Abstract.
The incidence of an indigenous malaria, defined as malaria acquired by a local mosquito transmission, declined from 2004 to 2015 in the Guangxi Zhuang Autonomous Region. However, imported malaria, defined as malaria acquired from other endemic regions outside of China, has been increasing in the region, as in the rest of the country, particularly the disease caused by Plasmodium falciparum. A retrospective study was conducted to explore malaria-endemic characteristics in Guangxi during the 2004–2015 timeframe; a total of 2,726 confirmed malaria cases were reported, and the majority (90.3%) were due to P. falciparum (N = 1,697 [62.2%]) and Plasmodium vivax (N = 765 [28.1%]). Thirty-four indigenous cases (1.2%) were observed, with no cases of transmission recorded since 2012. Imported P. vivax and Plasmodium ovale infections increased since 2013. The interval between returning to China and the onset of illness was longer for P. vivax and P. ovale infections than for P. falciparum and Plasmodium malariae infections. The difference interval among the species is likely because of the relapse of P. vivax and P. ovale caused by the activation of the latent hypnozoites. Therefore, health clinics should raise awareness and carry out epidemiological studies and follow-up surveys on migrant workers to avoid misdiagnosis and mistreatment. The evaluation of radical treatment should be carried out using a genotyping technology based on glucose-6-phosphate dehydrogenase deficiency levels, and some new drugs active against the hypnozoites should be developed to mitigate malaria in the region.
INTRODUCTION
The Guangxi Zhuang Autonomous Region (hereafter referred to as Guangxi) was once the site of a very high rate of malaria transmission. Malaria morbidity peaked in the region in 1954 at 296.7 cases per 10,000 per year. After the implementation of integrated strategies, such as insecticide-treated nets and indoor residual spraying (IRS), combined with environment improvement, case management, and mass drug administration of febrile individuals, the disease burden declined sharply.1,2 From 2000 to 2010, the reported malaria incidence was below 1 per 10,000 per year in Guangxi and entered the lowest level in 2008 (0.09/100,000) because of the National Malaria Control Program (2006–2015) and funding from both the Chinese government and external stakeholders, such as the Global Fund to Fight AIDS, Tuberculosis, and Malaria Program.3
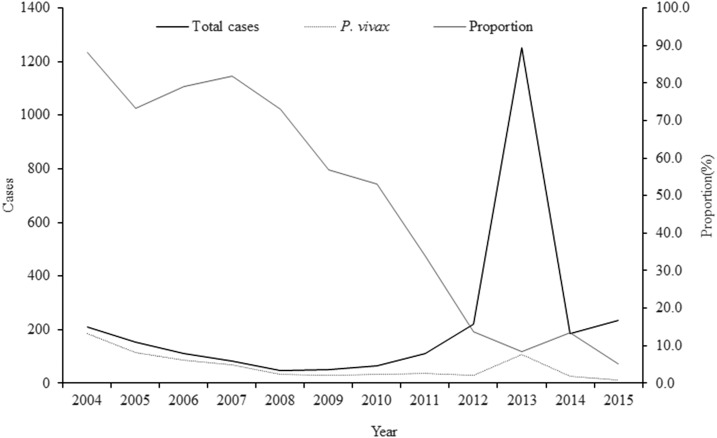
In Guangxi, Plasmodium vivax had been the primary malaria species before 2013. There were 621 reported cases of P. vivax infection from 2004 to 2012, which accounted for 58.9% of all reported malaria cases. However, the proportion of malaria caused by this parasite declined during that time period (Figure 1). One reason for this is the reduction of local transmission and the heavily increasing number of cases of imported Plasmodium falciparum from Africa, which has been observed in Guangxi as in China overall.4 Since 2013, the numbers and proportion of P. vivax increased from 30 P. vivax reported in 2012 to 107 P. vivax reported in 2013. It is well known that P. vivax is more difficult to control and eliminate than P. falciparum because of its tendency to relapse after treatment of the primary infection because of hypnozoite activity. In endemic areas, such as the tropics, the relapse of P. vivax malaria is a major contributor of the overall malarial incidence and an important source of malaria transmission.5 This is also true of Plasmodium ovale, and late relapse may occur because of the failure to receive or adhere to the prescribed chemoprophylaxis.6–9
Figure 1.
Plasmodium vivax in Guangxi, 2004–2015. The total cases (black solid line), P. vivax (black dash line) cases, and its proportion (grey line) were indicated.
