Abstract.
Leishmania donovani causes cutaneous leishmaniasis (CL) in Sri Lanka. Standard treatment is multiple, painful doses of intralesional sodium stibogluconate (IL-SSG). Treatment failures are increasingly reported, hence the need to investigate alternatives. Efficacy, safety, and cost-effectiveness of thermotherapy were assessed for the first time for L. donovani CL. A single blinded noninferiority randomized controlled trial was conducted on new laboratory-confirmed CL patients with single lesions (N = 213). Selected patients were randomly assigned to 1) test group (N = 98; single session of radiofrequency-induced heat therapy (RFHT) given at 50°C for 30 seconds) and 2) control group (N = 115; 1–3 mL IL-SSG given weekly, until cure/10 doses). Patients were followed-up fortnightly for 12 weeks to assess clinical cure. Cost of treatment was assessed using scenario building technique. Cure rates by 8, 10, and 12 weeks in RFHT group were 46.5%, 56.5%, and 65.9% as opposed to 28%, 40.8%, and 59.4% in IL-SSG group, with no major adverse events. Cure rate by RFHT was significantly higher at 8 weeks (P = 0.009, odds ratio [OR]: 2.236, confidence interval [CI]: 1.217–4.108) and 10 weeks (P = 0.035, OR: 1.881, CI: 1.044–3.388), but comparable thereafter. Cost of RFHT was 7 times less (USD = 1.54/patient) than IL-SSG (USD = 11.09/patient). A single application of RFHT is safe, cost-effective, and convenient, compared with multiple doses of IL-SSG in the treatment of L. donovani CL. Therefore, RFHT would be considered noninferior as per trial outcome when compared with standard IL-SSG therapy with multiple benefits for the patient and the national health care system.
INTRODUCTION
Leishmaniases are zoonoses caused by flagellate protozoa of the genus Leishmania, transmitted by phlebotomine sandflies. There are about 20 different species of the parasite worldwide, transmitted by over 30 Phlebotomus species.1 It is a neglected tropical disease affecting mostly the poor communities.2 An estimated 350 million people live at risk of contracting leishmaniasis, with a prevalence of 12 million cases and an annual case incidence of 2 million.3
Clinical presentation of the disease depends on the tropism of the parasite to various tissues, namely; skin, viscera, or mucous membranes. Cutaneous leishmaniasis (CL) is the commonest clinical form affecting more than 1.5 million people worldwide. The range of clinical entities of CL depend on the parasite species, host immune response, and duration of lesion. It presents typically as papules that progress to nodules and ulcers.3 Most lesions, including ulcerative lesions self-cure but the natural healing process is generally slow and can result in prominent scar formation.Therefore, large lesions with extensive scarring as a result of slow healing can cause distress to the patients.4,5
There is an array of treatment options for CL, which include local, systemic, and combination therapy. However, standard treatment remains as local or systemic pentavalent antimonials such as sodium stibogluconate or meglumineantimoniate administered intralesionally, intramuscularly, or intravenously.3 These therapies may have acceptable rates of efficacy but have many drawbacks such as high cost, systemic toxicity, and poor patient compliance because of lengthy courses of painful inoculations, which in turn can lead to partial treatment that promotes the emergence of drug-resistant parasite strains.6,7 Hence, single-session thermotherapy is an attractive alternative that is devoid of most limitations associated with standard antimonial therapy used for CL as shown by previous studies on CL caused by Leishmania tropica and Leishmania major from the old world.8–10 IL-SSG has been successfully used for L tropica in Afghanistan, Syria, and South Asia (except India) and the Mediterranean basin.3 Lesions caused by L major in Iran and Saudi Arabia, responded better to a combination of cryotherapy and intralesional injections of antimony than either treatment alone.3
In Sri Lanka, there has been a steady increase in the number of cases of CL over the last decade and now it is an established disease.11 Intriguingly, the etiological agent for CL in Sri Lanka is Leishmania donovani zymodeme MON 37, which causes visceral leishmaniasis elsewhere.12 Standard treatment of CL in the local setting is 1–3 mL of sodium stibogluconate (SSG; Pentostam GlaxoSmithKline 100 mL, 1 mg/mL) given intralesionally every 5–7 days for up to 10 times, or intramuscular or intravenous SSG 10–20 mg/kg/day given daily for 14–21 days or cryotherapy given weekly or fortnightly up to 7 cryo-sessions.13 Clinical cases of CL with poor or lack of responsiveness to standard antimonial therapy is now a known occurrence,14 with early evidence pointing toward the presence of a genetic variant of the local species that induces such complications (KKGDUL Kariyawasam and ND Karunaweera, unpublished data). However, drug resistance in L. donovani in Sri Lanka is yet to be fully established. Heat treatment of CL given through various other methods has been recorded previously.15,16 This study used thermotherapy or radiofrequency-induced heat therapy (RFHT) given with the aid of a machine (ThermoMed model 1.8, Thermosurgery Technologies, Inc., Phoenix, AZ), which has received clearance from the US Food and Drug Administration (FDA) for the treatment of CL.
The study was carried out to demonstrate the efficacy, safety, and cost-effectiveness of thermotherapy for L. donovani CL.
MATERIALS AND METHODS
Approvals.
