Abstract
Background
Osteoporosis is diagnosed by bone loss using a radiological parameter called T-score. Preclinical studies use DXA to evaluate bone status were the T-score is referenced on bone mineral density (BMD) values of the same animals before treatment. Clinically, the reference BMD represents values of an independent group of healthy patients around 30 years old. The present study established a clinically similar T-score standard to diagnose osteoporosis in a sheep model.
Material/Methods
We used 31 female merino land sheep (average 5.5 years old) to study osteoporosis. The following groups were compared using DXA measurement: 1) control; 2) ovariectomized (OVX); 3) OVX combined with a deficient diet (OVXD); and 4) OVXD combined with methylprednisolone administration (OVXDS). Further, an independent group of 32 healthy sheep (4–6 years old) were measured as an independent baseline. BMD was measured at 0 months, 3 months, and 8 months after treatment.
Results
The same significance pattern between the treated groups and either baseline groups was seen. However, using an independent baseline changed the “clinical” interpretation of the data from an osteoporotic bone status (T-score <−2.5) after 3 months of OXDS treatment into an osteopenic bone status (T-score <−1.5 to −2.4).
Conclusions
Using an independent baseline enhanced the statistical significance and showed the clinical relevance. Furthermore, an independent baseline is a reliable alternative to use of a new control group for future experiments and thus reduces the number of animals needed by eliminating the need for a control and corresponding to clinical practice.
MeSH Keywords: Animal Testing Alternatives; Bone Diseases, Metabolic; Databases as Topic; Osteoporosis
Background
Bone is a connective tissue that forms the skeleton to provide protection and mobility [1]. Bone remodeling maintains the integrity of bone tissue. However, different factors, such as menopause-related hormonal changes, affect bone homeostasis [2]. Osteoporotic fractures increase morbidity and mortality in the elderly population, which has socio-economic effects on society [3,4]. Therefore, understanding the biology of osteoporotic bone in preclinical animal models remains crucial [5].
Preclinical studies aim to recapitulate clinically reported osteoporotic bone changes in animal models [6]. Bilateral ovariectomy (OVX) is the usual approach of choice, either alone [7] or in combination with either a vitamin D-deficient diet [8], or a low-calcium diet [9]. The US Food and Drug Administration (FDA) guidelines propose that 2 species are needed for testing new therapies for osteoporosis [10,11]. We recently reported an in-depth characterization of an ovariectomized (OVX) rat model [12–14], which was also utilized to study the healing of osteoporotic metaphyseal fracture aided with newly developed bone substitutes [15,16]. The current study is the first step in a multi-center study to understand detailed alterations of bone tissue in a sheep model, which will be used to recapitulate bone healing and study its enhancement using novel biomaterials. In both studies, the reference group was an untreated internal group.
Unlike rats, sheep are brought to preclinical studies directly from the flock and are not bred specifically as experimental animals. However, this diversity makes sheep in a sense more clinically relevant, as it mimics the diversity of human patients. Ovariectomy (OVX) was reported to induce bone loss in sheep [17]. However, the ability to develop such bone loss was not always described [18]. On the other hand, it is difficult to produce bone loss in sheep by malnutrition alone. However, a diet deficient in calcium and vitamin D reflected osteoporotic structural parameters in sheep bone when combined to OVX [19]. Steroid therapy alone was reported to induce osteoporotic bone status in sheep [20]. Corticosteroid administration, such as methylprednisolone, accelerates bone loss in combination with OVX. Structural changes and biomechanical impairment resulting from steroidal therapy resemble those found in steroid-treated patients [21]. Longitudinal in vivo bone loss in preclinical models is usually determined by Dual Energy X-Ray Absorptiometry (DXA) by assessing bone mineral content (BMC in g) and areal bone mineral density (BMD, in g/cm2).
Clinically, osteoporotic bone status is determined by T-score [22], which is the correlation to a baseline of a healthy, skeletally mature patient population. Because such a baseline does not exist in preclinical studies, animals before treatment are often used as the reference. The lack of a unanimous baseline, as in human patients, affects the interpretation of the results. Therefore, many studies report a percentage of bone loss to avoid confusion.
The present study also used an independent baseline for the calculation of the T-score. To do so, the relevance of T-score calculation in establishing a significant and rapid osteoporotic bone status in sheep by a triple treatment strategy is addressed.
Proving that the independent baseline is reliable would preclude the future use of controls, adhering to the 3 R’s principles [23] for animal welfare. Furthermore, this would allow use of T-scores in osteoporosis research in addition to BMD loss [%], which makes it even more clinically relevant.
The motivation to use this extra group as independent control had several goals: 1) Achieving a more clinically relevant (real-life) control group; 2) Emphasizing that a control group and even the OVX group could be spared in future studies as in the clinical situation; and 3) The use of a pre-intervention time point (0 months) can be misleading in the interpretation of the data, especially when projected onto the clinical diagnosis.
