Abstract.
We compared the concentrations of Escherichia coli quantified with Colilert™ and the compartment bag test (CBT) in the source water and household stored drinking water (SDW) of 35 households in western Kenya. We also investigated the associations of the perceptions of organoleptic properties and overall quality with ≥ 1 MPN/100 mL E. coli in SDW. Participants who rated the taste or smell of their SDW “< 5” on a 1 = “poor” to 5 = “excellent” Likert scale were 8.71 or 7.04 times more likely, respectively, to have ≥ 1 MPN/100 mL E. coli. Organoleptic properties are innate, albeit imperfect, indicators of fecal pollution in water. Within their shared quantification range, concentrations of E. coli enumerated with Colilert and CBT were similar and had a significant correlation coefficient, 0.896 (95% confidence interval = 0.691–1.101). The methods had moderate agreement within the World Health Organization’s health risk levels (Cohen’s Kappa coefficient = 0.640). In low-resource settings, CBT provides comparable assessments of E. coli concentrations to Colilert.
The Sustainable Development Goal (SDG) 6.1 aims to ensure that “safely managed drinking water services” are free of fecal pollution.1 Evaluating the presence of fecal pollution is difficult in low-resource settings partially due to lack of financial, logistical, and technical resources. In such settings, organoleptic properties (smell, taste, and appearance) are relied upon to infer the quality of drinking water. There are no published studies that have evaluated the associations of organoleptic properties and the microbial quality of drinking water. In this study, we evaluated the associations of perceived quality and organoleptic properties (taste and smell) of stored drinking water (SDW) with the presence of Escherichia coli in 35 households in western Kenya.
The compartment bag test (CBT; Aquagenx, LLC, Chapel Hill, NC) is a novel method that shows promise to quantify E. coli in potable water in low-resource settings.2 Its attributes include incubation at ambient temperatures (25–40°C), no specialized equipment required, and an adequate quantification range (1–100 MPN/100 mL).3,4 Comparisons of E. coli concentrations measured using CBT and membrane filtration (USEPA Method 1604) in environmental waters around Atlanta, GA, were correlated.5 In three regions in Peru, concentrations of E. coli in SDW) measured by CBT in the field and membrane filtration in a laboratory were similar.6 Herein, we compared the concentrations of E. coli quantified by CBT and Colilert (IDEXX Laboratories, Inc., Westbrook, ME) in source water and SDW from 35 households in western Kenya.
Data were collected in the Nyanza Region, Kenya, within an observational pregnancy cohort (clinicaltrials.gov identifier: NCT02979418).7 We used STATA 14 (StataCorp LP, College Station, TX) to randomly select five households to visit in each of the seven clinic catchment areas from a pool of participating households. All participants were 9–12 months postpartum. If participants elected not to participate or were not available, another household was randomly selected. In January–February 2016, we collected samples of SDW at the participating households and surveyed participants on the organoleptic properties (taste and smell) and perceived overall quality of their SDW using a Likert ladder scale used in the parent cohort study (1 = “poor” to 5 = “excellent”; Supplemental Figure 1). We accompanied participants to source waters to collect samples, and photographed the supply for classification. The study protocol was reviewed and approved by the Kenyan Medical Research Institute Scientific and Ethics Review and Cornell University Institutional Review Board ([1504005493; 1205003043], May 8, 2015). Informed consent of the participants was obtained.
Six 100-mL replicates of SDW from each household and six 100-mL replicates of corresponding source water were collected using Whirl-Pak® Thio Bags® (Nasco, Fort Atkinson, WI). Source water was not collected if the household’s primary drinking water was rainwater or bottled water. There was no SDW in four households, but corresponding source water was collected. Samples were stored in a cooler at ambient temperature (≤ 21°C) for ≤ 6 hours because ice was not readily available. After transport, water samples were stored at 4°C for ≤ 24 hours. For each water sample, three 100-mL replicates each were analyzed using Colilert Quanti-Tray®/2000 (IDEXX Laboratories) and the CBT. We followed the manufacturers’ instructions and samples were incubated at 35°C for 24 hours. Bottled water was used for negative controls and analyzed alongside samples.
The range of quantification of the mean concentrations of E. coli enumerated with Colilert and CBT were 0.3–2,419.6 and 0.3–100 MPN/100 mL, respectively. Samples that were negative for the presence of E. coli were recorded as < 1 MPN/100 mL, and were evaluated at 0.15 MPN/100 mL (half the quantification limit of the mean of three replicates that individually had a detection limit of 1 MPN) in subsequent analyses. Results above the upper limit of quantification of Colilert and CBT were reported as > 2,419.6 and > 100 MPN/100 mL, respectively, and were evaluated at the upper limits of the methods in subsequent analyses (2,420 and 100 MPN/100 mL, respectively).