The trial was registered at the Australian and New Zealand Clinical Trial Registry (ANZCTR) (registration identification number ACTRN12614001288617).
Ethical clearance from the Ethics Review Committee, Faculty of Medicine, University of Colombo (http://www.med.cmb.ac.lk), and approval for the use of the thermotherapy device from the Drug Regulation Authority, Sri Lanka, were also obtained before the commencement of the study.
Study design, participants, and study area.
This study was a randomized controlled, single blinded trial based on a noninferiority design. The trial was carried out at the dermatology clinic, Teaching Hospital, Anuradhapura, Sri Lanka. The dermatology clinic is the main treatment center for CL in North–Central Province (8.33°N, 80.5°E), serving both the civilian and military patients within a perimeter of about 200 km. Eligible participants were patients above 12 years of age who were diagnosed clinically and confirmed parasitologically as CL. All had no prior treatment of lesions. Exclusionary criteria were other forms of leishmaniasis (e.g., visceral or muco-CL), multiple CL lesions, lesions in proximity to mucous membranes or cartilage, comorbidities such as diabetes, tuberculosis, malignancies or immunosuppressive conditions, known hypersensitivity to sodium stibogluconate or local anesthetics, and pregnancy and lactation. Participants were included in the study after written informed consent. Parents /guardians provided informed consent on behalf of all child participants.
Parasitological confirmation.
Laboratory confirmation of diagnosis (based on microscopy and culture of lesion aspirates or slit skin scrapings) was made at the Department of Parasitology, Faculty of Medicine Colombo, before enrollment to the study.
Randomization.
Patients confirmed as CL were assigned to either treatment group through a random selection process where the patients were asked to select a coded card from a collection placed in a box. The number to be included in each arm was determined using the software WINIPEP that assigned 98 for test arm and 114 for the control arm. The randomization was done by a senior nursing officer who was not a part of the management team. The consultants who monitored the treatment outcome were blinded to the treatment mode and strictly adhered to the practice of not discussing with patients regarding the treatment administered. The two arms of treatment included thermotherapy by RFHT as the test arm and IL-SSG as the control.
Procedures.
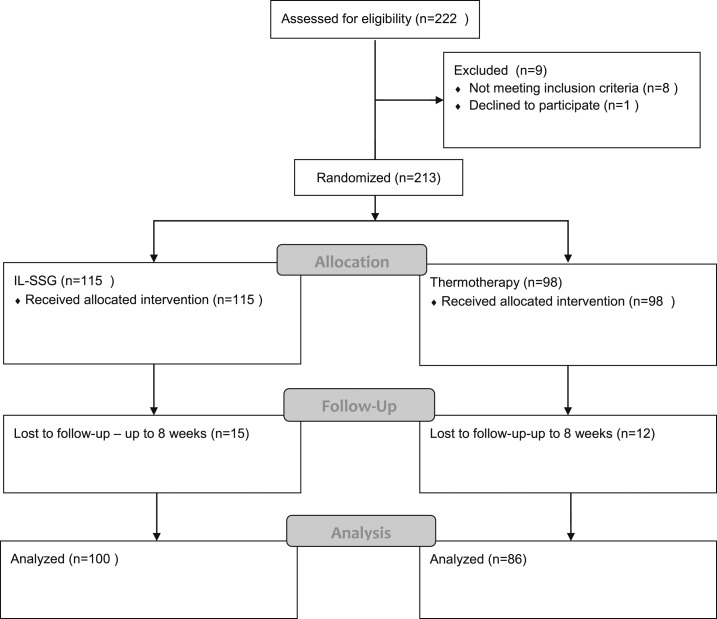
This randomized controlled trial compared single session RFHT administered (applications of 50°C for 30 seconds depending on lesion size) using the ThermoMed Model 1·8 device (test arm) to IL-SSG (Pentostam, GlaxoSmithKline) 1–3 mL administered intralesionally as weekly inoculations until cure or up to 10 doses (control arm). Treatment was administered to all participants by two qualified medical officers. The trial profile is as shown in Figure 1. Clinical trial data form was completed for each patient before treatment to record demographic information, cost incurred to visit the hospital for treatment, and clinical details with regard to baseline size and type of lesion. A physical examination was carried out to assess for any features of visceralization such as pallor and hepatosplenomegaly.
Figure 1.
Trial Profile.
Thermotherapy administration: The lesion was cleansed, anesthetized with 1% lignocaine, and moistened with normal saline before administration of thermotherapy. Heat was applied locally with a handset that included an applicator gauge with two electrodes which were placed on the lesion. The area between the electrodes covers 49–73 mm2, depending on the applicator gauge size used. Number of applications used to cover a lesion depended on lesion size and the applicator gauge was used in accordance with manufacturer’s instructions. Lesions less than 2 cm could be covered in approximately 1.5 minutes. After treatment, the lesion was covered with a sterile piece of gauze, and the patient was provided with framycetin skin cream (30 gram tube of soframycin, Sanofi) for application over the lesion twice daily for 3 days to prevent secondary infection.
