Abstract.
Orientia tsutsugamushi is a major cause of vector-borne infection in Asia. Prompt recognition and appropriate treatment are crucial because of its potentially fatal complications and lack of response to beta-lactam antibiotics. The present study retrospectively evaluated the clinical characteristics and laboratory findings of 16 patients with scrub typhus-related central nervous system (CNS) infections. Single titers ≥ 1:40 of total serum antibodies against O. tsutsugamushi detected by an indirect immunofluorescent assay were considered as positive results. The median age was 35.5 (range, 14–72) years, and 10 (62.5%) patients were female. The most common symptoms were headache (81.3%) and fever (81.3%). Eschar formation was found in three (18.8%) patients. Among patients with encephalitis, seizures and altered consciousness occurred in five (83.3%) and four (66.7%) patients, respectively. An abnormal liver function was noted in seven (43.8%) patients. The median antibody titer was 1:120 (range, 1:40–1:2,560). Typical cerebrospinal fluid profiles were lymphocytic pleocytosis, mild protein elevations, and normal glucose levels. All patients received an empirical treatment with doxycycline and most (93.8%) of them recovered without neurological sequelae. None of the patients reported side effects of the doxycycline treatment. An empirical treatment with doxycycline is needed in patients with CNS infections in scrub typhus endemic areas.
INTRODUCTION
Scrub typhus is a vector-borne infectious disease caused by Orientia tsutsugamushi, which is transmitted to humans by the bite of larval trombiculid mites. The infection usually occurs from June through November1,2 and is endemic to the Asia-Pacific region, extending from Japan, South Korea, China, Taiwan, Nepal, Pakistan, India, Papua New Guinea, and Australia. Orientia tsutsugamushi infection is typically characterized by fever, myalgia, headache, lymphadenopathy, skin rashes, and eschar formation; however, it can also affect the central nervous system (CNS) and cause meningitis and encephalitis.3,4 Therefore, O. tsutsugamushi should be considered to play a possible role in the pathogenesis of the CNS infection in areas where scrub typhus is endemic. Delay in diagnosis and treatment may result in complications such as pneumonitis, meningitis, hepatitis, acute kidney injury, disseminated intravascular coagulation, and eventually death.5–8
In South Korea, more than 5,000 cases have been annually reported since 2005, and the proportion of infected persons living in urban areas is rising.1,9 Meningitis or encephalitis occurs in approximately 10% of patients that are hospitalized because of scrub typhus.10 Despite the persistently high incidence of scrub typhus in South Korea, very little is known about its neurological manifestations. Therefore, we investigated the clinical and laboratory characteristics in patients with meningitis and encephalitis associated with O. tsutsugamushi infection.
METHODS
We retrospectively reviewed the electronic medical records for all patients admitted to the Seoul National University Hospital who were clinically diagnosed with meningitis or encephalitis between 2009 and 2014. All laboratory tests were performed within 24 hours of admission. The batched indirect immunofluorescent assays (IFAs) for the total serum antibodies against the O. tsutsugamushi strains Boryong, Gilliam, and Karp were conducted. The cutoff value for a positive antibody test was antibody titers ≥ 1:40. Furthermore, all patients underwent laboratory investigations to identify any other microbial etiologies, including the evaluation of blood and cerebrospinal fluid (CSF) cultures, polymerase chain reaction (PCR), and serological tests for the following pathogens: Mycoplasma, Legionella, Mycobacterium, Chlamydia, Brucella, Borrelia, measles virus, mumps virus, rubella, herpes simplex virus type 1, Epstein–Barr virus, cytomegalovirus, varicella zoster virus, enterovirus, respiratory virus, Japanese encephalitis virus, human herpes virus (HHV) type 6, HHV type 8, BK virus, JC virus, tick-borne encephalitis virus, Coxsackie B type 5, Coxsackie B type 6, parvovirus B19, Cryptococcus, Aspergillus, and Pneumocyctis. The PCR for O. tsutsugamushi was not performed because it was not available in our hospital. Among patients who tested positive for O. tsutsugamushi antibodies, we excluded patients who also tested positive for etiologies other than scrub typhus. Medical records of the selected patients were reviewed for clinical manifestations, laboratory or neuroimaging findings, and disease outcomes. Based on the case definition for aseptic meningitis from the Centers for Disease Control and Prevention (CDC), meningitis was diagnosed in cases with an acute onset of meningeal symptoms, fever, CSF pleocytosis, and an absence of microorganisms on Gram stain and/or on routine culture.11 According to the World Health Organization’s definition of acute encephalitis syndrome, cases with an acute onset of fever and a change in mental status and/or new onset of seizures were diagnosed as having encephalitis.12 Although three patients did not have fever, two patients were diagnosed with meningitis because of an acute onset of headache and CSF pleocytosis (patients #5 and #14), and one patient was diagnosed with meningoencephalitis based on an acute onset of seizures and a clear parenchymal involvement which were confirmed by using the brain magnetic resonance imaging (MRI) (patient #10). Patient data were anonymized to protect patient privacy. This study was approved by the Institutional Review Board of Seoul National University Hospital (1608-086-785).