Statistical analyses were evaluated using IBM SPSS Statistics for Windows, Version 22.0 (IBM, Corp., Armonk, NY), and significance was considered at α = 0.05. Logistic regression analyses evaluated the presence of E. coli (mean concentrations ≥ 1 MPN/100 mL) in SDW as measured by CBT or Colilert to the perceptions of taste, smell, and the overall quality of SDW at “5 = excellent quality” (reference group) compared with all other ratings “< 5 = below excellent” to normalize the categorical data. Wilcoxon matched-pairs signed rank test evaluated differences between the mean concentrations of E. coli using Colilert and CBT of source water and SDW from each household. A linear regression evaluated the relationship between the log transformed mean concentrations of E. coli measured by the two methods. MPN values outside of the quantification range of CBT were not included in these analyses. The mean concentrations of E. coli from all samples of SDW and source water were sorted into World Health Organization (WHO) health risk levels: < 1 MPN/100 mL “low risk”; 1–10 MPN/100 mL “intermediate risk”; > 10–100 MPN/100 mL “high risk”; and > 100 MPN/100 mL “very high risk.”8 Cohen’s Kappa coefficient evaluated the agreement of the WHO health risk levels between the two methods.
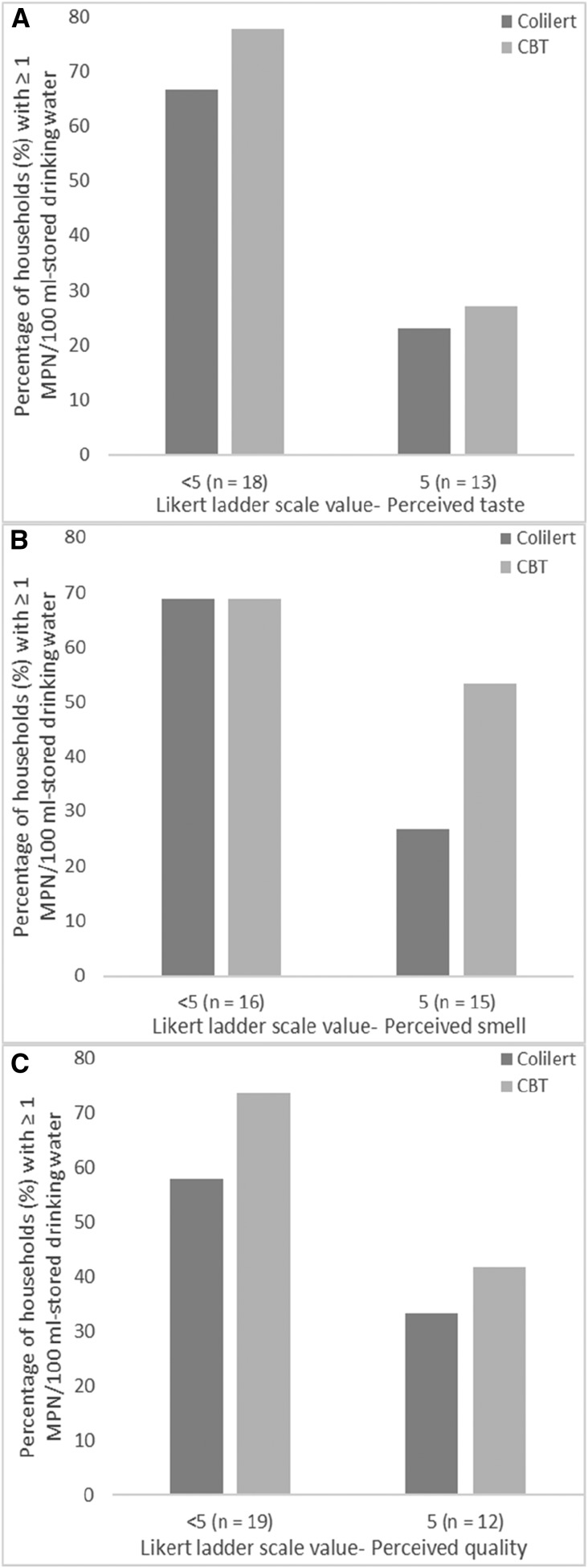
Using Colilert, we found that participants who rated the taste, smell, and overall quality of their SDW < 5 = “below excellent” were 8.71 (95% confidence interval [CI] = 1.10–68.94; P = 0.04), 7.04 (95% CI = 1.09–45.83; P = 0.04), and 0.452 (95% CI = 0.05–4.108; P = 0.481) times more likely, respectively, to have detectable E. coli (≥ 1 MPN/100 mL; Figure 1A–C) than participants that gave a rating of 5 = “excellent.” However using CBT, participants who rated the perceived taste, smell, and quality of their SDW < 5 = “below excellent” were 3.793 (95% CI = 0.634–22.692; P = 0.144), 1.302 (95% CI = 0.218–7.771; P = 0.772), and 1.706 (95% CI = 0.237–12.270; P = 0.595) times more likely to have E. coli present in the SDW (Figure 1A–C) than participants who rated the perceived characteristics 5 = “excellent,” although these effects were not significant. Independent of E. coli quantification method, some participants who rated the taste and smell of the SDW 5 = “excellent” had detectable E. coli (Figure 1A and B).