Plasmodium vivax and P. ovale causes two distinct infection syndromes: one actively proliferative and the other dormant or latent.10 Hypnozoites (the dormant liver stage) are one of the factors that cause the relapse of P. vivax and P. ovale malaria after drug treatment.11 Such relapse could provide parasites with new opportunities for sexual reproduction and transmission and cause recurring clinical episodes in people living in or visiting endemic areas. In temperate regions and part of the subtropics, P. vivax and P. ovale infections are characterized either by a long incubation or a long latent period between primary infection and relapse approximating 8–10 months.12 Although, the blood-stage infection of P. vivax and P. ovale could be effectively treated while prophylactic medication is administered, the relapses occurring after departure from an endemic area present a significant problem to human health and pose an obstacle to eliminating malaria.13,14 They can initiate local transmission to malaria-free localities, where Anopheles mosquitoes still remain.15 In this study, the malaria characteristics in Guangxi from 2004 to 2015 were investigated. This period was selected because the Chinese web-based reporting system was established in 2004 and data going back to 2005 are available. Because China and other countries working toward eliminations also face the same challenges as Guangxi, and studies such as these could have practical implications for the nation and the development of elimination strategies and interventions in a timely manner.16
MATERIALS AND METHODS
Study design.
This study included all patients clinically diagnosed with malaria by parasitological analysis between 2004 and 2015 in the Guangxi Autonomous Region in China. Clinically diagnosed cases are defined here as individuals with malaria-related symptoms (fever [axillary temperature ≥ 37.5°C], chills, severe malaise, headache, or vomiting) at the time of examination. In China, the “1-3-7” elimination strategy was conducted and described as a simplified set of targets that delineate responsibilities, actions, and their timeframe, which means case reporting within 24 hours, case confirmation and investigation within 3 days, and foci investigation and respond within 7 days.17,18 In this study, all the blood smear and blood-spot filter paper samples were taken from the case and delivered to the Guangxi provincial reference laboratory for microscopic verification and molecular verification using polymerase chain reaction (PCR). The definitions used in this study were listed in Table 1. The indigenous cases were defined as malaria-acquired by mosquito transmission in any endemic area within China. Imported cases were defined as patient-acquired from a known malaria-prevalent region outside Guangxi and were required to meet all three of the criteria described before.19 A county-level polygon map scale was produced for conducting a geographic information system (GIS)-based analysis on the spatial distribution of malaria in Guangxi. On this scale, the county-level point layer containing information regarding latitudes and longitudes of central points of each county was created. Annual parasitic incidence (API) = total confirmed cases in a year × 1,000/total population.
Table 1.
Definition used in this study
| Type of malaria | Description |
|---|---|
| Clinically diagnosed case | An individual with malaria-related symptoms (fever [axillary temperature ≥ 37.5°C], chills, severe malaise, headache, or vomiting) at the time of examination but detected negative using microscopy or rapid diagnostic tests. |
| Laboratory diagnosed case | A clinical case confirmed by microscopy or polymerase chain reaction or rapid diagnostic tests in the laboratory. |
| Indigenous case | Malaria acquired by mosquito transmission in an area within China. |
| Imported case | Patient acquired illness from a known malaria-prevalent region outside of China. |
| Relapsing | Relapses caused by the reactivation of dormant liver-stage parasites (hypnozoites) of Plasmodium vivax and Plasmodium ovale. |
| Death from malaria | Patient with signs and symptoms of complicated malaria, with confirmed diagnose of Plasmodium falciparum (or P. vivax) or associated infection. |
Data source.
All cases reported by the Guangxi from 2004 to 2015 in the web-based reporting system were carefully reviewed and analyzed. Health staff in both public and private medical facilities were required to report all confirmed and suspected cases. In China, a standard form was adopted by the physicians and the Center for Disease Control and Prevention (CDC) staff to collect individual information on each case of malaria, including name, gender, age, contact number, date of onset, results of laboratory diagnosis, and drugs. For the analysis of intervals from the onset of fever to diagnosis of malaria species and the geographic source of the infection, the interval from the onset of fever to diagnosis were included. Since the launch of the National Malaria Elimination Action Plan in 2010,20 the malarial species was confirmed for each case by laboratory methods and identified as imported or locally acquired. The current work was performed using data from 2010 to 2015 via the web-based reporting system. For analysis of the latency period of each malarial species, because the numbers of cases of P. vivax and P. ovale have risen significantly since 2013, herein the data from 2013 to 2015 were used. Local infections and imported malaria cases were obtained through the annual malaria reporting system. For the analysis of the distribution of local and imported cases, the API of each county in Guangxi from 2004 to 2015 were collected and imported into the ArcGIS 10.1 (ESRI Inc., Redlands, CA), categorized by 0, 0–0.01, 0.01–0.1, 0.1–1, and > 1. The population data for every county in Guangxi from 2004 to 2015 were obtained from the National Bureau of Statistics of China (http://data.stats.gov.cn/). Environmental data including rainfall and temperature were obtained from the China Meteorological Data Service Center (http://data.cma.cn/).
Seasonal feature analysis.
The seasonal index was used to understand the seasonal patterns of malaria incidence. The index was calculated by month, and it was the case number for a given month (i.e., January) divided by the mean number of cases in that corresponding month during the timeframe 2004–2015.21 No obvious seasonal pattern was expected if the seasonal index of each month was close to 1.0.
