramide signal amplification. The percent of BrdU cells colabeled with Olig2 were determined and the number of BrdU/Olig2 labeled cells were computed. These values were compared between groups using 2-way ANOVA (wheel access, 2 levels and ethanol history, 3 levels); significant interaction and/or main effects were further investigated using Holm-Sidak's post-hoc analysis.
A separate set of coronal sections of the brain (+3.0 to +3.7mm from bregma) were immunoprobed for quantifying the spatial distribution of newly born oligodendrocytes (BrdU) relative to cerebral endothelium (PECAM-1). To determine the distribution of newly born oligodendroglia, mPFC sections were immunoprobed for both BrdU and PECAM-1 (gift from Dr. Peter Newman), followed by biotinylated secondary and fluorescent labeling via tyramide signal amplification. Confocal analysis was conducted with Zeiss Axiovert 100M (Kim & Mandyam, 2014; Kim et al., 2014; Somkuwar et al., 2015). Note, PECAM-1 expression is not contingent on the functional integrity of endothelium (for review, (Newman, 1994)). The proximity between surviving BrdU cells and the nearest PECAM-1 labeled cell was measured using the “linear scale” function on a Zeiss AxioImager equipped with the software LSM510. Percentage of BrdU cells that were juxtaposed (physical touching with PECAM-1 cell), proximal (<5 μm apart from PECAM-1 cell), intermediate (between 5 μm and 30 μm apart from PECAM-1 cell) and distal (>30 μm apart from PECAM-1 cell) were calculated for each rat (5-40 cells/rat). Further, the number of BrdU cells within each location was calculated from the total number of BrdU cells obtained across mPFC (+2.6 to +4.5mm from bregma, 4 sections/rat) using quantitative immunohistochemical analysis (Somkuwar et al., 2016). Fraction and number of BrdU cells were analyzed using 3-way repeated-measures ANOVA with wheel access (2 levels) and ethanol history (3 levels) as between-subjects and distance (4 levels) as within-subjects independent variables. Huyhn-Feldt correction was applied to adjust for the violation of the sphericity hypothesis (i.e. the error covariance matrix of the orthonomalized transformed dependent variables is proportional to an identity matrix; Mauchly's test) for repeated-measures ANOVA. Significant effects at specific localization levels were further probed using 2-way ANOVAs (wheel access, 2 levels and ethanol history, 3 levels) and Sidak's post-hoc analyses.
Coronal sections (+3.0 to +3.7mm from bregma, 1 section/rat) were immunoprobed for SMI-71 (or EBA, 1:500, rat polyclonal), and biotin-tagged secondary antibodies and then visualized with DAB. Colored, white-balanced images were captured with StereoInvestigator software (MicroBrightField); and DAB levels (% area stained) in the infralimbic, prelimbic cortex and cingulate gyrus regions were evaluated using ImageJ software (NIH). Average area stained across the three mPFC subregions was compared between groups using 2-way ANOVA (wheel access, 2 levels and ethanol history, 3 levels) followed by Holm-Sidak's post-hoc analysis.
Results
The number of BrdU/Olig2 cells were enhanced by ethanol treatment. Two-way ANOVA revealed a significant ethanol treatment × wheel running interaction (FEthanolHistory × WheelAccess[2, 34] = 20.76, p<0.001), main effect of ethanol treatment (FEthanolHistory[2, 34] = 30.96, p<0.001), and main effect of wheel running (FWheelAccess[1, 34] = 74.55, p<0.001). Post hoc analysis demonstrated higher number of BrdU/Olig2 cells in ED and CIE-ED rats compared with sedentary controls (p<0.01), and lower number of BrdU/Olig2 cells in ED-WR and CIE-ED-WR rats compared to non-wheel groups (p<0.01; Figure 2a). Co-labeling analysis revealed no significant differences between the ratio of BrdU cells expressing Olig2 between any of the experimental groups, and there was greater than 95% co-labeling in all the groups (data not shown).
Figure 2.