RESULTS
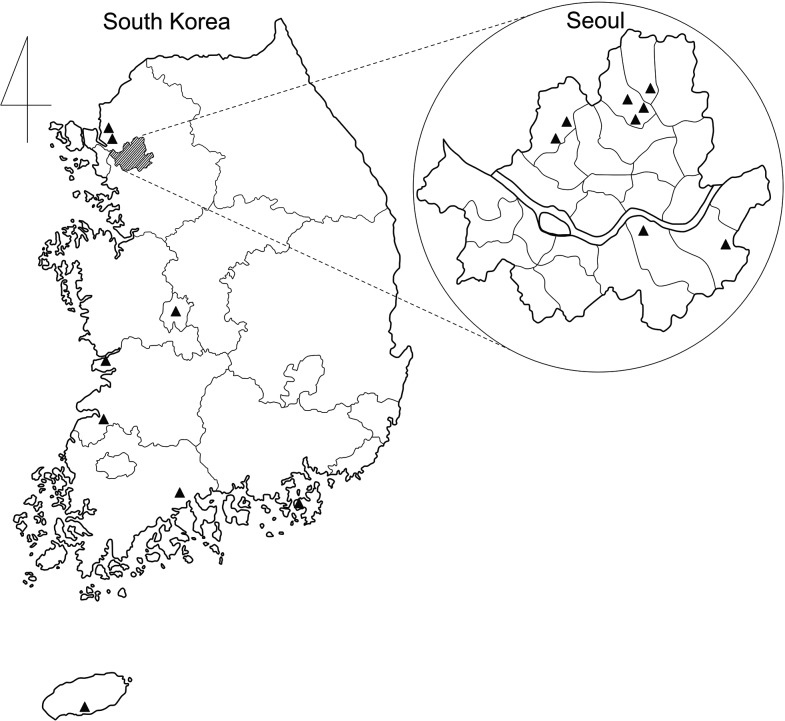
Sixteen patients diagnosed with O. tsutsugamushi-associated meningitis or encephalitis were identified during the study period. According to geographical regions, eight (50%) cases occurred in Seoul, the capital city of South Korea, whereas the other eight (50%) cases occurred in rural areas throughout the country (Figure 1). The median age was 35.5 (range, 14–72) years, and 10 (62.5%) patients were female. Overall, the most common symptoms were headache (81.3%) and fever (81.3%). Although four patients with meningitis (patients #1, #6, #7, and #13) had a history of fever 3 to 14 days before admission, their body temperature remained normal since the day of admission. Eschar formation was noted in three (18.8%) patients. According to clinical manifestations, meningitis was diagnosed in 10 (62.5%) patients, all of whom presented with a headache; meningeal irritation signs were found in four patients. Furthermore, six (37.5%) patients showed clinical features consistent with encephalitis. Altered consciousness was observed as stupor in three patients and confusion in one patient. Seizures were observed in five (83.3%) patients with encephalitis, of whom one had status epilepticus. In addition, neurological abnormalities presented in encephalitis cases included spastic dysarthria and limb ataxia. Three out of six patients (patient numbers #2, #8, and #15) initially showed fever with headaches and CSF pleocytosis, which would have been diagnosed as meningitis at the initial presentation but eventually developed symptoms indicating encephalitis 2–7 days after the initial headache occurred.
Figure 1.
The geographical distribution of scrub typhus-related meningitis and encephalitis cases in South Korea, 2009–2014. The enlarged figure shows the map of Seoul, the capital city of South Korea. The location of each case is noted as a triangle.