We hypothesized that alteration of bone status due to treatment in the ewe will remain visible when compared to the pretreated animals and the independent baseline.
Our future goal is to start an online open source panel for bone researchers to upload their DXA results of healthy skeletally mature sheep to serve as a clinically relevant baseline for the calculation of T-score. Thusly, a larger pool of individuals will allow us to assess T-score and Z-score without the need of operating and sacrificing a control group in upcoming studies.
Ethics statement
The study was conducted in strict accordance with the European Union legislation for the protection of animals used for scientific purposes; therefore, it was approved by the district’s Animal Ethics Committee “Government Presidium of Darmstadt, Germany”, permit no. Gen. Nr. F31/36.
Material and Methods
Experimental design
Sheep grouping
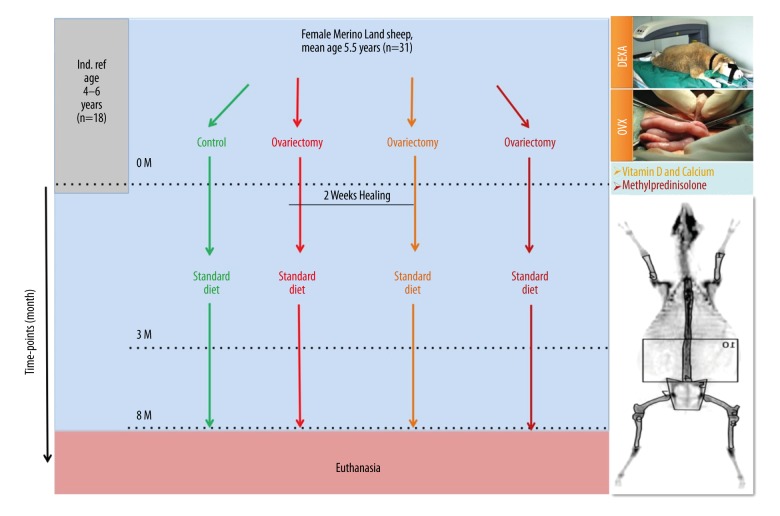
We randomly chose 31 skeletally mature merino sheep from herds around Wiesbaden, Germany. One sheep was excluded from the study because of pregnancy, leaving 31 animals for the study. Those sheep were divided into 4 different treatment groups with an age range of 3 to 9 years and an average of 5.5 years. Animals were divided into 4 groups: 1) non-operated sham group (Control, n=8); 2) bilaterally ovariectomized group (OVX, n=7); 3) bilaterally ovariectomized and treated with special diet deficient of calcium and vitamin D group (OVXD, n=8); and 4) triple treatment group, which received a biweekly dosage of subcutaneous glucocorticoid treatment (OVXDS, n=8) 8ml (40 mg/ml) methylprednisolone acetate in addition to the treatment received in OVXD (Figure 1). The accumulative treatment was designed to reduce number of controls. In this study design, the OVX acts as the direct control to the OVXD group to examine the dietary effect on bone. Furthermore, the effect of steroids alone could be deduced by having the OVXD as the direct control. Nonetheless, the impact of cumulative treatments could be compared to the untreated controls and the OVX groups. During the whole experiment, OVXD and OVXDS groups were held in small groups in a covered outside barn at the central research facility of the Johann Wolfgang Goethe University, Frankfurt, Germany.
Figure 1.
Induction of osteoporosis bone status in sheep model. Besides the control and OVX groups alone, 2 treatment regimens were combined to OVX operation in ewes. The first by combining calcium- and vitamin D-deficient diet (OVXD), and the second by adding steroidal therapy to the dietary restriction (OVXDS). The change in bone status was compared to all groups pretreatment and to an independent group of young skeletally mature ewes.
Sheep weight was measured at the beginning of the study (0 months) and at every succeeding time point (3 and 8 months) and veterinarians regularly examined every sheep. Furthermore, the sheep received prophylactic doses of 1 ml/5 kg KGW Febantel (Rintal 2.5% ad us. Vet., Bayer AG, Germany) against parasites.
Furthermore, an independent reference group (Ind. Baseline) of 18 Merino sheep between 4–6 years of age were scanned in vivo independently to acquire and compare T-score.
Surgical procedure preparation and anesthesia
Preoperatively, the sheep received premedication (10 mg/kg Ketamine hydrochloride (Ketavet® 10 mg/ml, Bela-Pharm GmbH und Co. KG, Germany), 0.01 ml/kg Xylazine (Rompun® 2%, Bayer AG, Germany), 0.3 mg/kg Midazolam (Midazolam Rotexmedica 5 mg/ml, ROTEXMEDICA GmbH, Germany), and 0.01 mg/kg Atropine (Atropine sulfate 0.5 mg/ml, B. Braun Melsungen AG, Germany) prior to anesthesia.