Figure 1.
The proportion of 31 households in western Kenya with mean concentrations ≥ 1 MPN/100 mL Escherichia coli (measured with Colilert and the compartment bag test) in the stored drinking water when participants rated the perceived (A) taste, (B) smell, and (C) overall quality the highest value on the Likert scale, 5 = “excellent,” compared with all other values, < 5 = “below excellent” (1–4).
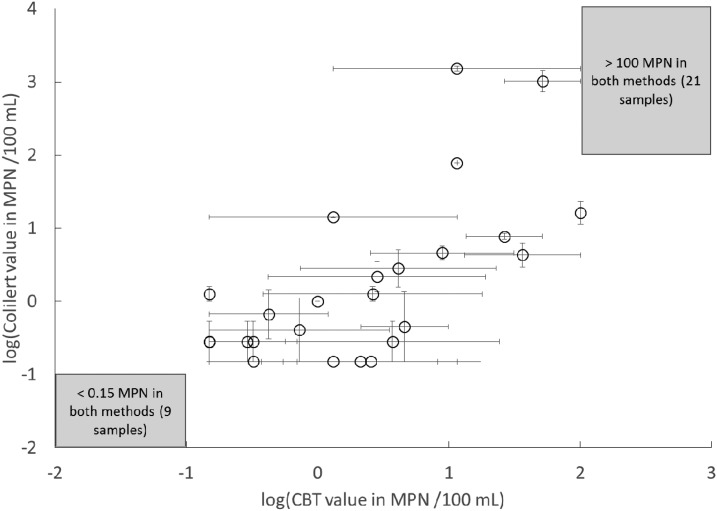
Within the shared quantification range of the two methods, a Wilcoxon matched-pairs signed rank test confirmed that the mean concentrations of E. coli enumerated with CBT and Colilert were similar (P = 0.367). There was a power-log correlation between mean concentrations of E. coli within the shared quantification range of CBT and Colilert (Figure 2): log (Cl) = 0.896 × log (Cb) − 0.047; P < 0.001; R2 = 0.673, standard error of the coefficient = 0.205, where Cl and Cb were the mean E. coli concentrations (MPN/100 mL) of samples measured with Colilert and CBT, respectively. Our dataset demonstrated that the mean concentrations of E. coli measured by Colilert and CBT had agreement between WHO health risk levels (Table 1; Cohen’s Kappa coefficient = 0.641; P < 0.001).
Figure 2.
Comparison of Escherichia coli concentrations (MPN/100 mL) measured with Colilert and compartment bag test (CBT) (N = 22). Each assay was run in triplicate and the mean values of the log-transformed concentrations of E. coli are plotted. Gray boxes represent samples that were outside the shared quantification range of the two methods (≥ 100 MPN/100 mL and < 0.3 MPN/100 mL). For samples where only one assay was within the quantification range, the other method’s value was plotted at the log-transformed value of one half of the lower limit of quantification (0.15 MPN/100 mL), or log-transformed value of the upper limit of quantification of CBT (100 MPN/100 mL). The error bars represent one standard error.
Table 1.
WHO health risk levels derived from the mean concentrations of Escherichia coli as determined by Colilert and the CBT in water samples collected from 35 households in western Kenya and included stored drinking water (N = 31) and source water (N = 21)
| Colilert—Frequency of water samples in each WHO health risk level (N = 52) | ||||||
|---|---|---|---|---|---|---|
| Low risk/safe (< 1 MPN/100 mL) | Intermediate risk (1–10 MPN/100 mL) | High risk (> 10–100 MPN/100 mL) | Very high risk (> 100 MPN/100 mL) | Total | ||
| CBT—frequency of water sample in each WHO health risk level (N = 52) | < 1 MPN/100 mL | 14 | 1 | 0 | 0 | 15 |
| 93.3% | 6.7% | 0% | 0% | 28.8% | ||
| 1–10 MPN/100 mL | 1 | 3 | 0 | 0 | 4 | |
| 25% | 75% | 0% | 0% | 7.7% | ||
| 10–100 MPN/100 mL | 3 | 6 | 1 | 1 | 11 | |
| 27.3% | 54.5% | 9.1% | 9.1% | 21.2% | ||
| > 100 MPN/100 mL | 1 | 0 | 0 | 21 | 22 | |
| 4.5% | 0% | 0% | 95.5% | 42.3% | ||
| Total | 19 | 10 | 1 | 22 | 52 | |
| 36.5% | 19.3% | 1.9% | 42.3% | 100% | ||
CBT = compartment bag test; WHO = World Health Organization.