Statistical analysis.
Statistical analyses were conducted using the SPSS 21.0 software and the R-3.3.2 software. A Kruskal–Wallis test was used to investigate the latent period, and the interval from the onset of fever to diagnosis was significantly different between all Plasmodium species, and a Nemenyi test was carried out to investigate the difference of Plasmodium species between multiple comparisons.
RESULTS
Malaria incidence in Guangxi from 2004 to 2015.
Of 2,726 malaria cases reported, a total of 2,692 (98.8%) were imported and 34 (1.2%, including 33 P. vivax and one clinically diagnosed case) were locally acquired. Among the imported cases, four Plasmodium species were identified: P. falciparum (N = 1,697 [63.0%]), P. vivax (N = 732 [27.2%]), P. ovale (N = 123 [4.6%]), Plasmodium malariae (N = 28 [1.1%]), and mixed infections (N = 44 [1.6%]) from 2004 to 2015.22–33 There were 68 (2.5%) clinically diagnosed cases reported among the imported cases. During this period, the number of cases was lowest in 2008 (N = 48). Since 2009, the number of cases of malaria has increased, peaking in 2013 because of the malaria outbreak affecting the returning workers from Ghana in Shanglin County.34 A total of 1,251 malaria cases were recorded in Guangxi during the entire year of 2013 from the web-based reporting system, and all were imported cases, corresponding to a 4-fold increase from 220 cases reported in 2012.35 Among them, 1,052 were Chinese miners returning from Ghana (84.1%, 1,052/1,251) to Guangxi’s Shanglin County, contributing to the high proportion of imported cases nationwide that year.36 This in turn led to a high proportion of malaria cases nationwide, with Guangxi contributing to 30.3% of all cases in 2013.
Plasmodium vivax was the predominant species in Guangxi before 2012. From 2004 to 2012, P. vivax malaria accounted for 58.9% of all reported malaria cases even though it comprised only 13.6% in 2012 (Figure 1). After the large number of imported P. falciparum cases in 2013, the number of P. vivax and P. ovale cases also increased (Table 2). During this year (2013), the numbers of P. vivax and P. ovale malaria cases were 107 and 19, respectively, representing 2-fold and 8-fold increases over data collected in 2012. In 2014, the number of P. ovale cases reportedly reached 59, most of these (N = 56) arrived from west and central African countries into three counties including Shanglin, Quanzhou, and Binyang causing 81.4% (48/59), 6.8% (4/59), and 3.4% (2/59) of malaria cases studied here, respectively, accounting for 32.1% of all reported cases in Guangxi, and representing 25.4% of all P. ovale cases (N = 232) reported nationwide. Of all imported P. vivax and P. ovale cases, those from African countries comprise 16.8% (N = 123) and 96.7% (N = 119), respectively.
Table 2.
Malaria in the Guangxi Zhuang Autonomous Region, 2004–2015
| Year | Total | Indigenous | Imported | |||||
|---|---|---|---|---|---|---|---|---|
| Clinically diagnosed | P. v | P. f | P. m | P. o | Mix | |||
| 2004 | 211 | 18 | 8 | 168 | 15 | 0 | 0 | 2 |
| 2005 | 154 | 7 | 19 | 106 | 19 | 0 | 0 | 3 |
| 2006 | 110 | 2 | 14 | 85 | 9 | 0 | 0 | 0 |
| 2007 | 83 | 4 | 7 | 64 | 8 | 0 | 0 | 0 |
| 2008 | 48 | 2 | 3 | 34 | 8 | 0 | 0 | 1 |
| 2009 | 51 | 0 | 2 | 29 | 17 | 1 | 0 | 2 |
| 2010 | 66 | 0 | 4 | 35 | 24 | 1 | 0 | 2 |
| 2011 | 112 | 0 | 6 | 38 | 62 | 2 | 1 | 3 |
| 2012 | 220 | 1 | 5 | 29 | 173 | 5 | 2 | 5 |
| 2013 | 1,251 | 0 | 0 | 107 | 1,105 | 8 | 19 | 12 |
| 2014 | 184 | 0 | 0 | 25 | 91 | 6 | 59 | 3 |
| 2015 | 236 | 0 | 0 | 12 | 166 | 5 | 42 | 11 |
| Total | 2,726 | 34 | 68 | 732 | 1,697 | 28 | 123 | 44 |
Guangxi established a provincial reference laboratory in 2012 to recheck all the blood smear and filter spot samples using microscopy and the PCR, and the proportion of clinically diagnosed cases has decreased significantly, to zero, since 2013.
Seasonal index.