After a few minutes, sedation allowed body-weight measurement and shaving of the ventral abdomen, and a claw inspection took place at every measuring time point.
Subsequently intravenous anesthesia was administered with 2 mg/kg Propofol (Propofol 2% (20 mg/1ml), Fresenius Kabi, Germany) and 2 μg/kg Fentanyl (Fentanyl-Hameln 50 μg/ml, Hameln pharmaceuticals GmbH, Germany). During the DXA scan after intubation and prior to bilateral ovariectomy, a Propofol perfusor with 50 ml/h was administered. Every sheep received a prophylactic antibiotic administration of 0.1 mL/kg penicillin (Veracin® RS, Albrecht GmbH, Germany) as well as an opiate for analgesia of 0.01 mg/kg buprenorphine hydrochloride (TEMGESIC® ampoules 0.3 mg, RB Pharmaceuticals GmbH, Germany) subcutaneously at all 3 time points.
Bilateral ovariectomy operation
Sheep in groups OVX, OVXD, and OVXDS were transported after the DXA measurements to the operation room and placed in supine position. Sheep received inhalation anesthesia with isoflurane. All 4 limbs were fixated with gauze bandages and the operation section was cleaned, disinfected, and covered sterilely. A skin incision cranial to the udder was performed. The subjacent layer of fat was then dissected and the linea alba was presented. A sting incision was made through the linea alba and the peritoneum. The uterus, fallopian tubes, and ovaries were exposed in situ. A transfixation ligature with a resorbable suture (Vicryl 4-0, Ethicon Johnson and Johnson Medical GmbH, Germany) was conducted and the ovaries were excised. Following a final abdominal inspection, the muscular layer was sutured as well as the skin. After surgery, sheep were put in an indoor barn, initially separated until regaining full consciousness. After veterinary assessment, sheep were released to pasture (OVX) or to the outside barn in small groups (OVXD and OVXDS) at the research facility.
Post-surgical assessment
After the operations, animals received an opiate administration for analgesia of 0.01 mg/kg buprenorphine hydrochloride (TEMGESIC® ampoules 0.3 mg, RB Pharmaceuticals GmbH, Germany) subcutaneously 2 times daily, as well as non-steroidal anti-inflammatory drug once a day intramuscular 0.5 mg/kg meloxicam (Metacam® 20 mg/ml ad. us. vet., Boehringer Ingelheim Vetmedica GmbH, Germany). Opiates were reduced after evaluation by the veterinarians. In the first 5 days after surgery, animals were injected intramuscularly with 0.1 ml/kg penicillin (Veracin® RS, Albrecht GmbH, Germany), every 48 h.
Sheep feeding
Two weeks after ovariectomy, sheep in groups OVXD and OVXDS received special diet (Cat No. S6189-S010, Sondermischung Schaf, 4 mm pellet, SNIFF Spezialdiäten GmbH, Germany). The diet was deficient in calcium and vitamin D3, and was given 1 time in the morning and 1 time in the evening, and water was given ad libitum. In addition to the diet, animals had access to straw as they were held the whole day in a covered outdoor barn filled with straw.
Animals of control and OVX group were fed during wintertime with standard feed (SNIFF Spezialdiäten GmbH, Germany) and in spring they were held on a pasture.
No difference in the food energy values between the standard feed and the special diet, both cover recommended daily requirement of a mature sheep.
Administration of corticosteroids
Two weeks after ovariectomy, every 14 days sheep in the OVXDS group received 320 mg methylprednisolone/sheep (Depot-Medrate® ad us. vet 40 mg/ml injection suspension, Pfizer Deutschland GmbH, Germany). Injections were intramuscular in alternative manner between forelimbs and hind limbs.
Euthanasia
After 8 months (end time point), animals were euthanized by intravenous administration of 50 mg/kg pentobarbital (Anestesal®, Pfizer, Mexico) under anesthesia as described above.
DXA measurements
To assess bone mineral density (BMD), bone mineral content (BMC), and BMD difference compared to baseline (T-score) and BMD difference compared to age-matched (Z-score), animals were scanned by DXA (lunar prodigy, GE Healthcare, Germany), as an easy, rapid, non-invasive, and precise method [24,25].
The sheep were anesthetized and placed under intubation in prone position with splayed limbs (Figure 1). The neck and head were adjusted and fixed using the provided accessories. A whole-body scan was carried out. Device calibration was performed according to the manufacturer’s protocol. The respective regions of interest (ROI) – lumbar vertebrae, and both proximal femora – were selected and manually contoured. Subsequently, BMD (g/cm2) and BMC (g) were determined.
The parameters were monitored under anesthesia at 0, 3, and 8 months for the experimental groups and at 0 and 12 months for the independent reference group. The quantitative analysis was done after contouring the ROI in the encore software (GE Healthware; v. 13.40) according to the manufacturer’s recommendations.