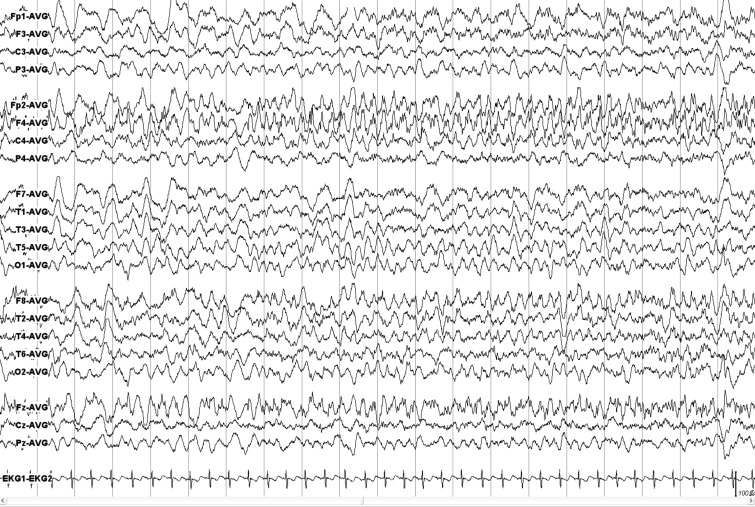
The median titer of antibodies against O. tsutsugamushi antigens was 1:120 (range, 1:40–1:2,560). Blood analysis revealed leukocytosis (leukocyte count > 10 × 109/L) in 5 (31.3%) patients and elevated high-sensitivity C-reactive protein levels (> 0.5 mg/dL) in 8 (50%) patients. The results of liver function tests were abnormal (aspartate transaminase > 40 units/L or alanine transaminase > 40 units/L) in 7 (43.8%) patients, and thrombocytopenia (< 150 × 109/μL) was noted in two (12.5%) patients. The CSF analysis revealed lymphocytic pleocytosis in 13 patients with a median leukocyte count of 180/µL and a differential lymphocyte count of 71%. The median CSF protein level was 0.57/(range, 0.15–3.47) g/L, and 12 (75%) patients had elevated CSF protein levels (> 0.4 g/L). The median CSF glucose levels were 3.4/(range, 2.3–5.9) mmol/L. The brain MRI showed a prominent leptomeningeal enhancement in 4 (25%) patients and a small nonenhanced T2 high-signal lesion involving the midbrain in one patient. Notably, one patient, who presented with meningitis and multiple cranial nerve deficits, revealed a marked contrast-enhancement of the facial nerve. Seven of 10 (70%) patients with meningitis underwent electroencephalography (EEG), and the results were normal for all of them. All of the patients with encephalitis underwent EEG; four (66.7%) of them showed diffusely slow activities, and one (16.7%) patient showed electrographic seizures originating in the right frontal area (Figure 2). The clinical characteristics and laboratory findings of the patients are summarized in Table 1. Most cases showed stable and favorable progression during the clinical course; however, one patient required critical care because of an acute kidney injury. All patients were treated with oral doxycycline combined with clarithromycin or azithromycin, and most (93.8%) of them completely recovered without significant neurological sequelae; only one case with multiple cranial neuropathies showed persistent facial nerve palsy. All of the five patients with seizures remained seizure-free after discharge (range, 25–55 months; median, 39 months), and the antiepileptic drug administration was successfully ended in one patient.
Figure 2.
Electroencephalography recordings of the patient (#3) with status epilepticus. Note generalized rhythmic delta activity with superimposed repetitive sharp waves originating in the right frontal areas. The average reference montage is used.
Table 1.