Patients included in the control arm were administered weekly IL-SSG treatment: SSG 1–3 mL was infiltrated around the lesion until there was complete blanching of the lesion and its margins. All participants were followed-up fortnightly until cure or up to 12 weeks. Evaluation of treatment was done independently by consultant dermatologists who were blinded to the treatment mode delivered. The clinical trial data form was also completed at every follow-up visit with details on the size of lesion, degree of reepithilialization, clinically cured or not, requirement of further treatment, and any other remarks. Photographs were taken at baseline, and subsequently at each visit and were saved in folders for each patient for assessment as shown in (Figure 2). The occurrence of adverse events was recorded through patients’ history and examination at each visit after commencement of therapy, and details were documented in the remarks section of the data form. Participants with clinical failure at 12 weeks were offered standard IL-SSG treatment, if they were given thermotherapy and IL-SSG–treated patients who failed to respond were given a combination of cryotherapy and IL-SSG as per standard local practice.
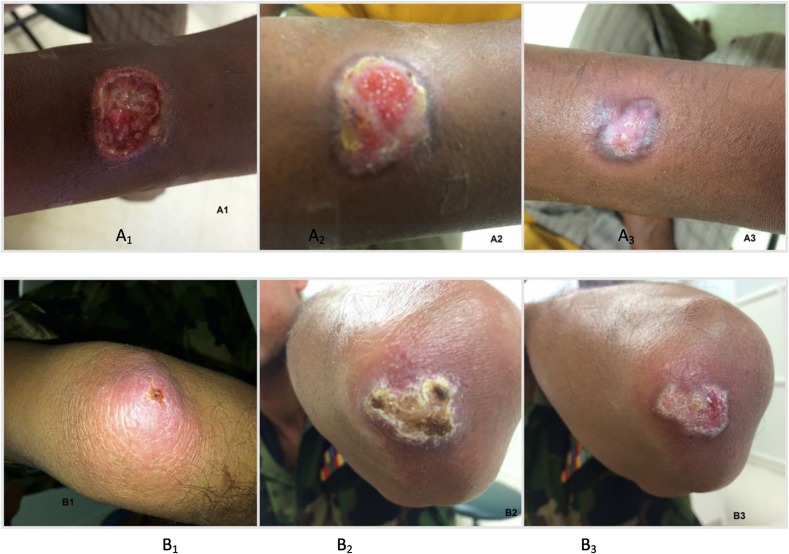
Figure 2.
Clinical manifestations of cutaneous leishmaniasis lesions before and after treatment with thermotherapy. (A1) Before treatment, an ulcer 1.2 cm in size on the forearm which was secondarily infected. (A2) At 2 weeks after treatment, ulcer size had enlarged to 2.5 cm with crusting. (A3) At 8 weeks ulcer had completely reepithelialized, leaving a 1 cm scar. (B1) Before treatment, a 5 cm noduler-ulcer with a 0.5 cm central ulcer. (B2) At 2 weeks ulcer enlarged to 2.5 cm, with the lesion being 3 cm. (B3) At 10 weeks lesion had healed with complete reepthelialization of the ulcer, leaving a 1.5 cm scar. This figure appears in color at www.ajtmh.org.
Study outcome.
Primary treatment outcome was considered as lesion cure. Cure of a lesion was defined as complete epithelialization or clinical healing of the lesion with no evidence of inflammation or induration as determined by direct assessment. Treatment outcome was measured by the percentage of patients cured at 8, 10, and 12 weeks. Treatment failure was defined as less than 50% clinical healing (or epithelialization) or the presence of any evidence of inflammation or induration at the end of 12 weeks.
Statistical analysis.
The total number of participants per arm of treatment was calculated using the null hypothesis that thermotherapy is inferior to IL-SSG by at least 10%. Assuming a 92% cure rate with IL-SSG9 with an 80% power and a 5% 2-sided type 1 error; the sample size calculated per arm of treatment was 92. To allow a loss to follow-up by 5%, the total number of patients per arm of treatment was taken as 97.
Subject data was entered and analyzed using SPSS version 20 (SPSS inc, Chicago, IL). Groups were compared using χ2 test for baseline demographic and lesion characteristics. The cure rate (at 8, 10, and 12 weeks) and its association with sex, age, size of lesion, and lesion characteristics were assessed using a stratified analysis with χ2 test. A P value of < 0.05 was taken to be significant. Logistic regression was used to assess the factors that determine cure or failure. For each associated factor, the odds ratio (OR) and 95% confidence intervals (CI) were estimated to evaluate the association of each variable with the outcome.
The scenario building technique that has been used for similar purposes previously was used to work out the cost of provision of standard treatment (IL-SSG) or thermotherapy for patients.17 Systemic costs such as cost of equipment, consumables and payments to staff in the provision of either treatment of a representative patient was identified together with the costs incurred by the patient to obtain treatment like travel time, travel cost, and waiting time. A modal estimate of average cost for each treatment option was thus obtained.
RESULTS
A total of 222 patients were screened from January to June 2014, and 213 patients who fit the inclusion criteria were randomized into either treatment arm viz; IL-SSG N = 115 or thermotherapy (N = 98) (Figure 1). Baseline demographic and clinical characteristics were comparable between the treatment groups (Table 1).
Table 1.