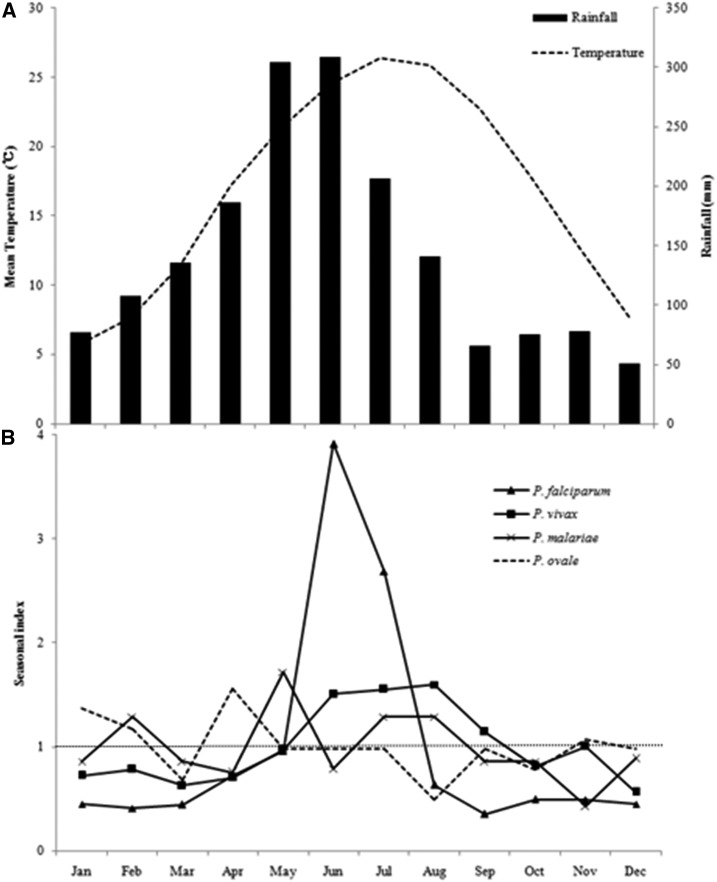
The environmental parameters showed the highest rainfall and temperature to take place in June and July, respectively (Figure 2A). All four species of malaria showed different seasonal characteristic during 2004 and 2015 (Figure 2B). Plasmodium vivax occurred most frequently in June–August and the high index observed in August was 1.6 at temperatures from 25°C to 27°C. All other months presented nearly no obvious seasonal characteristic because the index was close to 1.0 (Figure 2B). The highest incidence of P. vivax, P. falciparum, P. malariae, and P. ovale was observed from April to August, which was related to the imported cases and caused by mobile workers returning to Guangxi to perform agricultural work during this period.
Figure 2.
Seasonal distribution of malaria in Guangxi, 2004–2015. (A) Meteorological parameters comprising rainfall (black column) and temperature (black dash line) were indicated and (B) Seasonal index of Plasmodium falciparum (triangle), Plasmodium vivax (square), Plasmodium malariae (cross), and Plasmodium ovale (dash line). Seasonal index equal to 1 was indicated in the figure. The environmental data are mean values from 2004 to 2015.
Indigenous and imported malaria.
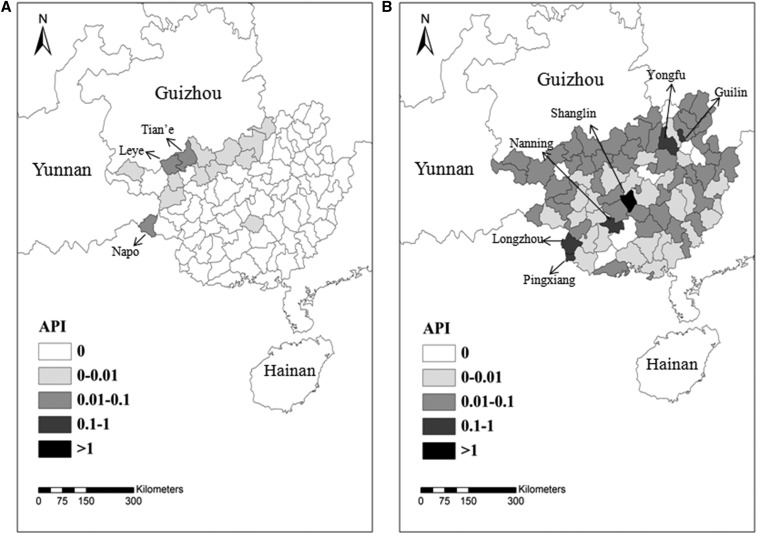
Among all reported cases, the indigenous cases accounted for 1.4% (N = 34) and declined after 2008. Indigenous malaria mainly occurred in the counties of Tian'e (API = 0.058), Leye (API = 0.023), and Napo (API = 0.014) (Figure 3A). Unlike other provinces in China, the proportion of imported cases remained high during the entire time from 2004 to 2015 (all > 95%). For example, from 2010 to 2015, the imported malaria came mainly from West and Central Africa and Southeast Asia, including Ghana (N = 1,534, 57.0%), Cameroon (N = 132, 4.9%), and Myanmar (N = 104, 3.9%) (Supplemental Table 1). Most imported cases were reported in Shanglin (API = 3.085), Nanning (API = 0.385), Guilin (API = 0.242), Longzhou (API = 0.213), Pingxiang (API = 0.179), and Yongfu (API = 0.120) (Figure 3B). The imported malaria was narrowed down to 20 counties in 2015, a 58.3% reduction from 2004 (N = 48).