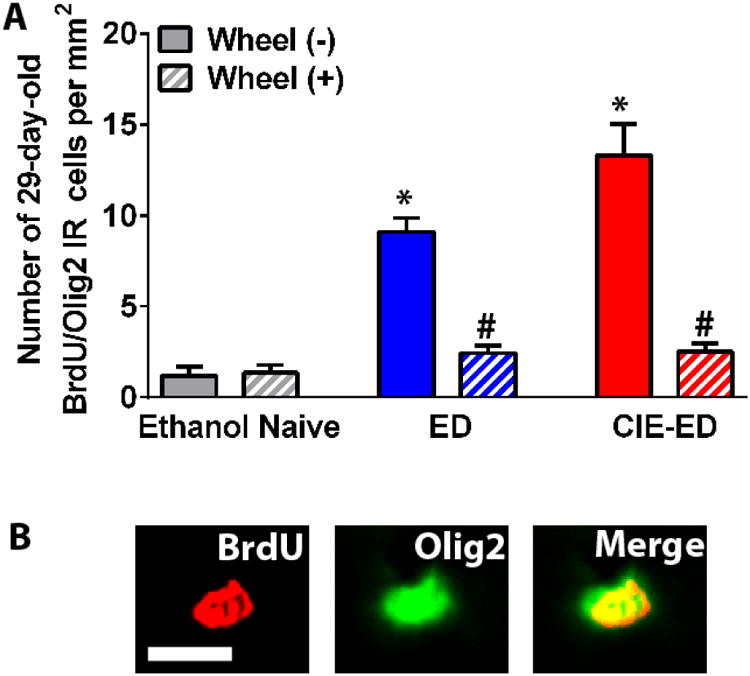
(a) Quantitative analysis of the number of 28-day-old BrdU/Olig2 colabeled cells in the mPFC. Data is indicated as number of cells per area of the mPFC, and represented as mean ± S.E.M. (b) Representative micrographs for immunofluorescent labeling of 28-day-old BrdU labeled cells colabeled with Olig2 from one control rat. BrdU is in red (CY3) and Olig 2 is in green (FITC). Scale bar indicated in the BrdU panel is 10 μm, applies to all the panels. *p<0.05 vs. ethanol naïve control in nonrunners. #p<0.05 vs. sedentary group in ED and CIE-ED groups. Values are mean ± S.E.M.
Analysis revealed that the distribution of surviving BrdU cells in relation to PECAM-1 expression showed intermediate>proximal>distal=juxtaposed (FDistance[3,120]=34.6, p<0.0001; Figure 3a-b). However, percent of juxtaposed, proximal, intermediate and distal BrdU cells relative to PECAM-1 immunofluorescence was not different between wheel access and ethanol history groups (data not shown). Analysis of number of cells in these localizations reveled significant interactions of distance and ethanol history (FDistance × EthanolHistory[3,120]=3.62, p=0.010) and distance and wheel access (FDistance × WheelAccess[3,120]=3.25, p=0.028), but not for 3-way interaction (FDistance × EthanolHistory × WheelAccess[3,120]=1.76, p=0.121). Further investigation into individual levels of localization revealed a difference between low and high ethanol intake histories only in the juxtaposed fraction, i.e. in the 14.5% of the surviving BrdU cells (Figure 3b-c). Specifically, significant main effects of wheel access, ethanol history and their interaction were found (FWheelAccess[1,40]=4.52, p=0.040; FEthanolHistory[2,40]=4.95, p=0.012; FEthanolHistory × WheelAccess[2,40]=3.93, p=0.028; Figure 3c). Despite the variability in the CIE-ED group, CIE-ED rats showed greater number of juxtaposed BrdU cells compared to both ED and naïve (ps<0.01), which was reduced by wheel access during abstinence (p<0.005), such that CIE-ED-WR was not different from WR and ED-WR. In contrast, BrdU cells proximal to and at intermediate separation from PECAM-1 were enhanced in both ED and CIE-ED compared to naïve, and this burst was normalized to WR levels in both ED-WR and CIE-ED-WR (data and statistics in Table 1).
Figure 3.