This is the first study to demonstrate a significant correlation between the organoleptic properties (taste and smell) of SDW and ≥ 1 MPN/100 mL E. coli–Colilert. The cohort and the participants of this study were 9–12 months postpartum. Therefore, we believe that the responses of the participants of this study were not affected by pregnancy and can be extrapolated to the cohort. Organoleptic properties of SDW may offer innate, albeit imperfect assessments of the presence of E. coli (≥ 1 MPN/100 mL). Our study was unable to establish a significant association between the perceived overall quality of drinking water and detectable E. coli in SDW. Similarly, there was a weak correlation between the concentrations of E. coli and the perceived safety of source water supplies (correlation coefficient = 0.0392; P = 0.64) in Cambodia,9 suggesting that perceived overall quality/safety may not be a reliable predictor of the actual presence and/or levels of fecal pollution in water.
This is the first peer-reviewed investigation to determine that the concentrations of E. coli enumerated with Colilert and CBT in low-resource settings are similar and correlated. In Peru, concentrations of E. coli in SDW were similar when measured in the field with CBT and in a laboratory with the membrane filtration method or with CBT.6 Comparably, in environmental waters sampled around Atlanta, GA, concentrations of E. coli measured with CBT and membrane filtration were correlated (correlation coefficient = 0.904, CI = 0.859–0.950).5 These studies provide further evidence that CBT provides comparable results to conventional methods. Therefore, CBT can be useful to monitor the progress toward meeting SDG 6.1 independent of resource settings. Broadening the monitoring of water supplies will provide a thorough and accurate evaluation of the progress toward meeting SDG 6.1: access to “safely managed drinking water” that is free of fecal pollution.
Supplementary Material
Acknowledgments:
We thank the Pii En Ngima study participants; study team Pauline Wekesa, Joy China, Joyce Bonke, Tobias Odwar, Sarah Obaje, Teresa Owade, Joshua Miller, and Benter Ogwano; Matthew Hickey (University of California-San Francisco); Daniel Omollo (Organic Health Response); Kevin Packard and Françoise Vermeylen (Cornell University’s Statistical Consulting Unit).
Note: Supplemental figure appears at www.ajtmh.org.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or ACSF.
REFERENCES
- 1.WHO/UNICEF Joint Monitoring Programme on Water Supply and Sanitation , 2017. Safely Managed Drinking Water Geneva, Switzerland: WHO. Available at: https://www.wssinfo.org/fileadmin/user_upload/resources/JMP-SMDW-TR-1-March-2017.pdf. Accessed March 7, 2017.
- 2.Bain R, Bartram J, Elliott M, Matthews R, Mcmahan L, Tung R, Chuang P, Gundry S, 2012. A summary catalogue of microbial drinking water tests for low and medium resource settings. Int J Environ Res Public Health 9: 1609–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss P, Aw TG, Urquhart G, Galeano MR, Rose JB, 2015. Well water quality in rural Nicaragua using a low-cost bacterial test and microbial source tracking. J Water Health 14: 199–207. [DOI] [PubMed] [Google Scholar]
- 4.Grady CA, Kipkorir EC, Nguyen K, Blatchley ER, 2015. Microbial quality of improved drinking water sources: evidence from western Kenya and southern Vietnam. J Water Health 13: 607–612. [DOI] [PubMed] [Google Scholar]
- 5.Stauber C, Miller C, Cantrell B, Kroell K, 2014. Evaluation of the compartment bag test for the detection of Escherichia coli in water. J Microbiol Methods 99: 66–70. [DOI] [PubMed] [Google Scholar]
- 6.McMahan L, Wang A, Rutstein S, Sobsey MD, Stauber C, Reyes J, 2017. Household microbial water quality testing in a Peruvian demographic and health survey: evaluation of the compartment bag test for Escherichia coli. Am J Trop Med Hyg 96: 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krumdieck NR, Collins SM, Wekesa P, Mbullo P, Boateng GO, Onono M, Young SL, 2016. Household water insecurity is associated with a range of negative consequences among pregnant Kenyan women of mixed HIV status. J Water Health 14: 1028–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO , 2011. Guidelines for Drinking-Water Quality, Vol. 38, 4th edition. Geneva, Switzerland: WHO Press. Available at: http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf.
- 9.Orgill J, Shaheed A, Brown J, Jeuland M, 2013. Water quality perceptions and willingness to pay for clean water in peri-urban Cambodian communities. J Water Health 11: 489–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.