Baseline characteristics
| Variable | IL-SSG % (n) | Thermotherapy % (n) | P value* |
|---|---|---|---|
| Sex | |||
| M | 77.6% (90) | 74.2% (73) | 0.633 |
| F | 22.4% (25) | 25.8% (25) | – |
| Age | 0.747 | ||
| Young (12–30 years) | 40.5% (47) | 36.1% (36) | |
| Middle age(31–60 years) | 41.4% (48) | 46.4% (45) | |
| Old (> 60 years) | 18.1% (20) | 17.5% (17) | |
| Occupation | 0.155 | ||
| Army | 26.7% (30) | 43.3% (42) | |
| Civilians | 73.3% (85) | 56.7% (56) | |
| Education | 0.274 | ||
| Primary | 26.7% (30) | 18.6% (18) | |
| Secondary | 69.8% (81) | 75.3% (74) | |
| Post-secondary | 3.4% (4) | 6.2% (6) | |
| Durationofillness | 0.832 | ||
| < 3 months | 16.4% (19) | 20·6% (20) | |
| 3–6 months | 37.9% (43) | 37·1% (37) | |
| 7–9 months | 20.7% (24) | 21.6% (21) | |
| 10–12 months | 11.2% (13) | 11.3% (11) | |
| > 12 months | 13.8% (16) | 9.3% (9) | |
| Type of lesion | 0.100 | ||
| Papule | 22.4% (26) | 21.6% (22) | |
| Nodule | 17.2% (20) | 21.6% (21) | |
| Plaque | 6.9% (8) | 0% (0) | |
| Ulcer | 6.9% (8) | 5.2% (5) | |
| Nodular ulcer | 46.6% (53) | 51.5% (50) |
IL-SSG = intralesional sodium stibogluconate.
Statistical analysis using χ2 test.
Table 2 shows the efficacy of treatment at each follow-up visits. Hundred and eighty six patients completed treatment by 8 weeks (86 were treated with single-session thermotherapy and 100 with IL-SSG (2–8 treatment sessions, median = 5) with 12 and 15 patients lost to follow-up in each arm, respectively. The total cured was 68 (36.6%), of which included 62.5% (N = 40) from thermotherapy group and 41% (N = 28) from the IL-SSG group. There was a significant statistical difference in cure rate (P = 0.009) between the treatment arms at 8 weeks.
Table 2.
Efficacy of treatment at 8, 10, and 12 weeks
| Treatment arm | Duration of treatment in weeks (total number of patients) | ||||||
|---|---|---|---|---|---|---|---|
| 8 (N = 186) | P value | 10 (N = 183) | P value | 12 (N = 178) | P value | ||
| IL-SSG | Cured n(%) | 28(28) | 0.009* | 40(40.8) | 0.035* | 57(59.4) | 0.374 |
| Not cured n(%) | 72(72) | 58(59.2) | 39(40.6) | ||||
| Thermotherapy | Cured n(%) | 40(46.5) | 48(56.5) | 54(65.9) | |||
| Not cured n(%) | 46(53.5) | 37(43.5) | 28(34.1) | ||||
IL-SSG = intralesional sodium stibogluconate. Number (n) and percentage (%) of patients who responded to each treatment.
Significant P value.
Hundred and eighty three patients were available for follow-up after 10 weeks, of which 88 were found to be cured. Cure rate with single-session thermotherapy was 56.5% (N = 48 of 85 treated) and IL-SSG (2–10 treatment sessions, median = 6) was 40.8% (N = 40 of 98 treated), and the difference was statistically significant (P = 0.035). Drop outs at 10 weeks were two in the IL-SSG group and one from the thermotherapy group.
A total of 178 patients were available for follow-up at 3 months after initiating treatment, of which 111 were found to be cured. Cured patients included in the thermotherapy group N = 54 of 82 and N = 57 of 96 in the IL-SSG groups (65.9% and 59.4%), respectively. There was, however, no significant difference in cure rates between the two arms of treatment at 3 months (P = 0.374) (Table 2) with none of the entrance characteristics being significantly associated with efficacy, hence it is not shown in Table 3. The number of treatment sessions of IL-SSG at this point ranged between 2 and 12 sessions (median 7 sessions).
Table 3.
Factors associated with treatment outcome (cure) at 8 and 10 weeks
| Variable | Crude OR (95% CI) 8 weeks | P value | Crude OR | P value |
|---|---|---|---|---|
| 8 weeks | (95% CI) 10 weeks | 10 weeks | ||
| Sex | ||||
| Females | 1.930 (0.997–3.738) | 0.050* | 2.444 (1.248–4.788) | 0.009* |
| Males | 1 | – | 1 | – |
| Type of lesion | ||||
| Papule | 2.731 (1.292–5.774) | 0.009* | 2.746 (1.260–5.982) | 0.012* |
| Nodule | 0.756 (0.323–1.772) | 0.520 | 0.985 (0.451–2.151) | 0.969 |
| Ulcer | 0.125 (0.125–3.446) | 0.618 | 0.738 (0.166–3.285) | 0.691 |
| Nodular ulcer | 1 | – | 1 | – |
| Size | ||||
| < 2 cm | 1.953 (0.983–3.871) | 0.050* | 1.861 (0.978–3.542) | 0.050* |
| > 2 cm | 1 | – | 1 | – |
| Treatment | ||||
| Thermotherapy | 2.236 (1.217–4.108) | 0.012* | 1.881 (1.044–3.388) | 0.032* |
| IL-SSG | 1 | – | 1 | – |
IL-SSG = intralesional sodium stibogluconate. Factors that likely influence cure were analyzed using binary logistic regression. Odds ratio (OR) and confidence interval (CI) were calculated for each variable.
