Abstract.
Individuals with newly diagnosed tuberculosis (TB) were screened for diabetes (DM) with fasting plasma glucose (FPG) in Pakistan. A significant decrease in FPG was observed when TB was treated. Of those with newly diagnosed DM, 46% and 62% no longer had hyperglycemia after 3 and 6 months, respectively. Individuals with known DM also showed a significant decrease in fasting plasma levels when treated for TB, but after 3 months none had normoglycemia, and after 6 months 9.2% were normoglycemic. Thus, TB-related DM may abate when the stress terminates, as is the case in gestational DM. However, because stress hyperglycemia may be associated with subsequent risk of developing DM, follow-up is recommended.
INTRODUCTION
Diabetes (DM) increases the risk of developing tuberculosis (TB) and subsequent treatment failure.1,2 Pakistan is experiencing a dramatic increase in DM and reported a prevalence of 6.9% in 2015. In Pakistan, DM health care is often left to family physicians with limited training and knowledge about diagnosis, monitoring, and prevention of complications.3 The collision of the two diseases is, therefore, of great concern in regions where TB is endemic. TB may, as is the case with other infections and conditions such as pregnancy, induce stress-related transient hyperglycemia, leading to overdiagnosis of DM. Stress hyperglycemia related to TB may be the result of insulin resistance caused by a complex interaction between catecholamines, cortisol, growth hormones, and cytokines.4,5 Stress-related insulin resistance results in hyperglycemia by two pathways: 1) hepatic overproduction of glucose, mainly through an inability to suppress gluconeogenesis and 2) peripheral defects in insulin-mediated glucose uptake. Stress hyperglycemia itself enhances the inflammatory response leading to further increase in blood glucose levels; a self-reinforcing cycle that may worsen outcomes and increase mortality.6 Despite the fast growing literature on DM enhancing the risk of TB, there are only few studies, mostly small, on the course of hyperglycemia in TB.7–10 The present study aimed to observe in more detail 1) changes in fasting plasma glucose (FPG) levels over time, 2) the extent to which these values are related to glycemic status when initiating TB treatment, and 3) the optimal time to screen and rescreen for DM in individuals with TB.
MATERIALS AND METHODS
The present study was part of a larger screening study described elsewhere.11 Individuals with newly diagnosed smear positive TB ≥ 25 years of age were invited to participate in the study between May 2014 and September 2015. Data were collected at Gulab Devi Chest Hospital, Lahore, Pakistan. Pregnant women and individuals with concomitant infections or type 1 diabetes mellitus were excluded. TB was diagnosed according to the national guidelines for control of TB in Pakistan, and directly observed treatment, short course was offered.12 All suspected TB cases delivered two sputum samples for microscopy of Ziehl–Neelsen stained smears. The results of sputum smear microscopy repeated at 2 and 5 months were also recorded. FPG was measured at enrolment in 462 individuals with newly diagnosed TB. Of these, 198 individuals were followed-up after 3 months of TB treatment, whereas 264 individuals had their FPG repeated after 6 months of anti-TB treatment. Having more than one initial and one follow-up FPG was refused by most participants and, therefore, not enforced. FPG was measured between 8 and 10 am after at least 8 hours of fasting on a capillary sample using Hemocue 201 RT (Ängelholm, Sweden). DM was classified as screen-detected DM (SDM) or known DM (KDM), according to history. SDM diagnosis was based on the World Health Organization (WHO) criteria, where values of FPG ≥ 7.0 mmol/L are diagnostic for DM.13 Individuals with SDM who remained hyperglycemic after 3 months of TB treatment were informed about the risks of adverse TB outcomes and were recommended to have their blood glucose levels retested after completion of the treatment, whereas those with hyperglycemia after 6 months were advised to visit a DM clinic. Individuals with KDM and hyperglycemia were recommended to consult the doctor responsible for their DM treatment. All participants gave written consent, and ethics approval was obtained from the National Bioethics Committee, Pakistan (No.4-87/14/NBC-139/RDC4936). The median difference in FPG over time was assessed using Wilcoxon signed-rank test, and differences in proportions of individuals who had hyperglycemia were assessed using the McNemar’s test. The association between hyperglycemia and treatment outcomes was evaluated by χ2 test.