Clinical characteristics and laboratory findings of patients with O. tsutsugamushi-associated meningitis and encephalitis
| Patient number | Age | Sex | Symptom onset to admission (days) | CNS symptom | Meningeal irritation sign | Associated symptom | Blood tests | CSF profiles | Brain MRI | EEG | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) | AST/ALT (units/L) | Total Ab titer | WBC (/µL) | Protein (g/L) | Glucose (mmol/L) | |||||||||
| 1 | 29 | F | 3 | M, HA | + | Fever | 8.9 | 20/8 | 1:160 | 324 | 0.72 | 4.0 | WNL | NA |
| 2 | 47 | M | 4 | ME, HA, confusion, seizure, stupor | + | Fever, eschar | 11.4 | 131/32 | 1:40 | 333 | 0.72 | 4.0 | Leptomeningeal enhancement | Generalized semirhythmic slow waves |
| 3 | 14 | M | 22 | ME, SE, drowsiness, confusion, seizure | − | Fever, skin rash, eschar | 10.3 | 125/111 | 1:2560 | 6 | 0.98 | 5.4 | WNL | Generalized rhythmic slow waves |
| 4 | 38 | M | 6 | M, HA | + | Fever | 4.7 | 81/111 | 1:160 | 20 | 0.42 | 2.3 | WNL | NA |
| 5 | 32 | M | 7 | M, HA, dizziness | + | – | 11.1 | 16/20 | 1:40 | 15 | 0.39 | 2.2 | WNL | WNL |
| 6 | 52 | F | 3 | M, HA | − | Fever, Myalgia | 7.29 | 184/260 | 1:1280 | 14 | 0.57 | 3.2 | Leptomeningeal enhancement | WNL |
| 7 | 27 | M | 13 | M, HA | + | Fever, Skin rash | 8.1 | 22/17 | 1:40 | 675 | 0.96 | 5.3 | WNL | WNL |
| 8 | 40 | F | 9 | ME, HA, dysarthria, limb ataxia, nystagmus | + | Fever | 10.8 | 51/61 | 1:80 | 40 | 0.57 | 3.2 | Leptomeningeal enhancement | Diffuse irregular slowing |
| 9 | 49 | M | 14 | ME, seizure, stupor | − | Fever, AKI, eschar | 6.2 | 75/57 | 1:40 | 1 | 0.41 | 2.3 | WNL | Diffuse irregular slowing |
| 10 | 53 | F | 10 | ME, seizure | − | – | 6.9 | 35/71 | 1:80 | 0 | 0.25 | 1.4 | T2 high signal in right midbrain | WNL |
| 11 | 31 | F | 3 | M | − | Fever, skin rash, vomiting | 7.2 | 21/12 | 1:40 | 360 | 0.63 | 3.5 | Leptomeningeal enhancement | WNL |
| 12 | 23 | F | 22 | M, HA | − | Fever | 4.3 | 39/32 | 1:160 | 180 | 0.64 | 3.6 | WNL | WNL |
| 13 | 72 | F | 12 | M, HA, multiple cr. neuropathy | − | Fever | 4.92 | 17/16 | 1:160 | 120 | 2.46 | 13.7 | Left facial nerve enhancement | NA |
| 14 | 24 | F | 1 | M, HA | − | – | 6.87 | 12/6 | 1:160 | 1,224 | 0.15 | 0.8 | WNL | WNL |
| 15 | 57 | F | 8 | ME, HA, confusion, dysarthria, seizure, stupor | − | Fever | 11.58 | 24/22 | 1:160 | 25 | 0.49 | 2.7 | WNL | Diffuse irregular slowing |
| 16 | 33 | F | 6 | M, HA | − | Fever | 5.45 | 15/5 | 1:80 | 910 | 3.47 | 19.3 | WNL | WNL |
CNS = central nervous system; M = meningitis; ME = meningoencephalitis; HA = headache; SE = status epilepticus; cr. = cranial; AKI = acute kidney injury; AST = aspartate transaminase; ALT = alanine transaminase; Ab = antibody; CSF = cerebrospinal fluid; MRI = magnetic resonance image; EEG = electroencephalography; WNL = within normal limit; NA = not available.
DISCUSSION
We described a case series of 16 patients with O. tsutsugamushi-associated meningitis or encephalitis in South Korea. The incidence of eschar formation in our study was 18.8%, which was a low proportion of patients presenting with the typical dermatological sign of scrub typhus compared with other studies,2,10,13 although the frequency of eschar might have been underestimated because of the retrospective nature of this study. Although 37.5% of patients developed encephalitis, the severity of neurological deterioration was modest, and patients responded well to the antibiotic treatment. Moreover, seizures were well-controlled with the antiepileptic drug treatment. Our observation suggested that the overall prognosis of the CNS involvement in scrub typhus was favorable, which is consistent with previous reports.14–16 Owing to the diagnostic difficulties based on clinical factors and favorable response to antibiotic treatment, we emphasize the use of doxycycline as an empirical treatment of O. tsutsugamushi-associated CNS infections in scrub typhus endemic areas.