P value less than 0.05 was taken as significant.
Based on the cure rates at 8 and 10 weeks in the two treatment arms, the rate of healing in the thermotherapy group appear faster when compared with the IL-SSG group; P = 0.009 and P = 0.035, respectively, for 8 and 10 weeks (Table 2).
The association between type of treatment and cure at 8 and 10 weeks was found to be significantly different (P = 0.012 and P = 0.034, respectively) with the odds of cure by thermotherapy being almost twice as that of IL-SSG at both time points of follow-up with the OR: 2.236 (95% CI: 1.217–4.108) and 1.88 (95% CI: 1.044–3.388), respectively. Furthermore, irrespective of the mode of therapy, the size, type of the lesion, and sex of the patient were significantly associated with the outcome at 8 and 10 weeks with female sex (P = 0.050), (P = 0.009), small lesion size < 2 cm (P = 0.050), (P = 0.050), and papular lesions P = 0.009), (P = 0.012) favoring faster healing (Table 3).
Treatment outcome at 8 and 10 weeks was significantly better with thermotherapy when compared with IL-SSG (Table 3). When factors that influenced individual treatment arm were considered female sex, and papular lesions were significantly associated with cure by thermotherapy at 8 or 10 weeks unlike in the IL-SSG arm (Table 4). Papules showed a tendency to heal followed by ulcers, nodules, and nodular ulcers (Table 4). Lesions less than 2 cm healed better with thermotherapy at 10 weeks (Table 4).
Table 4.
Factors associated with cure in each treatment arm
| Duration | 8 weeks | 10 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IL-SSG | RHFT | IL-SSG | RHFT | ||||||
| Variable | OR (95% CI) | P value | OR (95% CI) | P value | OR 95% CI | P value | OR 95% CI | P value | |
| Sex | Female | 1.200 (0.451–3.191) | 0.715 | 3.167 (1.176–8.528) | 0.023 | 1.347 (0.545–3.330) | 0.519 | 5.893 (1.800–19.288) | 0.003 |
| Male | 1 | – | 1 | – | 1 | – | 1 | – | |
| Lesion type | Papule | 1.200 (0.451–3.191) | 0.179 | 5.444 (1.546–19.177) | 0.008 | 1.471 (0.536–4.037) | 0.454 | 17.773 (2.177–145.102) | 0.007 |
| Nodule | 0.554 (0.135–2.276) | 0.412 | 0.907 (0.301–2.738) | 0.863 | 1.307 (0.420–4.064) | 0.643 | 0.760 (0.258–2.244) | 0.620 | |
| Ulcer | 0.861 (0.152–4.874) | 0.866 | 0.778 (0.066–9.217) | 0.842 | 0.882 (0.186–4.192) | 0.875 | 0.523 (0.044–6.184) | 0.670 | |
| Nodular ulcer | 1 | – | 1 | – | 1 | – | 1 | – | |
| Lesion size | < 2 cm | 1.596 (0.597–4.226) | 0.352 | 2.345 (0.881–6.242) | 0.088 | 1.137 (0.476–2.715) | 0.773 | 3.302 (1.247–8.743) | 0.016 |
| > 2 cm | 1 | – | 1 | – | 1 | – | 1 | – | |
IL-SSG = intralesional sodium stibogluconate; RFHT = radiofrequency-induced heat therapy. Factors that influence cure were analyzed using binary logistic regression. Odds ratio (OR) and confidence interval (CI) were calculated for each variable.
P value less than 0.05 was taken as significant.
Age and duration of lesion were not significantly associated with treatment outcome at 8 and 10 weeks (P = 0.216, 0.788 and P = 0.541, P = 0.874, respectively; data not shown).
The likelihood of cure at 8 weeks and 10 weeks analyzed through a backward logistic regression model indicated that factors which influenced cure in major part were thermotherapy and papular lesions, OR: 2.497 (CI: 1.288–4.283), OR: 3.154 (CI: 1.446–6.880) P = 0.007, P = 0.004 for 8 weeks and OR: 2.001 (CI: 1.050–3.813), OR: 2.626 (CI: 1.161–5.936) P = 0.035, P = 0.020 for 10 weeks, respectively (data not shown in table).
There were no major adverse events recorded in either arm of treatment. However, 31/50 (62%) of nodular ulcers and 3/5 (60%) ulcers showed a transient flaring up with increase in size up to 2 weeks after thermotherapy but healed rapidly thereafter (Figure 2).
Cost analysis.
Scenario building technique was used with modal class to calculate the cost of treatment of a representative CL patient. Most of the earning individuals in the study population were either farmers or army personnel, with a lesser number of traders and service providers. Average cost of daily earnings and travel was calculated based on questionnaire records.