Statistical analysis
Calculation of T-score was done by the formula T-score=(Measured BMD–mean BMD of baseline)/baseline population’s standard deviation (SD). Z-score was calculated with the formula; Z-score=(Measured BMD–mean BMD of age-matched)/Age-matched population SD [22]. Data were not normally distributed; therefore, the Mann-Whitney U test was used to examine the significance. Statistical analysis was done in IBM SPSS software V. 21.0 (CA, USA), and significance cutoff was considered P≤0.05 and highlight as a≤0.05, b≤0.01, and c≤0.001.
Results
Osteoporosis is diagnosed by means of DXA when showing inferior BMD reflected by a T-score below −2.5 and Z-score below −1.5.
Osteoporosis induction achieved after 3 months of triple treatment compared to initial bone status
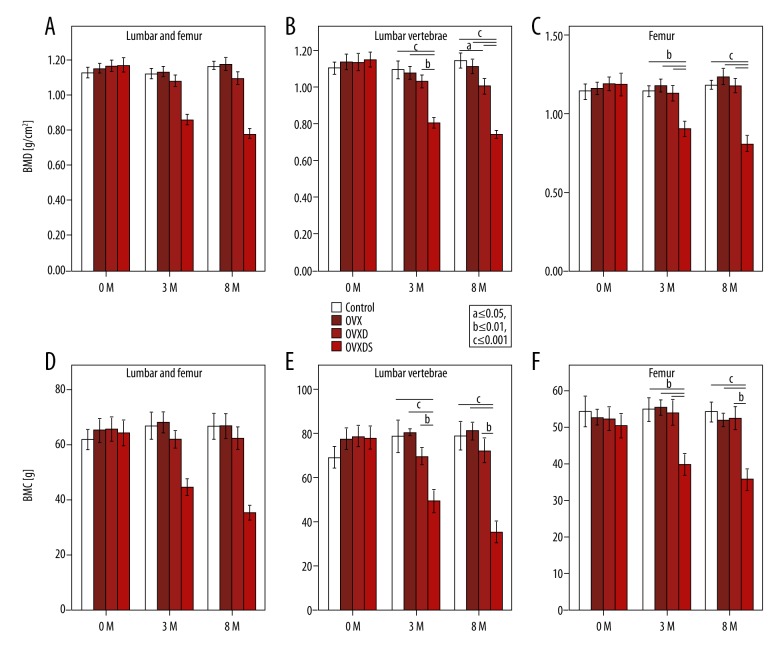
BMD values in the current sheep model showed no significant differences between the groups at 0 months. Nonetheless, OVXDS group showed significant reduction of BMD at 3 months and 8 months in the proximal femur (subsequently called femur) and the lumbar vertebra (LV) together (Figure 2A). Both regions showed significant differences between all groups and the triple treatment group (Figure 2B, 2C). However, in the LV at 8 months, the OVXD is significantly lower than the Control and the OVX group. Intriguingly, the difference between these 2 groups was lost when mean BMD of both regions was taken.
Figure 2.
Combined treatment has progressive effect on bone loss in ewes. (A) BMD mean in both regions reflects the systemic effect of the treatment. (B) LV showed lower values in OVXDS to all groups but also OVXD compared to OVX, suggesting more prevalent effect of the deficient diet on the LV. (C) Femur BMD was significantly lower in OVXDS at 3 months and 8 months when compared to all groups. (D) BMC asserts the BMD pattern showing difference only in the triple treatment group at 3 months and 8 months. (E) Beside lower BMC in OVXDS at 3 months and 8 months, less BMC was also seen in the OVXD compared to the OVX alone. (E) BMC in the femur completely reflects the BMD values (Bonferroni-corrected Mann-Whitney U test. P≤0.05; a≤0.05, b≤0.01, and c≤0.001).
Changes in BMD depend on the BMC values. Both LV and femur regions showed significant differences between all groups at all time points (Figure 2D). LV BMC values showed significant decrease in the OVXDS at 3 months compared to all other groups (Figure 2E). However, in the femur, significantly lower BMC in the OVXDS at 3 months and 8 months was seen in comparison to all groups (Figure 2F).
Z-Score is more representative in experimental data interpretation using age-matched controls
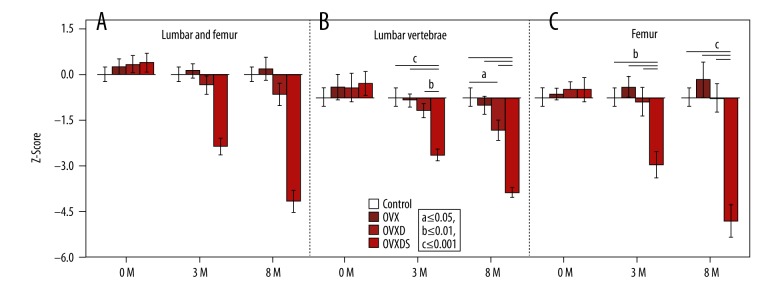
One of the important values in diagnosing the osteoporotic status in patients is the age-matched comparison using the Z-score. Thus, obtaining such a parameter in experimental animals is useful.