Figure 3.
Distribution of indigenous and imported malaria in Guangxi, 2004–2015. (A) Indigenous and (B) Imported cases were recorded and analyzed in the map created using ArcGIS 10.1. The counties with API > 0.01 for indigenous cases and API > 0.1 for imported cases are labeled on the map.
Latent period between return to Guangxi and onset of illness caused by P. vivax and P. ovale.
Considering the increasing proportion of P. vivax and P. ovale in Guangxi, herein we investigated the number of days that elapsed between the return to China from abroad and the onset of malarial symptoms to assess the latent period among all Plasmodium species. Analysis of all 1,645 malaria cases that occurred in 2013–2015 (26 cases failed to show up for follow-up studies) showed the median interval between the arrival and diagnosis to be longer for P. ovale (89 days) than for P. vivax (51 days), P. falciparum (7 days), or P. malariae (23 days; P value for all comparisons < 0.001) (Table 3). Between species, P. ovale was observed to have a latent period significantly different from all other species: P. ovale and P. falciparum (P = 0.000), P. ovale and P. vivax (P = 0.000), and P. ovale and P. malariae (P = 0.000). Plasmodium vivax was observed to be significant with P. falciparum (P = 0.000).
Table 3.
Latent period for all Plasmodium species in the Guangxi Zhuang Autonomous Region in China, 2013–2015*
| Species | No. of cases | Latency period (days) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Minimum† | Maximum‡ | ||
| P. falciparum | 1,353 | 14 | 23 | 7 | −29 | 287 |
| P. vivax | 139 | 42 | 54 | 23 | −17 | 180 |
| P. ovale | 109 | 125 | 108 | 89 | −5 | 364 |
| P. malariae | 19 | 65 | 68 | 51 | −7 | 349 |
| Mixed infection | 25 | 45 | 73 | 9 | −2 | 247 |
| Total | 1,645 | 26 | 51 | 8 | −29 | 364 |
The reported 1,645 malaria cases that occurred in 2013–2015 were investigated except for 26 cases lost to follow-up in this study.
This indicates that the interval of the last infection for this patient before he or she arrived in China.
This represents that the interval of primary infection for this patient before he or she arrived in China.
Interval from fever onset to diagnosis.
The number of days that elapsed from the onset of fever to diagnosis was recorded. Analysis of all malaria cases from 2010 to 2015 showed that the median duration from the fever onset to diagnosis was longest for P. malariae (4 days) and shortest for P. falciparum (1 day). Plasmodium ovale was observed with the maximum interval days of 226 among all species. Of the Plasmodium species, only P. vivax and P. falciparum exhibited any significant difference (P = 0.000) (Table 4).
Table 4.
Interval from the onset of fever to diagnosis of malarial species in Guangxi, 2010–2015
| Species | Interval from fever onset to diagnosis (d) | |||||
|---|---|---|---|---|---|---|
| N | Mean | SD | Median | Minimum* | Maximum† | |
| P. falciparum | 1,621 | 3 | 6 | 1 | 1 | 83 |
| P. vivax | 247 | 6 | 11 | 3 | 1 | 82 |
| P. ovale | 123 | 5 | 20 | 2 | 1 | 226 |
| P. malariae | 27 | 6 | 8 | 4 | 1 | 42 |
| Mixed infection | 36 | 6 | 8 | 4 | 2 | 39 |
| Clinically diagnosed | 15 | 6 | 9 | 3 | 1 | 34 |
| Total | 2,069 | 4 | 9 | 2 | 1 | 226 |
Herein “1” contains the interval that equal 1 day (24 hours) and those less than 1 day.
This represents that the interval between day of primary infection for this patient and diagnosis.
DISCUSSION
Historically, a high rate of malaria has been observed in China’s Guangxi Zhuang Autonomous Region. Malaria-induced morbidity has declined sharply from 1970s, largely because of the large-scale vector control interventions that have been performed through primary healthcare networks and community participation, which have helped eliminate malaria from the Guangxi region. However, the imported cases have been recently identified in returning workers from Africa. An increase in the mobility of human population and a likely change in the vectorial capacity of mosquitoes may have enhanced the risk of malaria resurgence or reintroduction.
Preventing the reintroduction of imported malaria has been a great challenge in Guangxi, particularly with respect to limiting the spread of P. falciparum, which is responsible for most malaria deaths and much of its increasing incidence.19 In 2013, because of the timely and proper implementation of the WHO “Test, Treat, Track” strategy and “1-3-7” strategy, secondary infections and deaths have been lowered to zero in Guangxi.