Most farmers lost Sri Lankan rupees (LKR) 536.00 (US Dollar: USD 4) off their daily wages while seeking treatment. When the modal travel cost back and forth at current exchange rate (1 USD = LKR 131) was added (i.e. LKR 300 or USD 2.29), the total cost per farmer patient was LKR 836.00 (USD 6.38) per visit for a single session of treatment. Cost for each treatment method per patient was calculated taking seven visits as the median number of visits required for those treated with IL-SSG, with the loss being (836 × 7 = LKR 5,852.00 [USD 44.67]). Modal loss of earnings for army patients were LKR 1,458.00 (USD 11.13). The travel cost for a soldier for a single visit was LKR 1,958.00 (USD 14.94) and if seven more additional visits were required then the loss per soldier patient would be LKR 13,706.00 (USD 104.60).
The cost of a 100 mL vial of SSG (Pentostam GlaxosmithKline) according to current market price is LKR 14,872 (USD 113),with the assumption that an average of 1 mL (100 mg SSG) is used per patient per visit, the cost of drug per patient per visit was calculated as LKR 148.72 (USD 1.13) and with patients requiring seven doses on average before cure, the cost of entire course of SSG per patient is LKR 148.72 × 7 = LKR 1,041.00 (USD 7.94). The price of the drug per patient was added to the cost of patient care (which included cost to pay staff and consumables such as syringes, gauze, cotton wool, saline, plaster etc., with total estimated as LKR 412 [USD 3.14]).Therefore, the cost incurred by the health care system to treat a patient with standard IL-SSG is LKR 1,453.00 (USD 11.09) .
The cost per patient treated with thermotherapy was calculated as LKR 164 (USD 1.20) for the single-session treatment. The cost of a new machine, ThermoMed Thermosurgery Inc., is LKR 850,000 (USD 6,488). Based on the assumption that the machine lasts for > 20 years (as per claims made by the manufacturer) and that 100 patients are treated per week, the annual value is LKR 42,500 (USD 324); per day value is LKR 817 (USD 6.2), and per patient value is LKR 8.17 (USD 0.06). The patient care cost was calculated for staff payments, consumables, electricity for charging the device (minimum), and transport of the device for calibration and servicing to be done every 4 years (according to manufacturer’s instructions) as LKR 164 (USD 1.25). However, if only 20 patients are treated per week (which was the approximate number as per hospital records), the per patient cost calculated to treat with thermotherapy is LKR 201.4 (USD 1.54) (per patient value is LKR 41 + 22.17 for staff payments + 132.5 for consumables + 5.76 for transport of device for calibration) and if the patient demand was presumed to be even lower, for example, 10 patients per week, then the cost per patient is LKR 248 (USD 1.90) (LKR 82 + 22.17 + 132.5 + 11.5).
DISCUSSION
This study demonstrates that a single session of treatment with thermotherapy for L. donovani–induced CL is effective and safe when compared with IL-SSG. Furthermore, the time to cure with thermotherapy was shown to be shorter than with IL-SSG, with more healed lesions in the thermotherapy arm (46.5% and 56.5%) than in the IL-SSG arm (28% and 40.8%) by 8 and 10 weeks, respectively. The chances of cure with thermotherapy was approximately double OR: 2.236 (95% CI: 1.217–4.108), OR: 1.881 (95% CI: 1.044–3.388) when compared with IL-SSG at 8 and 10 weeks, respectively; which might be due to an early favorable cytokine response.9 However, by 12 weeks the cure rate in both arms was shown to be comparable (thermotherapy 65.9% and IL-SSG 59.4%) with 111/178 (62%) patients cured by either treatment by this time. Therefore, thermotherapy is noninferior to IL-SSG treatment with the former mode of treatment of CL because of L. donovani having many advantages over the latter.
Standard treatment of L. donovani CL in Sri Lanka includes multiple sessions of intralesional (or intramuscular sodium stibogluconate) and/or local application of cryotherapy. These regimes present an array of problems as previously reported, that include painful inoculations or application, drug toxicity, costly medication, scarring with cryotherapy, poor patient compliance, poor treatment response4,18 and threat of emergence of drug-resistant strains.18 The current WHO recommendation for CL therapy in the old world (L. tropica and L. major) is IL-SSG 1–5 mL per session (every 3–7 days) for one to five sessions. It is not known, however, if the response to SSG in L. donovani CL differs depending on the frequency of its application and if more frequent sessions of IL-SSG (than the weekly treatment regimen that was used in this study) could lead to a more favorable outcome (e.g., cure with a lower total dose of SSG). Therefore, further studies may be required to optimize therapeutic options for L. donovani CL.
Several studies have been done in the past to demonstrate the efficacy of thermotherapy in the treatment of CL caused by other species of Leishmania. A study conducted in 2005 and 2013 on L. tropica–induced CL, demonstrated similar efficacy and effectiveness as observed in this study.8,9 Similarly a clinical trial on L. major–induced CL reported a cure rate of 48% with thermotherapy and 54% with intravenous SSG at 2 months.10 Differences in treatment response between the studies may be due to variations in sensitivity of Leishmania species to drugs and temperature.18,19 There is already evidence for such variations with L. tropica recognized as a more temperature-resistant species than L. major.18 Furthermore, the differences in cure rates also might be related to varying host genetic factors, immune status, and nutritional status of patients.