The Z-score was calculated for all individuals with the mean of both regions and then for each region separately. The values vary in pattern between the regions and their mean calculation. At 3 months, the mean Z-score reflecting the systemic effect of the treatment was higher but not significant in the OVX compared to the control. Nonetheless, the Z-score of OVXD was lower than both control and OVX whereas OVXDS was lower than OVXD (Figure 3A). The LV Z-scores were not significantly different at the initial time point; however, a decreased Z-score was seen at both 3 months in OVXDS (−2.1) and at 8 months in OVXD (−1.2) and OVXDS (−3.6). At the later time point, the OVXD group was also significantly lower than the OVX and control groups (Figure 3B). The femur results on the other hand showed only significant reduction of the Z-score in the OVXDS at both 3 months (−2.5) and 8 months (−4.6; Figure 3C). Although still not significant, the OVX increase was more obvious in the femur.
Figure 3.
Z-score is affected by the region of analysis reflecting general bone status. (A) Z-score of all ROIs has an intermediate pattern between the regions but still significantly lower in the triple treatment group. (B) Lower but not significant Z-scores in the OVXD at 3 months and 8 months in LV. (C) An intriguing not significant increase in Z-score of OVX in the femur at 3 months and 8 months (Bonferroni-corrected Mann-Whitney U test. P≤0.05; a≤0.05, b≤0.01, and c≤0.001).
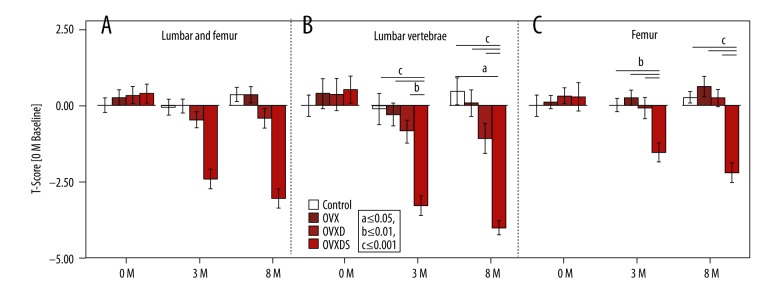
A clinically similar T-score using independent baseline is more stringent in interpreting bone status alterations
The T-score is the clinically indicative parameter of osteoporotic bone status. Experimentally, pretreated individuals are utilized for baseline calculations. The T-score reflects no differences at the initial time point in any region. Later, at 3 months and 8 months, the calculated T-score on both ROIs showed a significantly lower value in the OVXDS and a non-significantly lower T-score in the OVXD (Figure 4A). In LV, a T-score of −3.3 and −4.1 was detected in OVXDS at 3 months and 8 months, respectively (Figure 4B). Furthermore, a T-score of −0.2 at 3 months and -0.8 at 8 months in the OVXD was seen. Intriguingly, an increased T-score in the OVXD group femur was noted at both 3 months and 8 months. However, the OVXDS was decreased with −1.5 and −2.2 T-score, respectively (Figure 4C).
Figure 4.
Experimental T-score is calculated based on animals BMD values pretreatment. (A–C) Lower T-score indicated osteoporotic bone status in the OVXDS at 3 months and 8 months. (B) Progressive declination of T-score in OVXD from 3 months to 8 months. (C) An intriguing non-significant increase in OVX and OVXD from 3 months to 8 months. (Bonferroni-corrected Mann-Whitney U test. P≤0.05; a≤0.05, b≤0.01, and c≤0.001).
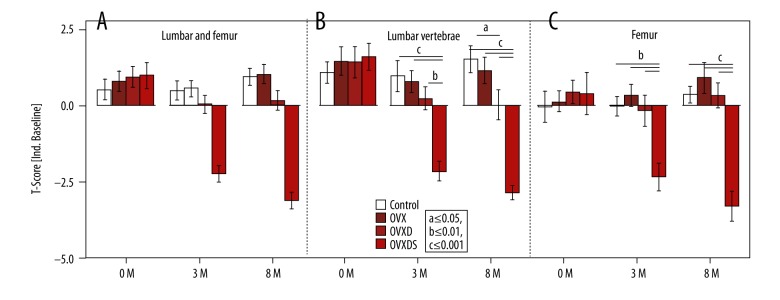
On the other hand, this study utilized also an independent reference group as the baseline (Figure 5). T-Score was lower only in the OVXDS at 3M (−2.1 for LV and −2.3 for femur) and at 8M (−2.8 for LV and −3.3 for femur) Table 1.