It is necessary to suspect scrub typhus when a patient with CNS infection shows atypical features, such as abnormal liver function test results, skin rashes, or thrombocytopenia. Doxycycline is the treatment of choice for scrub typhus infection and is recommended for the empirical antibiotics in patients with suspected rickettsial or ehrlichial infection.17–19 It should be noted that conventional antibiotics, including beta-lactams and aminoglycosides, have limited effects on O. tsutsugamushi infections.20 Moreover, although its etiology is bacterial, the CSF profile of scrub typhus meningitis may mimic that of viral meningitis with lymphocytic pleocytosis, making the appropriate diagnosis and treatment of scrub typhus meningitis difficult.2,10 Therefore, a treatment strategy with early administration of doxycycline may be reasonable, which would be effective in preventing further complications of scrub typhus infection. Our study shows excellent prognosis of patients when treated with this strategy. The rate of adverse reactions with doxycycline administration varies among clinical trials ranging from 0% to 61%, but the adverse effects are mostly benign conditions such as esophageal erosion, skin rashes, and pruritus.19,21 Furthermore, none of our patients had adverse reactions related to doxycycline, which supports the tolerability of the empirical treatment.
The IFA is the gold standard for the laboratory diagnosis of scrub typhus; however, the cutoff antibody titer varied among studies (1:10–1:400) and has not been clearly defined. More than a 4-fold increase in the antibody titer between the two serum samples is considered reliable for scrub typhus diagnosis; however, it essentially delays the diagnosis and antibiotic treatment. The PCR assay is an alternative diagnostic tool. The sensitivity of the PCR-based diagnosis of scrub typhus varied widely; its sensitivity ranged from 60% to 95% depending on the specific technique.22 The PCR has an advantage in detecting the pathogen, especially in early stages of the disease before antibodies become detectable.23 The PCR also provides a much higher specificity and a better chance to exclude false-positive cases compared with the IFA.22
The present study has several limitations. First, the laboratory diagnosis of scrub typhus was based on the antibody titer obtained from single serum samples rather than comparative results from two consecutive samples. Second, the cutoff value for the titer of 1:40 is low compared with those from other studies, especially within southeastern Asia. Although a study conducted in Thailand to evaluate the sensitivity and specificity of single-titer IFA values showed a relatively high sensitivity (91.7%) and specificity (95.6%) with a cutoff titer of 1:100 relative to indirect immunoperoxidase tests, this cutoff value cannot be directly applied to other regions since the seroprevalence of O. tsutsugamushi antibodies may differ across countries.24 A cutoff titer of 1:40 was widely adopted in South Korea for the diagnosis of scrub typhus since the early 1990s.25 However, a recent survey for O. tsutsugamushi in healthy Korean subjects showed 1.1% of the subjects with an immunoglobulin M antibody titer equal to or higher than 1:40 and 2.2% of the subjects with an immunoglobulin G antibody titer equal to or higher than 1:64.26 Furthermore, antibody titers in convalescent stages, which might have supported the diagnosis of O. tsutsugamushi infection, were not available in our study. In this regard, a single 1:40 titer used in this study cannot rule out the possibility of false positive results, although we excluded patients that tested positive for other microbial etiologies to minimize false positive rates. Other limitations include the retrospective design, small sample size, and hospital-based setting of this study. Therefore, our observations cannot represent the general population with O. tsutsugamushi-associated encephalitis. A nationwide study with a larger sample size is required to clearly define the clinical characteristics of these patients.
Scrub typhus should be considered as a possible etiology of acute meningitis or encephalitis particularly in endemic areas. A seasonal pattern should also be considered, but should not be a reason to exclude the diagnosis. Furthermore, patients with meningitis can present with nonspecific findings, where the serological tests play a critical role in the diagnosis of scrub typhus. Given the favorable response to doxycycline administration, the empirical treatment targeting scrub typhus must be initiated in patients with meningitis or encephalitis before laboratory confirmation.