There was a significant difference between the two sexes of participants in achieving cure at 8 and 10 weeks with larger proportion of females being cured with thermotherapy as compared with their male counter parts, despite having similar lesion types. A possible explanation might be a more favorable immune response in females with resultant enhanced clinical cure, as demonstrated in animal models.20
Smaller lesions (< 2 cm) and papular lesions were positively associated with cure outcome (< 2 cm lesions P = 0.050 at both 8 and 10 weeks times; papules P = 0.009 and 0.010, respectively) a finding that was similar to previous observations.20,15 Interestingly, the association with the size and type of lesion was independent of the duration of lesion.
None of the patients on either arm of treatment suffered from any major side effects of treatment, except pain that was experienced by 52/115 subjects during IL-SSG injections and few 16/98 after the administration of thermotherapy. Similar to previous observations, a proportion of patients in the thermotherapy arm experienced an apparent flaring up of lesion around 2 weeks after therapy that indicates the importance of providing relevant information to the patient on the likely events that could follow to avoid possible distress.8,9 Such temporary exacerbations of lesions are believed to be due to superficial second degree burns that could occur at the sites of application of the electrodes (Figure 2A2 and B2) that heals with no complications.15
Those patients who were included in the IL-SSG group received about seven treatment sessions overall, whereas the novel therapy introduced to treat CL (thermotherapy) required only a single session. Therefore, the cost that otherwise has to be borne by the patient to make additional visits to receive standard therapy with related loss of earnings is foregone.
Although the equipment required for thermotherapy (ThermoMED 1.8 model) is expensive, the overall cost to provide standard SSG treatment outweigh the cost of thermotherapy with the per patient cost calculated as only LKR 164 (USD 1.250) as opposed to LKR 1,453 (USD 11.09) for IL-SSG. If 20 patients are treated per week, then the cost is LKR 202 (USD 1.54) which is still less than IL-SSG treatment sessions. The per patient cost to treat with thermotherapy is about 7 times cheaper for the national health system. This would be a considerable saving for the health sector particularly in high transmission areas with average patient load of approximately 15–20 per week. Therefore, the health system averts an expense of LKR 1,251.00 (USD 9.5) per patient if the new mode of treatment, thermotherapy is introduced. The total sum saved by switching to thermotherapy from IL-SSG for a year, assuming that 20 patients are treated weekly, is LKR 1,301,040.00 (USD 9,931), which is considerable for a developing country like Sri Lanka that offers free health care to all its citizens.
Accurate estimations on the cost of leishmaniasis treatment with SSG, however, are difficult to make and depend on several variables such as the mode of delivery of treatment, if treatment is given on out-patient or in-patient basis, number of sessions of treatment given, and brand of SSG used. The total cost for treatment was estimated by adding the cost of treatment to patient care cost, with the latter factor also known to be variable. Cost of treatment of CL with SSG also vary depending on the geographic location; being USD 20 in Afghanistan,8 USD 280 in Guatemala,21 and USD 5,500 per patient in the USA.10 The cost determined for this study to treat a patient was on the basis of an out-patient given seven sessions of treatment that came to USD 11.09. In Sri Lanka, the cost to treat a CL patient with standard SSG therapy on out-patient basis is relatively cheaper as compared with other countries, which might be due to the cheap labor and the SSG brand used. This study identifies the use of thermotherapy for CL to be much more economical than SSG, especially in endemic areas as mentioned by Safi et al.22 in 2012. The retail price of the thermotherapy device reported by Reithinger et al.8 in 2005 was USD 23,450, the current price quoted by Thermosurgery Technologies, however, was USD 6,500.Therefore, the machine value may have depreciated overtime making it more affordable for low-income countries with high endemicity of CL.
Thermotherapy has the added advantage of being an effective alternative for patients with comorbidities such as renal, hepatic, and cardiac complications for whom systemic therapy with antimony is contraindicated.23 However, thermotherapy cannot be used too close to mucosae8 which is considered as a limitation. Single-session therapy requires the patient to visit the hospital once and hence it is a convenient mode of treatment, which reduces the economic and social burden on the patient. Furthermore, it is a portable battery-operated hand-held device that can be used in the field if necessary, to facilitate treatment of patients in far off remote areas. By contrast, IL-SSG intervention requires the patient to visit hospital at least 2–10 times which can be troublesome and hence reduce patient compliance, which may result in the development of resistance to SSG that has already been suspected in the local setting.14 Follow-up of 69 patients one year after treatment showed relapse of lesions in two patients in the IL-SSG group and none from thermotherapy group, which indicates better efficacy of thermotherapy in the long term, although this may need confirmation with a larger group of patients. Further studies are indicated to assess the efficacy of thermotherapy on patients who respond poorly to IL-SSG to assess the long-term effects of thermotherapy on CL and the impact of a repeat thermotherapy session on large lesions to further facilitate cure.
Thermotherapy by radio frequency could be considered as the treatment of choice in endemic areas for the treatment of L. donovani CL in Sri Lanka as it is effective and safe. Thermotherapy has the added advantage of being relatively noninvasive, cost-effective; it requires only a single hospital visit and responds early to treatment, all of which benefit both the patient and the national health sector. CL being a disease that is prevalent in many neighboring developing countries such as India, Pakistan, and Afghanistan, the outcome of this study will have a wider relevance and importance.