Figure 5.
Clinically relevant T-score is calculated to an independent baseline. (A–C) A positive T-score in all groups apart from OVXDS at 3 months and 8 months. (B) A significant but not osteoporotic decline in T-score values of OVXD at 3 months and 8 months. (C) Increasing trend in OVX T-score values in the femur (Bonferroni-corrected Mann-Whitney U test P≤0.05; a≤0.05, b≤0.01 and c≤0.001).
Table 1.
T-score values according to the baseline group for LV (A) and proximal femur (B).
| Group | Time point | Z-score | T-score [OM baseline] | T-Score [ind. baseline] |
|---|---|---|---|---|
| [A] | LV | |||
| Control | 0M | – | 0.0000 | 1.0812 |
| 3M | 0.0000 | −0.1100 | 0.9735 | |
| 8M | 0.0000 | 0.4599 | 1.5314 | |
| OVX | 0M | 0.3859 | 0.3859 | 1.4590 |
| 3M | −0.1278 | 0.2977 | 0.7899 | |
| 8M | −0.3001 | 0.0844 | 1.1639 | |
| OVXD | 0M | 0.3640 | 0.3640 | 1.4375 |
| 3M | −0.5026 | −0.8478 | 0.2513 | |
| 8M | −1.2438 | −1.0961 | 0.0083 | |
| OVXDS | 0M | 0.5205 | 0.5205 | 0.5908 |
| 3M | −2.1835 | −3.3151 | −2.1638 | |
| 8M | −3.6016 | −4.0460 | −2.8793 | |
| [B] | Femur | |||
| Control | 0M | 0.0000 | 0.0000 | −0.0291 |
| 3M | 0.0000 | 0.0140 | −0.0082 | |
| 8M | 0.000 | 0.2707 | 0.3744 | |
| OVX | 0M | 0.1154 | 0.1154 | 0.1429 |
| 3M | 0.3865 | 0.2522 | 0.3468 | |
| 8M | 0.6837 | 0.6333 | 0.9148 | |
| OVXD | 0M | 0.3219 | 0.3219 | 0.4507 |
| 3M | −0.1634 | −0.0867 | 0.1583 | |
| 8M | −0.0420 | 0.2484 | 0.3412 | |
| OVXDS | 0M | 0.2889 | 0.2889 | 0.4015 |
| 3M | −2.5433 | −1.5534 | −2.3445 | |
| 8M | −4.6647 | −2.2030 | −3.3127 | |
Discussion
In clinical practice, DXA is the criterion standard in bone density assessment. Despite its relatively limited spatial resolution and the lack of capacity to differentiate cortical and trabecular bone compartments, DXA is still dependable due to its reproducible, rapid, and relatively low-cost ability to measure longitudinal changes in BMD. The application of quantitative computer tomography (qCT) is rather less convenient for the volumetric BMD measurements. In preclinical bone research, due to their docile nature, comparatively low cost of housing, and similarities to human bone structure and metabolism, the ovariectomized ewe is becoming an invaluable model for investigating new implants and/or bone substitutes. Furthermore, the model could serve in testing newly developed anti-osteoporotic drugs.
This study was performed to establish an osteoporotic bone status in a sheep model resulting from combining estrogen deficiency via ovariectomy, malnutrition through calcium and vitamin D restriction, with addition to steroidal therapy by methylprednisolone injection. Moreover, the study highlights the role of an independent control group and its impact on stringency and interpretation of results.
Despite the lack of clinical relevance, experimental setups in bone research have always used the pretreated animals as the baseline to study changes in their bone density [26]. Ethically, the setup is more convenient than adding an independent control group of research animals. However, methodological advances in radiology, especially DXA scanning, and open source sharing allow researchers to use an independent control group.
The deduced results offer a concept matching the clinically employed concept, which diagnose patients depending on independently collected data form a validated baseline.
Therefore, we have conducted this study to compare the pretreated group as a control with an independent control that was prepared for a different experimental setup. Our study focused on 2 high fracture risk regions in an osteoporotic bone: femora and lumbar vertebrae (LV).
T-score and Z-score measurements depict the risk of osteoporosis or fracture among patients, respectively. Clinically, the value compares the patient’s bone status to a preset calibration based on a globally collected database utilized as baseline. The baseline represents young, healthy, and skeletally mature individuals. This shows bone density changes in relation to the peak bone mass. In animal research, Z-score baseline represents age-matched animals and this reflects the bone status of the individual compared to peers. However, in sheep, the Z-score is a rather vague definition due to the dependence on the mean age per group. As in sheep experiments, animals are often not bred for research purposes; therefore, different ages are used to reach a certain group mean age. However, taking only the mean age into consideration influences the deviation of the given group of 8 individuals; thus, the resulting data as this group can have different age homogeneity. For example, in this study, animals were between 3 and 9 years old and were distributed into the groups to reach a mean age of 5.5 years. On the other hand, the independent baseline was selected out of a larger pool of animals and the mean age was taken from animals 4–6 years old, resulting a more homogenous group with less deviation. This is exactly what is followed in the clinic due to the larger number of individuals, and the baseline can be set for T-score and Z-score with less deviation.