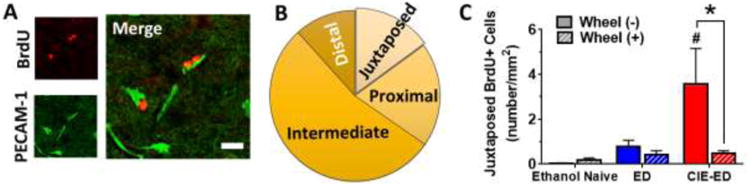
(a) Representative micrographs for immunofluorescent labeling of 28 day-old BrdU cells (CY3, red) and PECAM-1 (FITC, green) in the mPFC from one CIE-ED rat. Scale bar in the merged panel is 15 μm. (b) Pi-chart presenting the proportion of BrdU+ cells that are juxtaposed, proximal, intermediate or distal from the nearest PECAM-1 labeled cerebral endothelial cells (c) Quantification of the number of BrdU+ cells juxtaposed with cerebral endothelium. #p<0.05 vs. ED group; *p<0.05 vs. sedentary CIE-ED group.
Table 1.
History of ethanol experience increased BrdU+ cells that were at proximal and intermediate distance from PECAM1 in both CIE-ED and ED; both were normalized following wheel access during abstinence. F-statistics from 2-way ANOVAs are presented (Fe, ethanol history, 3 levels; Fw, wheel access, 2 levels; Fi, interaction)
| Ethanol Naïve | ED | CIE-ED | Fe | Fw | Fi | ||
|---|---|---|---|---|---|---|---|
| Proximal | Wheel(-) | 0.20 ± 0.08 | 2.3 ± 0.53 # | 3.0 ± 0.69 # | 8.21a | 14.2b | 2.87 |
| Wheel(+) | 0.08 ± 0.08 | 0.73 ± 0.32* | 0.78 ± 0.29* | ||||
| Intermediate | Wheel(-) | 0.67 ± 0.41 | 5.2 ± 0.46 # | 5.6 ± 0.89 # | 19.9a | 39.1b | 9.47c |
| Wheel(+) | 0.80 ± 0.45 | 1.5 ± 0.26 * | 1.8 ± 0.25* | ||||
| Distal | Wheel(-) | 0.13 ± 0.09 | 1.3 ± 0.46 | 0.97 ± 0.76 | 1.12 | 4.91b | 1.62 |
| Wheel(+) | 0.26 ± 0.14 | 0.17 ± 0.09 | 0.07 ± 0.07 |
p<0.001, significant main effect of ethanol history,
p<0.05, significant main effect of wheel access,
p<0.001, significant interaction,
p<0.05, compared to ethanol naïve control;
p<0.05, compared to respective Wheel(-)
SMI-71 expression, expressed as % of area contoured, revealed significant main effect of wheel access and interaction of wheel access and ethanol history (FWheelAccess[1,42]=18.0, p<0.0001; FEthanolHistory[2,42]=2.72, p=0.077; FEthanolHistory × WheelAccess[2,42]=6.52, p=0.003; Figure 4a-f). Wheel access did not enhance SMI-71 stained area in the ethanol naïve group (t(0.1)=42,p=0.9), but it increased SMI-71 stained area in both ED and CIE-ED rats (ED vs ED-WR: t(2.6)=42, p=0.02; CIE-ED vs CIE-ED-WR: t(5.5)=42, p<0.001). CIE-ED-WR had greater expression of SMI-71 compared to ED-WR (t(3.2)=42, p=0.006) and WR (t(3.99)=42, p=0.0008).
Figure 4.
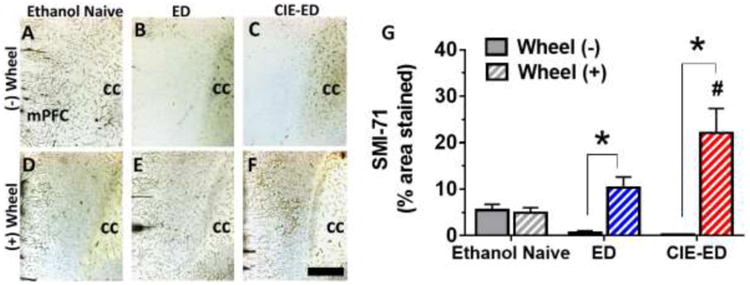
(a-f) Representative micrographs for immunohistochemical analysis of SMI-71 expression. Scale bar in f is 200 μm, applies a-f. cc, corpus callosum. (g) Quantification of SMI-71 (% area stained). Values are mean ± S.E.M. * p<0.001 compared to respective Wheel(-) group; #p<0.05 compared to respective ED and ethanol naïve control group.