Even though P. falciparum has continued to play a dominant role, P. vivax and P. ovale cases have increased in Guangxi. Some documents have indicated that Anopheles sinensis was the predominant vector and distributed in all counties in Guangxi.37 Duan has reported that the temperature between 25°C and 28°C was optimal for An. sinensis in China,38 and it was observed here that P. vivax was reported highest from June (24.6°C) to August (27.6°C). This high risk is brought on because of the increasing importation of P. vivax during the transmission season. The data herein provide the evidence that integrated control and prevention interventions should be enacted, particularly from June to September and December to February, which is when most people return to China from abroad. For example, the CDC staff can carry out health education programs in the villages and townships where returned workers reside, at airports, and at hospitals through TV, radio, cell phone messages, and delivery of information education communication materials. The CDC staff can also conduct vector investigations in areas where imported case clusters have been reported, and antimosquito activities such as the IRS and distribution of long-lasting insecticide nets have been adopted based on the results of the investigation.
The interval between returning to China and the onset of illness was longer for P. vivax and P. ovale infections than for P. falciparum and P. malariae infections. Likely reasons for this difference are as follows. First, most of these patients who are infected with P. vivax and P. ovale were diagnosed with malaria abroad in private clinics and had previously accepted improper treatments such as aspirin or paracetamol. For example, in 2014, 66 (78.6%) of all imported P. vivax and P. ovale were in individuals who had been given aspirin instead of antimalarial agents. Even after their return to their hometowns, many patients refused to follow the prescribed regimen. However, the patients were hard to follow-up because of the mobilization (they may have gone to other cities or regions and were not always available by cell phone); in this study, we have carried out a survey on 31 patients (P. vivax and P. ovale) and seven of them did not experience radical cures. This may lead to activation of hypnozoites in the liver causing the relapse of infection after returning from the endemic areas.
Second, migrant workers often use prophylactic antimalarial drugs improperly.16 In China, the International Travel Healthcare Center (ITHC) is responsible for the health education such as drugs used in African countries when people are infected with malaria. When people come to ITHC for physical examinations and vaccine injections such as those for yellow fever, the staff will tell them how to protect themselves from mosquito bites and provide the prophylactic antimalaria drugs for free. According to China’s antimalarial drug policy, piperaquine phosphate is the recommended antimalarial chemoprophylaxis drug used against mixed P. falciparum and P. vivax infections in endemic areas such as African countries, with the dosage is 600 mg once each month and should administrate never more than 4 months.39 However, the Chinese workers and travellers often take artemisinin combined therapies (ACTs) to treat malaria, which may not protect against relapses after cessation of drug use. This is true for P. ovale, which is also capable of producing hypnozoites. This could also explain the longer latent period of P. vivax and P. ovale caused by hypnozoites in the returning migrant workers.
Third, it is proposed here that, in endemic areas, a large amount of the population harbors latent hypnozoites that can be activated by a systemic illness such as P. falciparum malaria.40,41 This could explain the high rates of P. vivax and P. ovale malaria after P. falciparum malaria and the high proportion of heterologous genotypes in relapses reported in 2013.
The follow-up survey of all 210 P. vivax and P. ovale cases in 2013–2014 revealed that only five patients relapsed after primary infection with an average latent period of 116 days. A survey conducted by the Guangxi CDC staff indicated that these five patients are ethnic minorities with low glucose-6-phosphate dehydrogenase deficiency (G6PDd) levels, therefore they were not given primaquine for radical treatment. In Guangxi, the CDC staff carry out two responses: First, to strengthen the follow-up survey and inform the patients that the drugs (ACTs) will not cure the malaria because of the hypnozoites in the liver and refer them to hospitals or the CDC for diagnosis if the malaria symptom recur. Second, the CDC staff use artesunate combined with azithromycin to treat the patients with low G6PD levels. Mao and colleagues conducted a survey to show that after a combination therapy of a 7-day-dose of artesunate (800 mg in total) and 14-day-dose of azithromycin (3,750 mg in total), no parasites were detected via microscopy and PCR for the following 3–10 months. Apart from this, no relapse occurred to the patients during the following 12–14 months.42 Therefore, to combat with latent P. vivax and P. ovale, the follow-up surveys on the infections and the radical treatment should be carried out, which could be of useful to prompt diagnosis and treatment.
CONCLUSIONS
In summary, imported malaria has posed a challenge to the elimination of malaria not only in Guangxi but also nationwide. Since 2013, the proportion of imported P. vivax and P. ovale has seen an increasing trend, especially in cases imported from African countries with long latency periods. However, the hypnozoites of P. vivax or P. ovale are clearly obstacles to malaria eradication because of their latency period in the liver. Health clinics should raise awareness of the likelihood of P. vivax and P. ovale being imported from African countries and about their long latency periods. The county CDC staff should carry out epidemiological studies and follow-up surveys on migrant workers to prevent misdiagnosis and improper treatment. Meanwhile, the P. vivax and P. ovale patients should adopt the radical treatment using primaquine, which could reduce the number of infective vectors during the next transmission season. Primaquine remains the only antihypnozoite therapy in China, but it has been found to be dangerous because of the risk of hemolysis in the treatment of the G6PDd patients. In this way, the evaluation of radical treatment should be conducted carefully using the genotyping technology on the G6PDd level, and new drugs should be developed to target the hypnozoites.