REFERENCES
- 1.Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, Ryu SY, Yoo HS, Park O, 2009. Rapid increase of scrub typhus, South Korea, 2001–2006. Emerg Infect Dis 15: 1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittrich S, et al. , 2015. Orientia, Rickettsia, and leptospira pathogens as causes of CNS infections in Laos: a prospective study. Lancet Glob Health 3: e104–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pai H, Sohn S, Seong Y, Kee S, Chang WH, Choe KW, 1997. Central nervous system involvement in patients with scrub typhus. Clin Infect Dis 24: 436–440. [DOI] [PubMed] [Google Scholar]
- 4.Silpapojakul K, Ukkachoke C, Krisanapan S, Silpapojakul K, 1991. Rickettsial meningitis and encephalitis. Arch Intern Med 151: 1753–1757. [PubMed] [Google Scholar]
- 5.Ebisawa II, 1995. Current epidemiology and treatment of tsutsugamushi disease in Japan. J Travel Med 2: 218–220. [DOI] [PubMed] [Google Scholar]
- 6.Seong SY, Choi MS, Kim IS, 2001. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect 3: 11–21. [DOI] [PubMed] [Google Scholar]
- 7.Kim DM, Kim SW, Choi SH, Yun NR, 2010. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis 10: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang SJ, Park KH, Jung SI, Jang HC, Ji SY, Ahn JS, Kim HJ, Shin JH, Kim DM, 2010. Scrub typhus induced by peripheral blood stem cell transplantation in the immunocompromised patient: diagnostic usefulness of nested polymerase chain reaction. Transfusion 50: 467–470. [DOI] [PubMed] [Google Scholar]
- 9.Jeong MA, Youn SK, Kim YK, Lee H, Kim SJ, Sohn A, 2013. Trends in the incidence of scrub typhus: the fastest growing vector-borne disease in Korea. Osong Public Health Res Perspect 4: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DM, Chung JH, Yun NR, Kim SW, Lee JY, Han MA, Lee YB, 2013. Scrub typhus meningitis or meningoencephalitis. Am J Trop Med Hyg 89: 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention , 1997. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep 46: 1–55. [PubMed] [Google Scholar]
- 12.Solomon T, Thao TT, Lewthwaite P, Ooi MH, Kneen R, Dung NM, White N, 2008. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bull World Health Organ 86: 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abhilash KP, Gunasekaran K, Mitra S, Patole S, Sathyendra S, Jasmine S, Varghese GM, 2015. Scrub typhus meningitis: an under-recognized cause of aseptic meningitis in India. Neurol India 63: 209–214. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan S, Muthu V, Iqbal N, Remalayam B, George T, 2013. Scrub typhus meningitis in south India–a retrospective study. PLoS One 8: e66595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NP, 2007. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis 44: 391–401. [DOI] [PubMed] [Google Scholar]
- 16.Kalita J, Mani VE, Bhoi SK, Misra UK, 2016. Status epilepticus in scrub typhus. Epilepsia 57: e125–e128. [DOI] [PubMed] [Google Scholar]
- 17.Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ, Infectious Diseases Society of America, 2008. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 47: 303–327. [DOI] [PubMed] [Google Scholar]
- 18.Phimda K, et al. , 2007. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob Agents Chemother 51: 3259–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Y, Huang Z, Tu C, Zhang L, Ye D, Zhu BP, 2012. Meta-analysis of drug treatment for scrub typhus in Asia. Intern Med 51: 2313–2320. [DOI] [PubMed] [Google Scholar]
- 20.Raoult D, Drancourt M, 1991. Antimicrobial therapy of rickettsial diseases. Antimicrob Agents Chemother 35: 2457–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith K, Leyden JJ, 2005. Safety of doxycycline and minocycline: a systematic review. Clin Ther 27: 1329–1342. [DOI] [PubMed] [Google Scholar]
- 22.Janardhanan J, Trowbridge P, Varghese GM, 2014. Diagnosis of scrub typhus. Expert Rev Anti Infect Ther 12: 1533–1540. [DOI] [PubMed] [Google Scholar]
- 23.Kim DM, Yun NR, Yang TY, Lee JH, Yang JT, Shim SK, Choi EN, Park MY, Lee SH, 2006. Usefulness of nested PCR for the diagnosis of scrub typhus in clinical practice: a prospective study. Am J Trop Med Hyg 75: 542–545. [PubMed] [Google Scholar]
- 24.Coleman RE, et al. , 2002. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg 67: 497–503. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Sung BJ, Youn TY, Chang WH, 1991. The longevity of immunofluorescent antibody in the patientsconfirmed as Tsutsugamushi disease. Korean Journal of Infectious Diseases 23: 19–23. [Google Scholar]
- 26.Kim DM, Lee YM, Back JH, Yang TY, Lee JH, Song HJ, Shim SK, Hwang KJ, Park MY, 2010. A serosurvey of Orientia tsutsugamushi from patients with scrub typhus. Clin Microbiol Infect 16: 447–451. [DOI] [PubMed] [Google Scholar]