Discussion
The current findings in accordance with our recent report demonstrate that enhanced oligodendrogenesis in the mPFC of ethanol abstinent rats (both ED and CIE-ED) was associated with enhanced ethanol seeking, and wheel running during abstinence reduced both ethanol seeking and oligodendrogenesis (Somkuwar et al., 2016). Our recent report also revealed that PECAM-1 expression (using immunoblotting analysis on mPFC homogenate) was also increased in ethanol abstinent rats, and normalized to control levels in rats with wheel access during abstinence (Somkuwar et al., 2016). Since cerebral endothelial cells promote oligodendrogenesis and vice versa, in part, via exchange of trophic factors (Arai & Lo, 2009; Miyamoto et al., 2014), it was not surprising that PECAM-1 and oligodendroglial proliferation in the mPFC were modulated in tandem during ethanol abstinence (Somkuwar et al., 2016). The major findings reported herein are that prolonged abstinence and reinstatement of ethanol seeking in CIE-ED rats increased oligodendrogenesis within a 30μ radius around the damaged endothelium expressing PECAM-1 and these effects were associated with reduction in SMI-71 expression on endothelial cells. Reduced SMI-71 expression was also observed in ED rats, however, these effects did not correlate with closer proximity of oligodendrocytes with endothelial cells as seen in CIE-ED rats. Our findings of reduced SMI-71 immunostaining in the mPFC in ED and CIE-ED rats demonstrate that protracted abstinence from chronic ethanol experience produces unheralded effects on endothelial cells and BBB integrity that may be associated with dependence-like syndrome.
Chronic ethanol exposure induces oxidative stress and neuroinflammation which may contribute to BBB damage (Haorah et al., 2005; Wang et al., 2013; Hernandez et al., 2016), and to cognitive deficits (Tiwari et al., 2009; Hernandez et al., 2016). In fact, oxidative stress in endothelial cells suppresses release of trophic factors necessary for oligodendrogenesis (Arai & Lo, 2009), which explains the reduced proliferation of oligodendrocyte progenitors in the mPFC observed during CIE (Richardson et al., 2009). Thus, BBB damage noted herein may be an effect of ethanol exposure that persisted into abstinence. As a corollary, the increased PECAM-1 expression and oligodendrogenesis during abstinence could emphasize physiological mechanisms of recovery from ethanol-induced oxidative damage. However, these effects appear to indicate maladaptive plasticity, such that, they were associated with enhanced cue-induced reinstatement of ethanol seeking, a behavioral paradigm for relapse, and with decreased neuronal activation in the mPFC (Somkuwar et al., 2016), which together suggests reduced executive function and behavioral inhibition (Warthen et al., 2016). A potential limitation of the currently used methodology is that it is not clear whether the observed increase in PECAM-1 expression is due to increases in the number of vascular endothelial cells or increased expression of PECAM-1 protein within the residual vascular endothelial cells. The current findings of reduced SMI-71 on endothelial cells in ED and CIE-ED rats during abstinence suggest that the expression of PECAM-1 within the existing vasculature is increased which may lead to angiogenesis with longer abstinence; however, this hypothesis needs to be validated. Similar increase in PECAM-1 expression and decrease in SMI-71 expression was reported in the basal ganglia of a rodent model of dyskinesia, where an increase in BBB permeability was also observed (Westin et al., 2006), suggesting a pre-angiogenic state in the basal ganglia. Although the current findings do not provide any causation between alterations in PECAM-1 and SMI-71 and relapse to ethanol seeking, and these studies are beyond the scope of the current report, future studies should investigate the functional consequence of enhanced PECAM-1 expression with BBB dysfunction during ethanol abstinence.