RESULTS
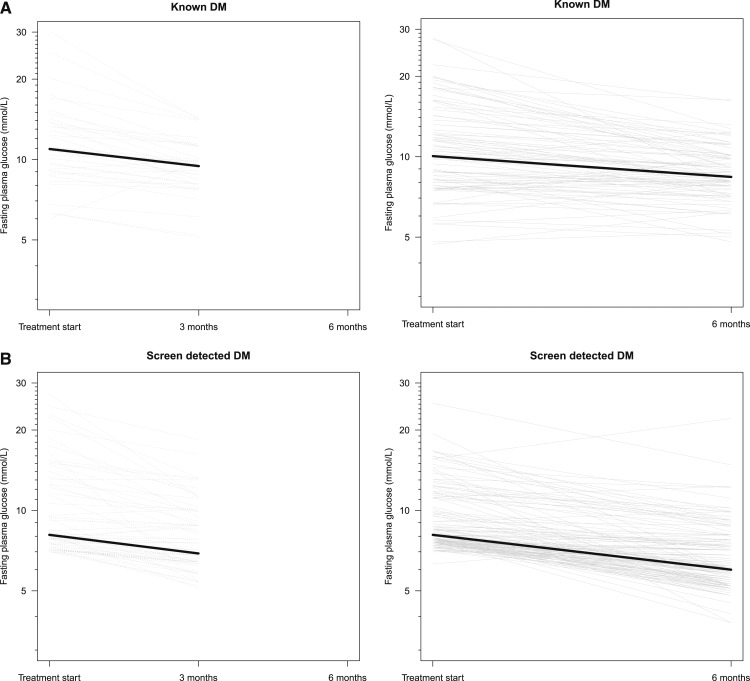
Baseline characteristics for the two DM groups are described in Table 1; SDM individuals were younger and had significantly lower body mass index, waist–hip ratio, and blood pressure. The participants did differ significantly from the cohort from whom they were enrolled regarding anthropometric or biochemical parameters (results not shown). At enrolment, 42.2% (N = 195) of the participants with TB were diagnosed with SDM, and 26.0% (N = 120) had KDM. FPG decreased over time for both groups (SDM and KDM); in SDM, the median decrease (interquartile range [IQR] 25;75) was 1.2 mmol/L (0.5;3.1) and 2.1 mmol/L (1.2;3.0) after 3 and 6 months, respectively. For individuals with KDM, the median decrease in FPG was 1.5 mmol/L (1.0;3.6) and 1.7 mmol/L (0.6;3.5) after 3 and 6 months, respectively. The decrease in FPG over time was significant for both groups at 3 months (P < 0.001) and 6 months follow-up (P < 0.001) (Figure 1). For individuals with SDM, 46% reverted to normoglycemia after 3 months of anti-TB treatment, whereas 62% became normoglycemic after 6 months. For TB patients with KDM, 88.2% were hyperglycemic at baseline and, none of these became normoglycemic after 3 months of anti-TB treatment (P = 0.31), whereas 9.2% of those who were hyperglycemic at baseline became normoglycemic after 6 months (P = 0.34). Most of the participants with KDM (80%) were receiving DM treatment at the time of TB diagnosis, but we did not have information on whether DM treatment was adjusted during TB treatment.
Table 1.