Our measurements were focused on T-score to see the effect of utilizing an independent group.
The results concluded the induction of osteopenic and osteoporotic bone status after triple treatment at 3 months and 8 months. Other treatment regimens were not effective in altering the bone status into a clinically recognized definition (i.e., osteoporotic, osteopenic). The triple treatment effect was more prevalent on the trabecular bone than the cortical bone. This is suggested by the decrease in BMD and BMC parameters in the LV in contrast to the proximal femur. This accords with the described composition of trabecular bone (95% trabecular, 5% cortical bone) [27,28]. Furthermore, the effect of methylprednisolone on the LV is inferred by the reports of higher susceptibility of vertebrae to steroidal therapy than the distal radius and proximal femur [29].
Our findings reflect a change in the data interpretation from osteoporotic into osteopenic bone status in the LV and from osteopenic to osteoporotic in the proximal femur when replacing the internal baseline with the independent one. Nonetheless, the main conclusion remains the same.
The use of an independent baseline showed valid data that can be utilized for further experiments.
Using Google Scholar search (keywords: sheep, ewe, osteoporosis, DXA, DXA) revealed 2016 studies in the last 10 years and the vast majority used DXA to assess bone quality. With current technological advancement and database management, we trust that sparing the control group in any future or current osteoporotic sheep model is possible for both control and OVX groups.
Our data will be provided as an open source for researchers to be utilized in T-score calculation. A preliminary start is found at http://DXAdb.glycosciences.de/. Furthermore, we assigned an email address (DXA@chiru.med.uni-giessen.de) for those who would like to share their data into the open source website.
Moreover, the central research facility in Frankfurt is the main animal research facility in Hessen capable of housing and care. Taking of sheep as a large animal model, we have decided to keep a DXA device placed in the facility and asked the animal experiment controllers to advise research groups to scan their animals prior to surgery. The labor for operating the DXA and assessment of the data will be provided by the Institute of Experimental Trauma Surgery in Giessen.
Conclusions
Finding alternatives to preclinical animal experiments is urgent and crucial. However, such alternatives shall have the same capacities to serve the purpose and must be clinically relevant.
We showed that transferring clinical practice into the preclinical research practice in this particular case will provide more clinically relevant results and, most importantly, reduce the number of research animals needed.
The current study showed that the use of an independent group affects the “clinical” interpretation of the bone loss results without changing the main significance of the data.
Beside the advances in in vivo imaging and organ culture, an alternative to animal experiments following the 3R principles can be achieved with data management.
Footnotes
Conflict of interest
None.
Source of support: This study was funded by DFG, the German Research Foundation (SFB/TRR 79, Projects T1, B7, and B12), and the BMBF Federal Ministry of Education and Research VIP project, HA-screw
References
- 1.Hercz G. Regulation of bone remodeling: Impact of novel therapies. Semin Dial. 2001;14(1):55–60. doi: 10.1046/j.1525-139x.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 2.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–45. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsson MK, Gerdhem P, Ahlborg HG. The prevention of osteoporotic fractures. J Bone Joint Surg Br. 2005;87(10):1320–27. doi: 10.1302/0301-620X.87B10.16578. [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 5.Turner AS. Animal models of osteoporosis – necessity and limitations. Eur Cell Mater. 2001;1:66–81. doi: 10.22203/ecm.v001a08. [DOI] [PubMed] [Google Scholar]
- 6.Wang G, Wang J, Sun D, et al. Short-term hypoxia accelerates bone loss in ovariectomized rats by suppressing osteoblastogenesis but enhancing osteoclastogenesis. Med Sci Monit. 2016;22:2962–71. doi: 10.12659/MSM.899485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francisco JI, Yu Y, Oliver RA, Walsh WR. Relationship between age, skeletal site, and time post-ovariectomy on bone mineral and trabecular microarchitecture in rats. J Orthop Res. 2011;29(2):189–96. doi: 10.1002/jor.21217. [DOI] [PubMed] [Google Scholar]
- 8.Melhus G, Solberg LB, Dimmen S, et al. Experimental osteoporosis induced by ovariectomy and vitamin D deficiency does not markedly affect fracture healing in rats. Acta Orthop. 2007;78(3):393–403. doi: 10.1080/17453670710013988. [DOI] [PubMed] [Google Scholar]