Wheel running performed voluntarily during abstinence was protective against cue-triggered ethanol seeking, suggesting a preservation of the response inhibition learned during extinction (Somkuwar et al., 2016), a behavior that is heavily reliant on the mPFC (Lasseter et al., 2010). Wheel running also increased neuronal activation, normalized oligodendrogenesis and PECAM-1 expression (Somkuwar et al., 2016) and increased expression of SMI-71 supporting restoration of a healthy BBB in mPFC (current findings). Exercise has been shown to be BBB protective in other disease models, such as cerebral ischemia and multiple sclerosis (Souza et al., 2016), and to reduce disease progression and restore neuronal function in several other neuropathologies (Phillips et al., 2014). Future studies should investigate the mechanistic link between PECAM-1 and ethanol seeking (via pharmacological and/or genetic manipulation) in the context of hyperproliferation of oligodendrocytes in the mPFC.
An interesting observation in the current study was the lack of difference in SMI-71 expression in mPFC in CIE-ED and ED rats; these effects may be due to floor effect in SMI-71 expression, since the magnitude of SMI-71 recovery following wheel access during alcohol abstinence was greater in CIE-ED-WR compared to ED-WR. Alternatively, the nature of BBB damage may be qualitatively different between CIE-ED and ED rats that could not be harnessed with SMI-71 expression. Notably, only in the CIE-ED rats, increased oligodendrogenesis was observed juxtaposed with the damaged cerebral endothelium. One limitation of this analysis is that PECAM-1-BrdU spatial relation is quantified in tissue slices and not in a more physiologically accurate 3D space. Thus, it is possible that PECAM-1 may be closer to BrdU than apparent here, although that will likely not alter the number of juxtaposed cells. Further, others have noted oligodendrocyte progenitor cells are located close to cerebral endothelia, and under pathological conditions, such as oxidative stress, weaken the endothelial barrier in a paracrine manner (Seo et al., 2013). Thus, one could predict that in rats with an AUD phenotype, oligodendrocyte progenitor cells contribute to BBB damage and that during abstinence, the impaired BBB provides a conducive environment for maladaptive oligodendrogenesis.
Highlights.
Protracted abstinence from chronic alcohol enhances the proximity of oligodendrocytes with endothelial cells
Enhanced oligo-vasculature proximity is associated with enhanced blood-brain barrier permeability
Wheel running during abstinence prevents oligo-vascular maladaptations and normalizes blood-brain barrier permeability
Acknowledgments
The authors thank Dr. Charles Stiles, Harvard Medical School for the Olig2 antibody and Dr. Peter J. Newman, BloodCenter of Wisconsin for PECAM-1 antibody. The study was supported by funds from the National Institute of Health (AA020098, AA06420 and DA034140 to CDM) and the Veterans Medical Research Foundation start-up funds. TBN was supported by funds from Skaggs School of Pharmacy, UCSD for summer internship.