Baseline biological characteristics for tuberculosis patients with screen-detected diabetes (SDM) and known diabetes (KDM); presented as mean (95% CI)
| Variable | SDM (N = 195) | KDM (N = 120) | P value* |
|---|---|---|---|
| Male (n (%) | 129 (65.8) | 73 (60.8) | 0.10 |
| Age (years) | 43 (41:45) | 47 (46:49) | < 0.001 |
| Weight (kg) | 50.3 (48.8:52.0) | 57.0 (54.8:59.0) | < 0.001 |
| Height (cm) | 163 (161:164) | 162 (160:163) | 0.46 |
| Body mass index (kg/m2) | 19.0 (18.4:19.6) | 21.7 (21.0:22.5) | < 0.001 |
| Waist (cm) | 65.7 (63.8:67.6) | 73.2 (71.3:75.5) | < 0.001 |
| Hip (cm) | 79.2 (78.0:80.4) | 84.0 (82.5:85.5) | < 0.001 |
| Waist–Hip ratio | 0.82 (0.80:0.84) | 0.87 (0.85:0.89) | < 0.001 |
| Hemoglobin (g/dL) | 11.7 (11.4:11.9) | 11.8 (11.4:12.1) | 0.56 |
| Systolic blood pressure[BP] (mm of Hg) | 103 (100:105) | 116 (113:119) | < 0.001 |
| Diastolic BP (mm of Hg) | 67 (66:68) | 75 (73:77) | < 0.001 |
| Pulse (beats/minutes) | 107 (104:109) | 107 (104:109) | 0.95 |
Between-group comparison of TB patients with SDM and KDM was performed using a t-test and χ2 test for continuous and categorical variables, respectively.
Figure 1.
(A) Longitudinal course of fasting plasma glucose levels for tuberculosis (TB) patients. Fasting plasma glucose levels for both screen-detected diabetes (SDM) and known diabetes (KDM) at start of TB treatment and after 3 or 6 months are illustrated. The median of fasting plasma glucose is highlighted for each group: for the SDM group, the median decrease (IQR 25;75) was 1.2 mmol/L (0.5;3.1) and 2.1 mmol/L (1.2;3.0) after 3 and 6 months, respectively. For persons with KDM, the median decrease was 1.5 mmol/L (1.0;3.6) and 1.7 mmol/L (0.6;3.5) after 3 and 6 months, respectively. (B) Longitudinal course of fasting plasma glucose levels for tuberculosis (TB) patients. Fasting plasma glucose levels for both screen-detected diabetes (SDM) and known diabetes (KDM) at start of TB treatment and after 3 or 6 months are illustrated. The median of fasting plasma glucose is highlighted for each group: for the SDM group, the median decrease (IQR 25;75) was 1.2 mmol/L (0.5;3.1) and 2.1 mmol/L (1.2;3.0) after 3 and 6 months, respectively. For persons with KDM, the median decrease was 1.5 mmol/L (1.0;3.6) and 1.7 mmol/L (0.6;3.5)after 3 and 6 months, respectively.
A positive sputum smear at 2 or 5 months after anti-TB treatment start was not associated with hyperglycemia (FPG ≥ 7.0 mmol/L) at baseline (P = 0.30). Of the 462 participants, six (1.3%) died. There was no association between hyperglycemia at baseline and mortality (P = 0.44) nor between hyperglycemia at 6 months and death (P = 0.66).
DISCUSSION AND CONCLUSION
Individuals with TB and DM, whether SDM or KDM, experienced a highly significant decrease in FPG over time after anti-TB treatment. Hyperglycemia is associated with poor TB outcome, and even though a notable number of individuals with SDM had transient hyperglycemia, 38% remained with hyperglycemia after 6 months of anti-TB treatment, underlining the importance of screening for DM in individuals with TB.7 Previous studies have found that stress hyperglycemia may unveil underlying DM in up to 60% of hospital-admitted patients.14,15 Most individuals with KDM remained hyperglycemic at follow-up, despite receiving DM treatment, but it is important to note that the decrease in FPG was significant. Therefore, optimization of DM treatment in individuals with TB is important. Although we did not obtain detailed information on whether DM treatment or diet was adjusted during TB treatment, the persistent hyperglycemia suggests that DM treatment was not intensified. Another limitation of our study is that FPG measurement was not repeated at 3 and 6 months in all participants. Individuals with SDM were informed about and were aware of the possibility of stress-related hyperglycemia. Less than 10% of those with SDM had marked hyperglycemia (FPG ≥ 14.0 mmol/L); all those with marked hyperglycemia were advised to have their FPG repeated within a couple of days by a private doctor. Because of limited resources, we assumed that most participants did not invest in a visit to a private doctor or initiate DM treatment during the course of TB treatment, but the lack of information on treatment of DM during TB follow-up was an important limitation of our study.