- 9.Costa GP, Leite DS, do Prado RF, et al. Effect of low-calcium diet and grind diet on bone turnover of ovariectomized female rats. Med Oral Patol Oral Cir Bucal. 2011;16(4):e497–502. doi: 10.4317/medoral.16.e497. [DOI] [PubMed] [Google Scholar]
- 10.Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA guidelines and animal models for osteoporosis. Bone. 1995;17(4):S125–33. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Meng H, Wang X, et al. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med Sci Monit. 2016;22:226–33. doi: 10.12659/MSM.897044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocker W, El Khassawna T, Bauer N, et al. Short-term glucocorticoid treatment causes spinal osteoporosis in ovariectomized rats. Eur Spine J. 2014;23(11):2437–48. doi: 10.1007/s00586-014-3463-z. [DOI] [PubMed] [Google Scholar]
- 13.El Khassawna T, Bocker W, Govindarajan P, et al. Effects of multi-deficiencies-diet on bone parameters of peripheral bone in ovariectomized mature rat. PLoS One. 2013;8(8):e71665. doi: 10.1371/journal.pone.0071665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindarajan P, Bocker W, El Khassawna T, et al. Bone matrix, cellularity, and structural changes in a rat model with high-turnover osteoporosis induced by combined ovariectomy and a multiple-deficient diet. Am J Pathol. 2014;184(3):765–77. doi: 10.1016/j.ajpath.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Thormann U, Ray S, Sommer U, et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials. 2013;34(34):8589–98. doi: 10.1016/j.biomaterials.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Ray S, Thormann U, Sommer U, et al. Effects of macroporous, strontium loaded xerogel-scaffolds on new bone formation in critical-size metaphyseal fracture defects in ovariectomized rats. Injury. 2016;47:S52–61. doi: 10.1016/S0020-1383(16)30013-4. [DOI] [PubMed] [Google Scholar]
- 17.Turner AS, Alvis M, Myers W, et al. Changes in bone mineral density and bone-specific alkaline phosphatase in ovariectomized ewes. Bone. 1995;17(4 Suppl):395S–402S. doi: 10.1016/8756-3282(95)00317-7. [DOI] [PubMed] [Google Scholar]
- 18.Pogoda P, Egermann M, Schnell JC, et al. Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res. 2006;21(10):1591–99. doi: 10.1359/jbmr.060709. [DOI] [PubMed] [Google Scholar]
- 19.Lill C, Fluegel A, Schneider E. Effect of ovariectomy, malnutrition and glucocorticoid application on bone properties in sheep: A pilot study. Osteoporosis Int. 2002;13(6):480–86. doi: 10.1007/s001980200058. [DOI] [PubMed] [Google Scholar]
- 20.Kiełbowicz Z, Piątek A, Bieżyński J, et al. The experimental osteoporosis in sheep – clinical approach. Pol J Vet Sci. 2015;18(3):645–54. doi: 10.1515/pjvs-2015-0083. [DOI] [PubMed] [Google Scholar]
- 21.Ding M, Cheng L, Bollen P, et al. Glucocorticoid induced osteopenia in cancellous bone of sheep: Validation of large animal model for spine fusion and biomaterial research. Spine (Phila Pa 1976) 2010;35(4):363–70. doi: 10.1097/BRS.0b013e3181b8e0ff. [DOI] [PubMed] [Google Scholar]
- 22.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J. 2007;83(982):509–17. doi: 10.1136/pgmj.2007.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell WMS, Burch RL, Hume CW. The principles of humane experimental technique. 1959 [Google Scholar]
- 24.Gillette-Guyonnet S, Andrieu S, Nourhashemi F, et al. Comparison of bone mineral density and body composition measurements in women obtained from two DXA instruments. Mech Ageing Dev. 2003;124(3):317–21. doi: 10.1016/s0047-6374(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Piao J, Pang L, et al. The diagnostic criteria for primary osteoporosis and the incidence of osteoporosis in China. J Bone Miner Metab. 2002;20(4):181–89. doi: 10.1007/s007740200026. [DOI] [PubMed] [Google Scholar]
- 26.Zarrinkalam MR, Beard H, Schultz CG, Moore RJ. Validation of the sheep as a large animal model for the study of vertebral osteoporosis. Eur Spine J. 2009;18(2):244–53. doi: 10.1007/s00586-008-0813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonacci MD, Hanson DS, Leblanc A, Heggeness MH. Regional variation in vertebral bone density and trabecular architecture are influenced by osteoarthritic change and osteoporosis. Spine. 1997;22(20):2393–401. doi: 10.1097/00007632-199710150-00014. [DOI] [PubMed] [Google Scholar]
- 28.Huiskes R, Ruimerman R, Van Lenthe GH, Janssen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405(6787):704–6. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- 29.Graves L, Lukert BP. Glucocorticoid-induced osteoporosis. Clin Rev Bone Miner Metab. 2004;2(2):79–90. [Google Scholar]