Supplementary Material
Acknowledgments:
We thank the staff in the county CDCs in the Guangxi Autonomous Region. The research was supported by the National Natural Science Foundation of China (Grant No. 81602904).
Note: Supplemental table appear at www.ajtmh.org.
REFERENCES
- 1.Yin JH, Zhou SS, Xia ZG, Wang RB, Qian YJ, Yang WZ, Zhou XN, 2014. Historical patterns of malaria transmission in China. Adv Parasitol 86: 1–19. [DOI] [PubMed] [Google Scholar]
- 2.Li JH, Li J, Qin YX, Guo CK, Huang YM, Lin Z, 2014. Appraisal of the effect and measures on control malaria for 60 years in Guangxi. J Trop Med 14: 361–364. [Google Scholar]
- 3.Ministry of Health , 2006. National Malaria Control Programme (2006–2015). Beijing, China: Ministry of Health. [Google Scholar]
- 4.Feng J, Xia ZG, Vong S, Yang WZ, Zhou SS, Xiao N, 2014. Preparedness for malaria resurgence in China: case study on imported cases in 2000–2012. Adv Parasitol 86: 231–265. [DOI] [PubMed] [Google Scholar]
- 5.Wampfler R, Robinson L, Hofmann N, Waltmann A, Betuela I, Silkey M, Siba P, Smith T, Mueller I, Felger I, 2014. Dynamics of P. vivax clones in a cohort of children with or without primaquine treatment at baseline. Malar J 13 (Suppl 1): O24. [Google Scholar]
- 6.Centers for Disease Control and Prevention , 2005. Late relapse of Plasmodium ovale malaria–Philadelphia, Pennsylvania, November 2004. MMWR Morb Mortal Wkly Rep 54: 1231–1233. [PubMed] [Google Scholar]
- 7.Siala E, Gastli M, Essid R, Jemal S, Ben Abdallah R, Ben Abda I, Aoun K, Bouratbine A, 2015. Late relapse of imported Plasmodium ovale malaria: a case report. Tunis Med 93: 347–350. [PubMed] [Google Scholar]
- 8.Coldren RL, Jongsakul K, Vayakornvichit S, Noedl H, Fukudas MM, 2007. Apparent relapse of imported Plasmodium ovale malaria in a pregnant woman. Am J Trop Med Hyg 77: 992–994. [PubMed] [Google Scholar]
- 9.Mellon G, Ficko C, Thellier M, Kendjo E, Aoun O, Adriamanantena D, Rapp C, French National Reference Center for Imported Malaria Study Group , 2014. Two cases of late Plasmodium ovale presentation in military personnel. J Travel Med 21: 52–54. [DOI] [PubMed] [Google Scholar]
- 10.Baird JK, 2016. Attacking Plasmodium vivax. Am J Trop Med Hyg 95: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imwong M, et al. , 2007. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis 195: 927–933. [DOI] [PubMed] [Google Scholar]
- 12.White NJ, 2011. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J 10: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotwal RS, Wenzel RB, Sterling RA, Porter WD, Jordan NN, Petruccelli BP, 2005. An outbreak of malaria in US Army Rangers returning from Afghanistan. JAMA 293: 212–216. [DOI] [PubMed] [Google Scholar]
- 14.Wells TN, Burrows JN, Baird JK, 2010. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol 26: 145–151. [DOI] [PubMed] [Google Scholar]
- 15.Hanna JN, Ritchie SA, Eisen DP, Cooper RD, Brookes DL, Montgomery BL, 2004. An outbreak of Plasmodium vivax malaria in far north Queensland, 2002. Med J Aust 180: 24–28. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Xiao H, Zhang L, Yan H, Feng X, Fang W, Xia Z, 2015. The Plasmodium vivax in China: decreased in local cases but increased imported cases from southeast Asia and Africa. Sci Rep 5: 8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Liu J, Feng XY, Zhang L, Xiao HH, Xia ZG, 2016. Towards malaria elimination: monitoring and evaluation of the “1-3-7” approach at the China–Myanmar border. Am J Trop Med Hyg 95: 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou SS, et al. , 2015. China’s 1-3-7 surveillance and response strategy for malaria elimination: is case reporting, investigation and foci response happening according to plan? Infect Dis Poverty 4: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng J, Xiao H, Xia Z, Zhang L, Xiao N, 2015. Analysis of malaria epidemiological characteristics in the People’s Republic of China, 2004–2013. Am J Trop Med Hyg 93: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health , 2010. National Malaria Elimination Action Plan (2010–2020). Beijing, China: Ministry of Health. [Google Scholar]