Acknowledgments:
We thank Gena Zischke from Thermosurgery Inc for providing the ThermoMed 1.8 machine. We are grateful to Upul Senarath, Community Physician, Faculty of Medicine, Colombo, for advice regarding statistical analysis and K.K.G.D.U.L Kariyawasam for formatting of the figures and technical advice.
REFERENCES
- 1.Centers for Diease Control and Prevention, 2013 Parasites - Leishmaniasis: Epidemiology and Risk Factors. Available at: www.cdc.gov/parasites/leishmaniasis/index.html.
- 2.Alvar J, Yactayo S, Bern C, 2006. Leishmaniasis and poverty. Trends Parasitol 22: 552–557. [DOI] [PubMed] [Google Scholar]
- 3.WHO, Control of the leishmaniasis , 2010. In Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Technical Report Series No 949 Geneva, Switzerland: World Health Organization, 1–185.
- 4.Kassi M, Afghan AK, Rehman R, Kasi PM, 2008. Marring leishmaniasis: the stigmatization and the impact of cutaneous leishmaniasis in Pakistan and Afghanistan. PLoS Negl Trop Dis 2: e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart CC, Brieger WR, 2009. Community views on cutaneous leishmaniasis in Istalif, Afghanistan: implications for treatment and prevention. Int Q Community Health Educ 29: 123–142. [DOI] [PubMed] [Google Scholar]
- 6.Berman JD, 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis 24: 684–703. [DOI] [PubMed] [Google Scholar]
- 7.Bryceson A, 2001. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop Med Int Health 6: 928–934. [DOI] [PubMed] [Google Scholar]
- 8.Reithinger R, et al. , 2005. Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled trial. Clin Infect Dis 40: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 9.Bumb RA, et al. , 2013. Long-term efficacy of single-dose radiofrequency-induced heat therapy vs. intralesional antimonials for cutaneous leishmaniasis in India. Br J Dermatol 168: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 10.Aronson NE, et al. , 2010. A randomized controlled trial of local heat therapy versus intravenous sodium stibogluconate for the treatment of cutaneous Leishmania major infection. PLoS Negl Trop Dis 4: e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karunaweera ND, 2009. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: a wolf in sheep’s clothing? Trends Parasitol 25: 458–463. [DOI] [PubMed] [Google Scholar]
- 12.Karunaweera ND, Pratlong F, Siriwardane HVYD, Ihalamulla RL, Dedet JP, 2003. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans R Soc Trop Med Hyg 97: 380–381. [DOI] [PubMed] [Google Scholar]
- 13.Karunanayake PH, Karunaweera ND, Siriwardana HVYD, 2013. Management of leishmaniasis. Sri Lanka Prescriber 21: 1–5. [Google Scholar]
- 14.Refai FW, Madarasingha N, Fernandopulle R, Karunaweera ND, 2016. Nonresponsiveness to standard treatment in cutaneous leishmaniasis: a case series from Sri Lanka. Trop Parasitol 6: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neva FA, Petersen EA, Corsey R, Bogaert H, Martinez D, 1984. Observations on local heat treatment for cutaneous leishmaniasis. Am J Trop Med Hyg 33: 800–804. [DOI] [PubMed] [Google Scholar]
- 16.Junaid AJ, 1986. Treatment of cutaneous leishmaniasis with infrared heat. Int J Dermatol 25: 470–472. [DOI] [PubMed] [Google Scholar]
- 17.Bloom DE, et al. , 1997. Socio-economic dimensions of the HIV/AIDS epidemic in Sri Lanka. Bloom DE, Godwin P, eds. The Economics of HIV and AIDS: The Case of South and South East Asia. Delhi, India: Oxford University Press. [Google Scholar]
- 18.Garnier T, Croft SL, 2002. Topical treatment for cutaneous leishmaniasis. Curr Opin Investig Drugs 3: 538–544. [PubMed] [Google Scholar]
- 19.Berman JD, Neva FA, 1981. Effect of temperature on multiplication of Leishmania amastigotes within human monocyte-derived macrophages in vitro. Am J Trop Med Hyg 30: 318–321. [DOI] [PubMed] [Google Scholar]
- 20.Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG, 2002. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun 70: 2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arana BA, Mendoza CE, Rizzo NR, Kroeger A, 2001. Randomized, controlled, double-blind trial of topical treatment of cutaneous leishmaniasis with paromomycin plus methylbenzethonium chloride ointment in Guatemala. Am J Trop Med Hyg 65: 466–470. [DOI] [PubMed] [Google Scholar]
- 22.Safi N, 2012. Evaluation of thermotherapy for the treatment of cutaneous leishmaniasis in Kabul, Afghanistan: a randomized controlled trial. Int J Infect Dis 16: e174. [DOI] [PubMed] [Google Scholar]
- 23.Cardona-Arias JA, Vélez ID, López-Carvajal L, 2015. Efficacy of thermotherapy to treat cutaneous leishmaniasis: a meta-analysis of controlled clinical trials. PLoS One 10: e0122569. [DOI] [PMC free article] [PubMed] [Google Scholar]