In conclusion, the number of participants reverting to normoglycemia increased as anti-TB treatment progressed. Even though the WHO recommends screening for DM at the start of TB treatment, a definite diagnosis of newly diagnosed DM cannot be ascertained before anti-TB treatment has been completed.16 Transient-elevated plasma glucose may predict the development of DM. Screening for DM after recovery from stress has been shown to be cost effective and may decrease the risk of associated complications.17 Because transient hyperglycemia in TB may also indicate subsequent increased risk for manifest DM, we still recommend following individuals whose FPG normalized during TB treatment; however, at present there is not enough evidence to suggest a length of follow-up time, or whether individuals with SDM should be recommended diet and lifestyle changes.
Acknowledgments:
We are very grateful to all the coinvestigators at Gulab Devi Hospital. We thank Aqeel ur Rehman (DMS), Zaheer Akthar (associate professor in pulmonology) for their valuable guidance. We thank Mehwish Ali and Fozia Mehtab (nurses) for their great effort and help, and sincerely thank all the study participants.
REFERENCES
- 1.Jeon CY, Murray MB, 2008. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez-Corona ME, et al. , 2013. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 68: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shera AS, Jawad F, Basit A, 2002. Diabetes related knowledge, attitude and practices of family physicians in Pakistan. J Pak Med Assoc 52: 465–470. [PubMed] [Google Scholar]
- 4.Chrousos GP, 1995. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332: 1351–1362. [DOI] [PubMed] [Google Scholar]
- 5.Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E, 2007. Glucose metabolism and catecholamines. Crit Care Med 35: S508–S518. [DOI] [PubMed] [Google Scholar]
- 6.Dungan KM, Braithwaite SS, Preiser JC, 2009. Stress hyperglycaemia. Lancet 373: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boillat-Blanco N, et al. , 2016. Transient hyperglycemia in patients with tuberculosis in Tanzania: implications for diabetes screening algorithms. J Infect Dis 213: 1163–1172. [DOI] [PubMed] [Google Scholar]
- 8.Oluboyo PO, Erasmus RT, 1990. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle 71: 135–138. [DOI] [PubMed] [Google Scholar]
- 9.Jawad F, Shera AS, Memon R, Ansari G, 1995. Glucose intolerance in pulmonary tuberculosis. J Pak Med Assoc 45: 237–238. [PubMed] [Google Scholar]
- 10.Basoglu OK, Bacakoglu F, Cok G, Sayiner A, Ates M, 1999. The oral glucose tolerance test in patients with respiratory infections. Monaldi Arch Chest Dis 54: 307–310. [PubMed] [Google Scholar]
- 11.Aftab H, Ambreen A, Jamil M, Garred P, Petersen JH, Nielsen SD, Bygbjerg IC, Christensen DL, 2017. High prevalence of diabetes and anthropometric heterogeneity among tuberculosis patients in Pakistan. Trop Med Int Health. 22: 465–473. [DOI] [PubMed] [Google Scholar]
- 12.National Tuberculosis Programme Pakistan , 2015. National Guidelines for the Control of Tuberculosis in Pakistan. Available at: http://ntp.gov.pk/uploads/NATIONAL_GUIDELINE_ON_TB_CASE_MANAGEMENT_REV_JAN_2015.pdf. Accessed March 15, 2015.
- 13.World Health Organization , 2006. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia Available at http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf.
- 14.Greci LS, Kailasam M, Malkani S, Katz DL, Hulinsky I, Ahmadi R, Nawaz H, 2003. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care 26: 1064–1068. [DOI] [PubMed] [Google Scholar]
- 15.Gray CS, Scott JF, French JM, Alberti KG, O’Connell JE, 2004. Prevalence and prediction of unrecognised diabetes mellitus and impaired glucose tolerance following acute stroke. Age Ageing 33: 71–77. [DOI] [PubMed] [Google Scholar]
- 16.Maurice J, 2011. WHO framework targets tuberculosis-diabetes link. Lancet 378: 1209–1210. [DOI] [PubMed] [Google Scholar]
- 17.Kim C, Herman WH, Vijan S, 2007. Efficacy and cost of postpartum screening strategies for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care 30: 1102–1106. [DOI] [PubMed] [Google Scholar]