- 21.Xiao D, Long Y, Wang S, Wu K, Xu D, Li H, Wang G, Yan Y, 2012. Epidemic distribution and variation of Plasmodium falciparum and Plasmodium vivax malaria in Hainan, China during 1995–2008. Am J Trop Med Hyg 87: 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou SS, Tang L, Sheng HF, Wang Y, 2005. Malaria situation in the People’s Republic of China in 2004. Chin J Parasitol Parasit Dis 24: 1–3. [PubMed] [Google Scholar]
- 23.Zhou SS, Wang Y, Tang LH, 2006. Malaria situation in the People’s Republic of China in 2005. Chin J Parasitol Parasit Dis 24: 401–403. [PubMed] [Google Scholar]
- 24.Zhou SS, Wang Y, Tang LH, 2007. Malaria situation in the People’s Republic of China in 2006. Chin J Parasitol Parasit Dis 25: 439–441. [PubMed] [Google Scholar]
- 25.Zhou SS, Wang Y, Fang W, Tang LH, 2008. Malaria situation in the People’s Republic of China in 2007. Chin J Parasitol Parasit Dis 26: 401–403. [PubMed] [Google Scholar]
- 26.Zhou SS, Wang Y, Fang W, Tang LH, 2009. Malaria situation in the People’s Republic of China in 2008. Chin J Parasitol Parasit Dis 27: 455–456. [PubMed] [Google Scholar]
- 27.Zhou SS, Wang Y, Xia ZG, 2011. Malaria situation in the People’s Republic of China in 2009. Chin J Parasitol Parasit Dis 29: 1–3. [PubMed] [Google Scholar]
- 28.Zhou SS, Wang Y, Li Y, 2011. Malaria situation in the People’s Republic of China in 2010. Chin J Parasitol Parasit Dis 29: 401–403. [PubMed] [Google Scholar]
- 29.Xia ZG, Yang MN, Zhou SS, 2012. Malaria situation in the People’s Republic of China in 2011. Chin J Parasitol Parasit Dis 30: 419–422. [PubMed] [Google Scholar]
- 30.Xia ZG, Feng J, Zhou SS, 2013. Malaria situation in the People’s Republic of China in 2012. Chin J Parasitol Parasit Dis 31: 413–418. [PubMed] [Google Scholar]
- 31.Zhang L, Feng J, Xia ZG, 2014. Malaria situation in the People’s Republic of China in 2013. Chin J Parasitol Parasit Dis 32: 407–413. [PubMed] [Google Scholar]
- 32.Zhang L, Zhou SS, Feng J, Fang W, Xia ZG, 2015. Malaria situation in the People’s Republic of China in 2014. Chin J Parasitol Parasit Dis 33: 319–326. [PubMed] [Google Scholar]
- 33.Zhang L, Feng J, Zhang SS, Xia ZG, Zhou SS, 2016. Malaria situation in the People’s Republic of China in 2015. Chin J Parasitol Parasit Dis 34: 477–481. [PubMed] [Google Scholar]
- 34.Li Z, et al. , 2015. Malaria imported from Ghana by returning gold miners, China, 2013. Emerg Infect Dis 21: 864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng J, Li J, Yan H, Feng X, Xia Z, 2015. Evaluation of antimalarial resistance marker polymorphism in returned migrant workers in China. Antimicrob Agents Chemother 59: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling KM, Li J, Yang YC, Wei SJ, Huang YM, Li JH, Guo CK, Wei HY, 2015. Characteristics of imported malaria epidemic in Guangxi, 2013. Mod Prev Med 24: 2439–2442. [Google Scholar]
- 37.Guo CK, Li JH, Jing YX, 2007. Investigation on geographical distribution, ecological feature and malaria transmission of Anopheles in Guangxi. Chin J Vector Bio & Control 18: 112–115. [Google Scholar]
- 38.Wang WM, Zhou HY, Liu YB, Li JL, Zhu GD, Tang JX, Cao YY, Gu YP, 2012. Establishment of early warning system of malaria epidemic situation in Jiangsu Province I impact of temperature on development of Plasmodium vivax in Anopheles sinensis in Jiangsu Province. Chin J Schistosomasis Control 24: 581–584. [PubMed] [Google Scholar]
- 39.National Health and Family Planning Commission , 2016. Technical Regulations for Application of Antimalarials. Beijing, China: National Health and Family Planning Commission. [Google Scholar]
- 40.White NJ, 2011. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J 10: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battle KE, et al. , 2014. Geographical variation in Plasmodium vivax relapse. Malar J 13: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao W, Li J, Wei HY, Lin KM, Huang YM, 2013. Long-term effects of artesunate combined with azithromycin on Plasmodium vivax malaria. Chin Trop Med 13: 614–615, 628. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.