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alikunju S, Abdul Muneer PM, Zhang Y, Szlachetka AM, Haorah J. The inflammatory footprints of alcohol-induced oxidative damage in neurovascular components. Brain Behav Immun. 2011;25 Suppl 1:S129–136. doi: 10.1016/j.bbi.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134:219–245. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Finnie JW, Manavis J, Chidlow G. Loss of endothelial barrier antigen immunoreactivity as a marker of Clostridium perfringens type D epsilon toxin-induced microvascular damage in rat brain. Journal of comparative pathology. 2014;151:153–156. doi: 10.1016/j.jcpa.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008;Chapter 9 doi: 10.1002/0471142301.ns0929s44. Unit 9 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT, Heilig M. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int J Neuropsychopharmacol. 2010;13:583–593. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Gorantla S, Zheng J, Persidsky Y. Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J Neurochem. 2007;100:324–336. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Hernandez JA, Lopez-Sanchez RC, Rendon-Ramirez A. Lipids and Oxidative Stress Associated with Ethanol-Induced Neurological Damage. Oxid Med Cell Longev. 2016;2016:1543809. doi: 10.1155/2016/1543809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Mandyam CD. Methamphetamine affects cell proliferation in the medial prefrontal cortex: a new niche for toxicity. Pharmacol Biochem Behav. 2014;126:90–96. doi: 10.1016/j.pbb.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct. 2015;220:1705–1720. doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, Shindo A, Choi YK, Taguchi A, Matsuyama T, Takahashi R, Ihara M, Arai K. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett. 2015;597:164–169. doi: 10.1016/j.neulet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Lankford KL, Arroyo EJ, Sasaki M, Neyazi M, Radtke C, Kocsis JD. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp Neurol. 2015;267:152–164. doi: 10.1016/j.expneurol.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71:1055–1066. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. The role of PECAM-1 in vascular cell biology. Ann N Y Acad Sci. 1994;714:165–174. doi: 10.1111/j.1749-6632.1994.tb12041.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Shin JY, Kim HN, Oh SH, Song SK, Lee PH. Mesenchymal stem cells stabilize the blood-brain barrier through regulation of astrocytes. Stem cell research & therapy. 2015;6:187. doi: 10.1186/s13287-015-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz J, Hartig W, Weise C, Hobohm C, Schneider D, Krueger M, Kacza J, Michalski D. Endothelial barrier antigen-immunoreactivity is conversely associated with blood-brain barrier dysfunction after embolic stroke in rats. Eur J Histochem. 2013;57:e38. doi: 10.4081/ejh.2013.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cell Neurosci. 2014;8:170. doi: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon-Pavlich MJ, Ghofranian A, Quigley JA, Dutta RR, Galinato MH, Mandyam CD. Wheel running reduces ethanol seeking by increasing neuronal activation and reducing oligodendroglial/neuroinflammatory factors in the medial prefrontal cortex. Brain Behav Immun. 2016;58:357–368. doi: 10.1016/j.bbi.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon MJ, Staples MC, Zamora-Martinez ER, Navarro AI, Kim A, Quigley JA, Edwards S, Mandyam CD. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Staples MC, Galinato MH, Fannon MJ, Mandyam CD. Role of NG2 expressing cells in addiction: a new approach for an old problem. Frontiers in pharmacology. 2014;5:279. doi: 10.3389/fphar.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PS, Goncalves ED, Pedroso GS, Farias HR, Junqueira SC, Marcon R, Tuon T, Cola M, Silveira PC, Santos AR, Calixto JB, Souza CT, de Pinho RA, Dutra RC. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0014-0. [DOI] [PubMed] [Google Scholar]
- Sternberger NH, Sternberger LA. Blood-brain barrier protein recognized by monoclonal antibody. Proc Natl Acad Sci U S A. 1987;84:8169–8173. doi: 10.1073/pnas.84.22.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Kuhad A, Chopra K. Suppression of neuro-inflammatory signaling cascade by tocotrienol can prevent chronic alcohol-induced cognitive dysfunction in rats. Behav Brain Res. 2009;203:296–303. doi: 10.1016/j.bbr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Du H, Jiang L, Ma X, de Graaf RA, Behar KL, Mason GF. Oxidation of ethanol in the rat brain and effects associated with chronic ethanol exposure. Proc Natl Acad Sci U S A. 2013;110:14444–14449. doi: 10.1073/pnas.1306011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthen DM, Lambeth PS, Ottolini M, Shi YT, Barker BS, Gaykema RP, Newmyer BA, Joy-Gaba J, Ohmura Y, Perez-Reyes E, Guler AD, Patel MK, Scott MM. Activation of Pyramidal Neurons in Mouse Medial Prefrontal Cortex Enhances Food-Seeking Behavior While Reducing Impulsivity in the Absence of an Effect on Food Intake. Frontiers in Behavioral Neuroscience. 2016;10 doi: 10.3389/fnbeh.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin JE, Lindgren HS, Gardi J, Nyengaard JR, Brundin P, Mohapel P, Cenci MA. Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia. J Neurosci. 2006;26:9448–9461. doi: 10.1523/JNEUROSCI.0944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


